Pathological cardiac hypertrophy is a major predictor for the development of cardiac diseases. Neuroendocrine factors such as norepinephrine and angiotensin II trigger Ca2+-dependent processes leading to cell growth and cardiac hypertrophy. Here, we report the identification of a constitutively active background Ca2+ entry (BGCE) pathway that is not affected by inhibition of voltage-gated Ca2+ channels but critically depends on the presence of TRPC1 and TRPC4 proteins, members of the TRP family of cation channels. It fine-tunes Ca2+ cycling in beating cardiomyocytes under basal conditions and during neurohumoral stimulation. Suppression of BGCE protects against development of maladaptive cardiac remodelling without evidence for alterations in cardiac or extra-cardiac functions and may represent a potential new therapeutic strategy to attenuate the pathogenesis of associated diseases.
Keywords: Calcium, Ion channels, Cardiac remodelling, Background Ca2+ entry, TRPC1/TRPC4
Abstract
Aims
Pathological cardiac hypertrophy is a major predictor for the development of cardiac diseases. It is associated with chronic neurohumoral stimulation and with altered cardiac Ca2+ signalling in cardiomyocytes. TRPC proteins form agonist-induced cation channels, but their functional role for Ca2+ homeostasis in cardiomyocytes during fast cytosolic Ca2+ cycling and neurohumoral stimulation leading to hypertrophy is unknown.
Methods and results
In a systematic analysis of multiple knockout mice using fluorescence imaging of electrically paced adult ventricular cardiomyocytes and Mn2+-quench microfluorimetry, we identified a background Ca2+ entry (BGCE) pathway that critically depends on TRPC1/C4 proteins but not others such as TRPC3/C6. Reduction of BGCE in TRPC1/C4-deficient cardiomyocytes lowers diastolic and systolic Ca2+ concentrations both, under basal conditions and under neurohumoral stimulation without affecting cardiac contractility measured in isolated hearts and in vivo. Neurohumoral-induced cardiac hypertrophy as well as the expression of foetal genes (ANP, BNP) and genes regulated by Ca2+-dependent signalling (RCAN1-4, myomaxin) was reduced in TRPC1/C4 knockout (DKO), but not in TRPC1- or TRPC4-single knockout mice. Pressure overload-induced hypertrophy and interstitial fibrosis were both ameliorated in TRPC1/C4-DKO mice, whereas they did not show alterations in other cardiovascular parameters contributing to systemic neurohumoral-induced hypertrophy such as renin secretion and blood pressure.
Conclusions
The constitutively active TRPC1/C4-dependent BGCE fine-tunes Ca2+ cycling in beating adult cardiomyocytes. TRPC1/C4-gene inactivation protects against development of maladaptive cardiac remodelling without altering cardiac or extracardiac functions contributing to this pathogenesis.
Translational perspective.
Pathological cardiac hypertrophy is a major predictor for the development of cardiac diseases. Neuroendocrine factors such as norepinephrine and angiotensin II trigger Ca2+-dependent processes leading to cell growth and cardiac hypertrophy. Here, we report the identification of a constitutively active background Ca2+ entry (BGCE) pathway that is not affected by inhibition of voltage-gated Ca2+ channels but critically depends on the presence of TRPC1 and TRPC4 proteins, members of the TRP family of cation channels. It fine-tunes Ca2+ cycling in beating cardiomyocytes under basal conditions and during neurohumoral stimulation. Suppression of BGCE protects against development of maladaptive cardiac remodelling without evidence for alterations in cardiac or extra-cardiac functions and may represent a potential new therapeutic strategy to attenuate the pathogenesis of associated diseases.
Introduction
Pathological cardiac hypertrophy is a major predictor for the development of cardiac diseases.1 In cardiomyocytes, neurohumoral stimuli such as catecholamines or angiotensin II (AngII) activate G-protein-dependent signalling pathways that stimulate Ca2+ dependent processes leading to myocyte growth and cardiac hypertrophy.2 Beating cardiomyocytes display fast cytosolic Ca2+ cycling originating from a spatiotemporally coordinated interplay of voltage-gated Ca2+ channels, Na+–Ca2+ exchangers, ryanodine receptors, and plasma membrane and SERCA pumps.3 It is unclear to which extent cardiac remodelling processes associated with cardiac hypertrophy are caused solely by modulating channels and pumps involved in fast Ca2+ cycling or whether alternative Ca2+ entry pathways may fine-tune cardiac Ca2+ homeostasis persistently and contribute to cardiac hypertrophy.4 A longer lasting modulation of diastolic Ca2+ levels has been suggested as a regulatory mechanism for the development of cardiac hypertrophy, which can result from alterations of sarcoplasmic reticulum (SR) Ca2+ release and/or by altered expression of proteins involved in plasma membrane Ca2+ transport.4 The complexity of Ca2+-dependent regulation of cardiac hypertrophy in vivo becomes evident as a large increase or reduction of voltage-gated L-type Ca2+ currents both result in mild cardiac hypertrophy.5,6
TRPC proteins form ion channels that contribute to Ca2+ entry directly or indirectly through activation of voltage-gated Ca2+ channels.7 The effects of over-expressing TRPC transgenes or dominant negative variants and knockdown of TRPC genes suggested that members of the TRPC subfamily may be involved in cardiac Ca2+ signalling and hypertrophy development.8–12 However, their impact on the diastolic level and amplitude of fast cytosolic Ca2+ cycling in beating adult cardiomyocytes and during neurohumoral stimulation triggering hypertrophy is unknown. Nevertheless, it was proposed that substances antagonizing TRPC channels may work as drugs to suppress cardiac hypertrophy. To develop such antagonists, it has to be considered, which specific members of the TRPC subfamily are essential constituents of channels involved in cardiac remodelling processes. Additionally, it is still unknown whether these proteins affect other cardiovascular functions and whether extra-cardiac TRPC channels alter the development of cardiac hypertrophy. Here, we identify TRPC1 and TRPC4 as constituents of a novel background Ca2+ entry (BGCE) pathway in adult cardiomyocytes, which determines diastolic and systolic Ca2+ concentrations in beating cells. TRPC1 and TRPC4 together are required for maladaptive cardiac remodelling without affecting cardiac contractility and rhythmicity or systemic blood pressure.
Methods
An expanded Materials and methods is available at Supplementary material online.
Animal experiments
All animal experiments were reviewed and approved in accordance with the ethic regulations and the animal welfare committees of the Universities of Saarland and Heidelberg. Isoproterenol (Iso, 30 mg/kg/day) and AngII (3 mg/kg/day) were infused via Alzet pumps over 7 and 14 days, respectively; pumps filled with saline (NaCl 0.9%) were used as control. Blood pressure and heart rate were measured with telemetric transmitters (PA-C10, Data Science International). Ivabradine was administered via chow pellets. Transverse aortic constriction (TAC, 5 weeks) was performed by tying a suture ligature against a 27-gauge needle and control mice underwent a sham operation. Plasma angiotensinogen and AngII concentrations were determined by Angiotensinogen (JP27413, IBL, Germany) and AngII (Uscn, Life Science Inc.) ELISA assays. Renin activity from the perfusate of isolated kidneys was determined from quantification of AngI by radioimmunoassay (Byk and DiaSorin). To measure cardiac function, a conductance catheter (SPR-835, Millar) inserted into the left ventricular from hearts mounted in a working heart apparatus (Hugo Sachs Elektronik-Harvard) was used. Cardiac dimensions and contractility were assessed by echocardiography under anaesthesia (1.5% Isoflurane) using a Vevo 770 system (Visualsonics).
Fluorescence imaging of adult mouse cardiomyocytes
Electrically evoked Ca2+ transients were measured in Fura2-loaded ventricular adult mouse myocyte using a ‘post-rest’ protocol. Sarcoplasmic reticulum-Ca2+ content was analysed in cardiomyocytes from caffeine-induced Ca2+ transients. Ca2+ sparks were studied in Fluo4-loaded myocytes at imaging frequencies of 100 Hz. Mn2+-induced Fura2-fluorescence quenching was employed to measure Ca2+ entry using nominally Ca2+-free solutions. Iso and AngII stimulation of adult mouse ventricular myocytes was performed after reaching steady-state conditions by field stimulation (5 min, 1 Hz, 12 V, alternating polarities).
RNA isolation and expression analysis
Total heart RNA was isolated with peqGOLD-RNAPure reagent (peqLab) and purified by extraction steps with diethyl ether. Quantity, purity, and integrity of the RNA samples were controlled by spectrophotometry (NanoQuant, Tecan) and microfluidic analysis (Bioanalyzer 2100, Agilent Technologies). For real-time quantitative PCR, 10 ng cDNA were used for TaqMan Gene Expression Assay. Quantitative PCR was performed with a Universal Probe Library (Roche) for Rcan1-4 and Myomaxin, and the specific TaqMan probe for mouse GAPDH (Applied Biosystems, Product Nr. Mm03302249) and detection on a 7500-Fast Cycler (Applied Biosystems). For expression analysis by NCounter NanoString technology RNA was hybridized with a Nanostring Gene Expression CodeSet and analysed using the nCounter Digital Analyzer. Corresponding counts (normalized counts) are presented relative to expression levels of control genes (Actb, Hprt1, Tbp, Ubc, and Gapdh).
Histopathological analysis
Images were acquired with a digital colour camera (MRc5, Zeiss) and were analysed with the AxioVision software (Zeiss). To quantify the cross-sectional area >100 cells/mouse were analysed through the left ventricle free wall. Quantification of interstitial cardiac fibrosis was performed with the AutoMeasure module of AxioVision software 4.8.1 (Zeiss).
Data analysis
Analyses were made with Origin v8.1.13.88 (OriginLab Corporation, USA) or Prism 5.0c (GraphPad Software Inc.). Data are displayed as bar graphs or scatter plots with indication of mean ± SD. All analyses were made using each mouse as experimental unit. Statistical analyses were performed using the appropriate tests (unpaired or paired t-test, one- or two-way ANOVA, Mann–Whitney or Kruskal–Wallis) depending on the result of normality tests (Shapiro-Wilk or D‘Agostino-Pearson omnibus) and Levene's test for equal variance. Differences with P < 0.05 were considered as statistically significant. Significances were depicted as actual P-values or as *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
TRPC1 and TRPC4 form a background Ca2+ entry pathway that determines diastolic and systolic [Ca2+]i and responses to neurohumoral stimulation
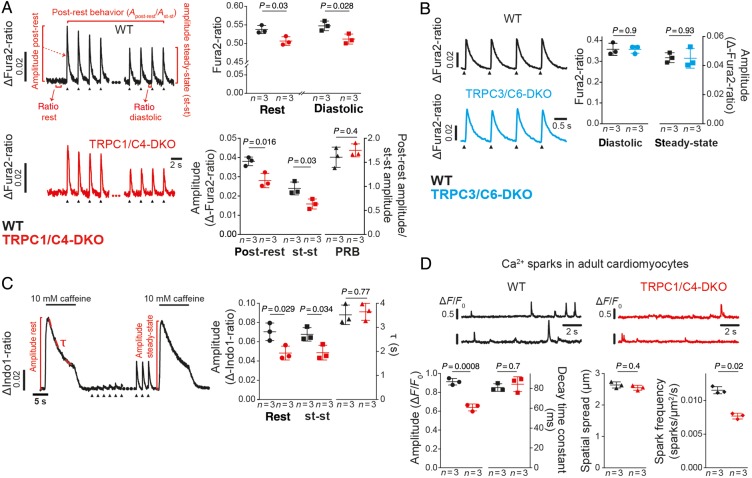
We studied putative effects of TRPC1/C4 and TRPC3/C6 double knockout (DKO) on changes of intracellular Ca2+ in electrically paced adult ventricular myocytes. Resting and diastolic Ca2+ concentrations and the amplitude of Ca2+ transients were reduced in TRPC1/C4-DKO but not in TRPC3/C6-DKO (Figure 1A and B) whereas the post-rest behaviour of global calcium transients (Figure 1A) and the current density of voltage-gated L-type Ca2+ channels (see Supplementary material online, Figure S1A) were unaltered in TRPC1/C4-DKO.
Figure 1.
Ca2+ signalling in cardiomyocytes from TRPC1/C4-DKO is reduced. (A) Representative electrically evoked Ca2+ transients (0.5 Hz, arrow heads) in adult ventricular myocytes. Here resting Ca2+ (rest) and diastolic Ca2+ levels during steady-state (st-st) pacing, and amplitudes of Ca2+ transients post-rest and during steady-state pacing are depicted. The post-rest behaviour, PRB, (post-rest amplitude/st-st amplitude) is shown (44–55 cells/group). (B) Analysis of electrically evoked Ca2+ transients (1 Hz) in TRPC3/C6-DKO myocytes (119–191 cells/group). (C) Analysis of caffeine-induced Ca2+ transients in resting cardiomyocytes or immediately after a 3-min pacing period (st-st conditions). Typical transients are depicted highlighting the amplitudes (65–75 cells/group) and decay time constant (τ) (52–68 cells/group). (D) Recordings of two different spark sites and the corresponding analysis of decay time constant, spatial spread and frequency (24–32 cells/group).
Sarcoplasmic reticulum-Ca2+ content, estimated from the amplitude of caffeine-induced Ca2+ transients, was reduced under resting and steady-state conditions; however, Ca2+ extrusion, which is mainly mediated by NCX activity in mice13 and can be correlated with the decay time constant of the caffeine-induced transients (Figure 1C), was unchanged in TRPC1/C4-DKO mice as was the decay of electrically evoked Ca2+ transients, which correlates with SERCA activity (see Supplementary material online, Figure S1B). Analysis of Ca2+ sparks revealed that the amplitude and frequency of Ca2+, but not the decay time constant and spatial spread, were reduced in cells from TRPC1/C4-DKO (Figure 1D).
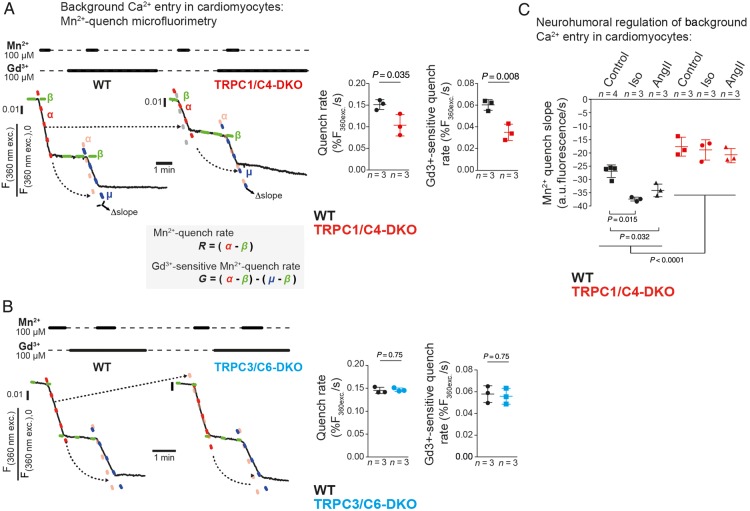
To investigate Ca2+ entry across the plasma membrane, we used Mn2+-quench microfluorimetry. Figure 2A shows a typical Fura2-fluorescence trace with the rate of quenching (α) being proportional to the magnitude of Mn2+ entry. The basal Mn2+ quench rate and the Gd3+-sensitive portion of this Mn2+-entry were reduced in TRPC1/C4-DKO cells (Figure 2A). This Mn2+-entry was not affected by voltage-gated calcium channel blockers (see Supplementary material online, Figure S1C) or deletion of TRPC3/C6 proteins (Figure 2B). Importantly, we found a significantly larger Mn2+ entry in WT cardiomyocytes after chronic neurohormonal stimulation with Iso or AngII but this increase was abrogated in TRPC1/C4-DKO cells (Figure 2C).
Figure 2.
TRPC1/C4 form a background Ca2+ entry pathway in adult cardiomyocytes. (A) For quantifying constitutively active background Ca2+ entry pathways, we linearly approximated Mn2+-quench rates (α or μ) and corrected them for bleaching of Fura2 (β). Quenching rates in the absence (R) and presence of Gd3+ (G, as ‘Δslope’) were analysed (24–32 cells/group). (B) Mn2+-quench in WT and TRPC3/C6-DKO cardiomyocytes (24–26 cells per group) as described in (A). (C) Mn2+-quench (1 mM, 37°C) in cells from mice treated with Iso or AngII (174–471 cells/group).
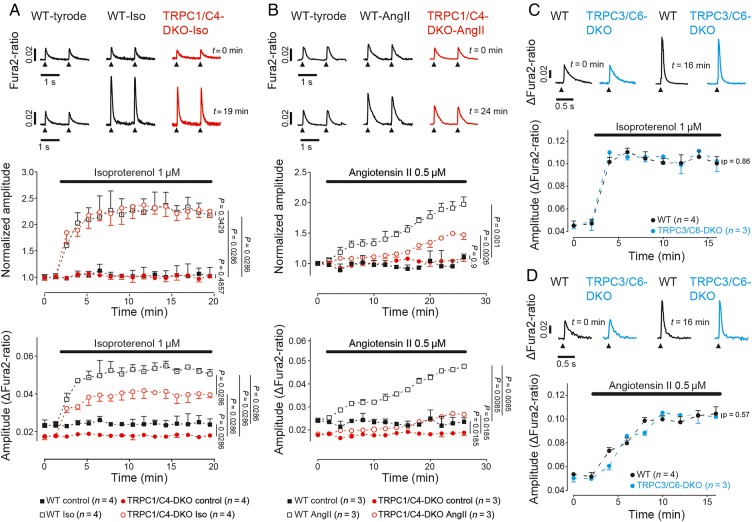
Next, we addressed the question whether and how the lack of TRPC1/C4 might modulate Iso- and AngII-evoked changes of Ca2+ transients. In cardiomyocytes from WT and TRPC1/C4-DKO mice application of Iso resulted in an equal relative increase in the amplitude of Ca2+ transients, whereas peak Fura2-ratios were substantially reduced in TRPC1/C4-DKO mice (Figure 3A; see Supplementary material online, FigureS2A). Interestingly, both, peak Fura2-ratios and their relative increases during AngII-application, were significantly reduced (Figure 3B; see Supplementary material online, FigureS2B). In cardiomyocytes from TRPC3/C6-DKO mice, Ca2+ responses to Iso or AngII stimulation were not altered (Figure 3C and D; see Supplementary material online, FigureS2C and D).
Figure 3.
Contribution of the TRPC1/C4-mediated Ca2+ entry pathway to cellular Ca2+ responses after neurohumoral stimulation. Effect of Iso (A and C) or AngII (B and D) on electrically evoked Ca2+ transients (1 Hz) in cardiomyocytes from TRPC1/C4-DKO (A and B, 15–40 cells/ group) or TRPC3/C6-DKO (C and D, 105–190 cells/group). The relative changes of the Ca2+ transient amplitude normalized to the control period at the time point t = 0 (A and B), and as absolute Fura2-ratio changes (A–D) are presented.
TRPC1/C4-DKO are protected from development of maladaptive cardiac hypertrophy
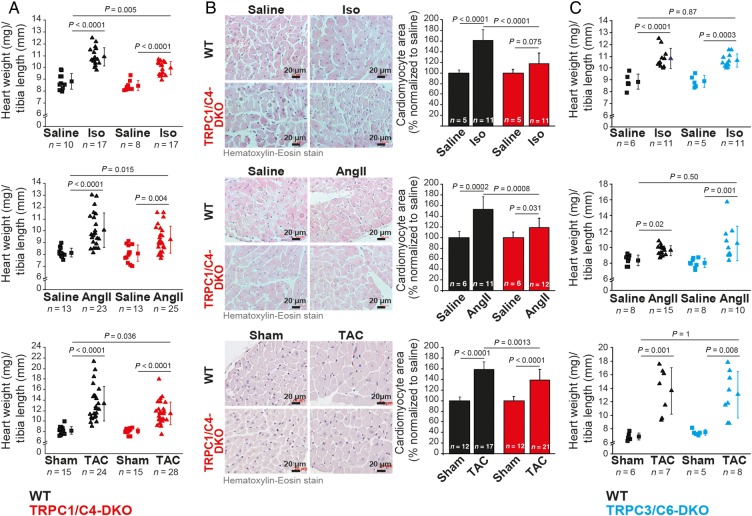
To identify the contribution of BGCE and TRPCs (see Supplementary material online, Figure S3) to the development of cardiac hypertrophy, we analysed responses of TRPC1/C4-DKO mice to systemic infusion of Iso or AngII, or to increased afterload (TAC). In TRPC1/C4-DKO mice, cardiac hypertrophy was significantly reduced in all three conditions (Figure 4A and B). TRPC1 and TRPC4 are thought to interact with TRPC5,14 which appears to be up-regulated in patients with cardiac failure.15 We therefore analysed TRPC1/C4/5 triple KO mice and found no additional reduction in Iso-induced hypertrophy compared with TRPC1/C4-DKO mice (see Supplementary material online, Figure S4). However, mice lacking either TRPC1 or TRPC4 showed no reduced Iso- or AngII-induced hypertrophy responses (see Supplementary material online, Figure S5). Because cardiac-specific over-expression of TRPC6 and TRPC3 resulted in cardiac hypertrophy and failure,11,12 we tested whether both proteins have a causative role in these processes. We analysed TRPC3/C6-DKO mice to avoid compensatory effects of these TRPCs,16 but cardiac hypertrophy and fibrosis were not reduced (Figure 4C; see Supplementary material online, Figure S6).
Figure 4.
Cardiac hypertrophy is reduced in TRPC1/C4-DKO mice. Analysis of cardiac hypertrophy in TRPC1/C4-DKO (A and B) and in TRPC3/C6-DKO (C) mice after Iso, AngII, or aortic banding. (B) Analysis of cardiomyocytes cross-sectional area from mice in (A).
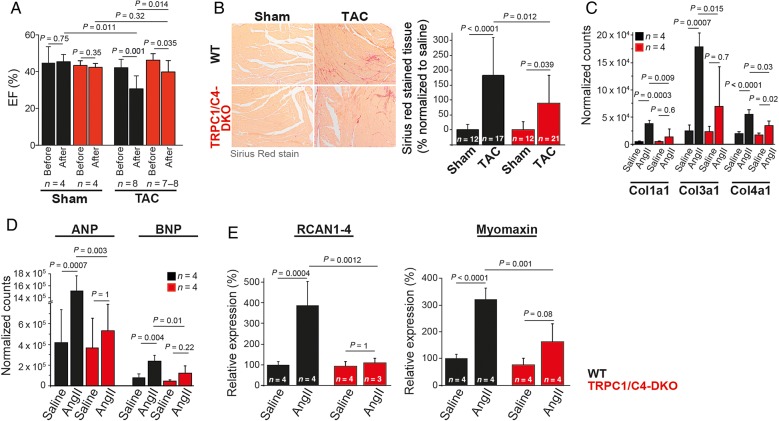
In adverse cardiac remodelling, myocardial hypertrophy is accompanied by reduction of cardiac function, interstitial fibrosis, and the activation of a foetal gene program. In TRPC1/C4-DKO mice, both the TAC-evoked reduction in ejection fraction (WT: −28.6%, DKO: −14.1%; Figure 5A and Table 1) and the interstitial fibrosis (Figure 5B) were reduced by ∼50%. Accordingly, AngII-induced increases in col1a1, col3a1, and col4a1 (Figure 5C) and in foetal gene expression, i.e. Nppa and Nppb (Figure 5D), were diminished. To characterize Ca2+-dependent downstream signalling pathways involved in adverse cardiac remodelling, we quantified the expression RCAN1-4 as an endogenous calcineurin reporter17 and myomaxin as a transcriptional target of MEF2a.18 Expression of both was reduced in TRPC1/C4-DKO mice (Figure 5E).
Figure 5.
Reduced pathological cardiac hypertrophy in TRPC1/C4-DKO mice. (A) Ejection fraction (EF) measured by echocardiography before and 5 weeks after TAC. (B) Reduced ventricular fibrosis in TRPC1/C4-DKO mice after TAC. Expression analysis of collagen genes (C), ANP and BNP (D), and the reporters of Ca2+-dependent hypertrophy signalling through NFAT and MEF2, RCAN and Myomaxin (E) after AngII infusion.
Table 1.
Cardiac function analysed by echocardiography
| Parameters | WT | TRPC1/C4-DKO | WT |
TRPC1/C4-DKO |
P-value TAC vs. TAC | ||
|---|---|---|---|---|---|---|---|
| Basal (n = 12) | Basal (n = 12) | Sham (n = 4) | TAC (n = 8) | Sham (n = 4) | TAC (n = 7) | ||
| Body weight (g) | 30.8 ± 1.5 | 30.4 ± 2.8 | 28.5 ± 5.2 | 31.6 ± 1.8 | 31.2 ± 0.96 | 31.1 ± 2.8 | |
| P-value | 0.683 | 0.146 | 0.935 | 0.690 | |||
| HR (BPM) | 425 ± 32 | 382 ± 34 | 400 ± 36 | 481 ± 35 | 368 ± 32 | 507 ± 53 | |
| P-value | 0.015 | 0.004 | 0.0012 | 0.280 | |||
| LV volume diastole (µL) | 96.1 ± 9.9 | 88.7 ± 9.9 | 101.3 ± 6.7 | 112.9 ± 18 | 95.0 ± 7.9 | 89.8 ± 13.1 | |
| P-value | 0.161 | 0.326 | 0.491 | 0.016 | |||
| LV volume systole (µL) | 55.1 ± 11.3 | 47.9 ± 7.4 | 53.1 ± 4.1 | 77.7 ± 21 | 54.8 ± 5.6 | 55.2 ± 13.1 | |
| P-value | 0.095 | 0.089 | 0.961 | 0.030 | |||
| EF (%) | 43.0 ± 5.5 | 46.2 ± 3.4 | 45.4 ± 4.0 | 30.6 ± 7.0 | 42.3 ± 2.0 | 39.8 ± 6.2 | |
| P-value | 0.303 | 0.008 | 0.468 | 0.019 | |||
| MVE-wave (mm/s) | 993 ± 142 | 930 ± 157 | 859 ± 35 | 1099 ± 142 | 913 ± 99 | 1283 ± 227 | |
| P-value | 0.330 | 0.021 | 0.014 | 0.078 | |||
| MVA-wave (mm/s) | 643 ± 49 | 620 ± 137 | 577 ± 46 | 448 ± 296 | 542 ± 62 | 333 ± 244 | |
| P-value | 0.593 | 0.498 | 0.149 | 0.596 | |||
| IVRT (ms) | 15.0 ± 2.2 | 16.0 ± 2.1 | 14.7 ± 2.3 | 11.1 ± 1.9 | 16.2 ± 1.6 | 9.9 ± 2.9 | |
| P-value | 0.318 | 0.014 | 0.003 | 0.358 | |||
| MV E/A | 1.55 ± 0.28 | 1.52 ± 0.18 | 1.46 ± 0.15 | 3.58 ± 3.9 | 1.69 ± 0.23 | 5.75 ± 3.7 | |
| P-value | 0.739 | 0.329 | 0.073 | 0.457 | |||
LV, left ventricle; EF, ejection fraction; IVRT, isovolumetric relaxation time; MVE, mitral valve early wave peak; MVA, mitral valve atrial wave peak.
Systemic pathways possibly contributing to the reduced cardiac hypertrophy in TRPC1/C4-DKO mice
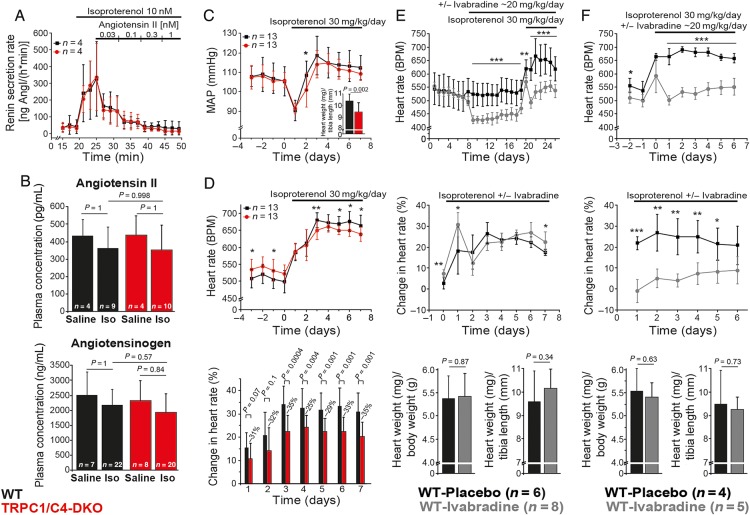
Systemic administration of Iso or AngII activates signalling pathways in multiple organ systems that could influence the development of cardiac hypertrophy. However, Iso-induced renin release and its suppression by AngII were unaffected in TRPC1/C4-DKO (Figure 6A), as well as plasma AngII and angiotensinogen concentrations (Figure 6B). Likewise, Iso infusion resulted in a transient increase in mean arterial blood pressure (MAP), but it was indistinguishable from pre-implantation levels at Day 7 in both genotypes, while hypertrophic responses were still reduced (Figure 6C, inset). In contrast, AngII infusion in WT mice resulted in an ∼45 mmHg increase in MAP (145.4 ± 10.2 mmHg; n = 5, P = 0.0006), but hypertrophy development was smaller compared with Iso treatment (see Supplementary material online, Figure S7A) supporting our notion that reduced hypertrophy in TRPC1/C4-DKO mice was not dependent on altered MAP responses. Intra-ventricular pressure measurements performed at the end of Iso treatment revealed no alterations in contractility in TRPC1/C4-DKO mice (see Supplementary material online, Table S1). Comparable results were acquired from working heart experiments using untreated mice except of a 12% increase in time constant of relaxation (Table 2). Nevertheless, parameters of the end-diastolic pressure–volume relationship were not altered in untreated TRPC1/C4-DKO mice and a detailed echocardiographic analysis revealed normal systolic and diastolic functions (Table 1).
Figure 6.
Systemic pathways contributing to the development of cardiac hypertrophy in TRPC1/C4-DKO. (A) Renin secretion in isolated perfused kidneys. (B) AngII and angiotensinogen concentration after Iso infusion. (C) Mean arterial blood pressure from conscious mice and corresponding cardiac hypertrophy (inset). (D) Mean heart rate from conscious mice before and during Iso treatment, and percent Iso-induced change of heart rate (normalized to mean of days −3 to 0). (E and F) No effect of heart rate reduction on cardiac hypertrophy indexes by treatment with Ivabradine before and during (E) or only during (F) Iso treatment.
Table 2.
Cardiac function analysed in working heart apparatus
| Parameters | WT (n=9) | TRPC1/C4-DKO (n = 10) | P-value |
|---|---|---|---|
| Heart rate (BPM) | 418 ± 48.0 | 425 ± 30.1 | 0.689 |
| Systolic parameters | |||
| Cardiac output (mL/min) | 13.1 ± 2.5 | 12.7 ± 2.6 | 0.718 |
| Ejection fraction (%) | 54.2 ± 9.8 | 56.5 ± 9.6 | 0.599 |
| dP/dtmax (mmHg/s) | 5298 ± 732 | 5240 ± 574 | 0.850 |
| End-systolic volume at 100 mmHg (µL) | 42.1 ± 10.6 (n = 8) | 34.4 ± 7.5 (n = 9) | 0.102 |
| ESPVR | |||
| Ees (mmHg/µL) | 2.2 ± 0.8 (n = 8) | 3.1 ± 1.0 (n = 9) | 0.079 |
| Vo (µL) | 9.6 ± 34.3 | −6.0 ± 43.0 | 0.427 |
| Diastolic parameters | |||
| Time constant of relaxation (τ) during isovolumetric diastole (ms) | 9.8 ± 0.7 | 11.0 ± 0.8 | 0.005 |
| End-diastolic volume (µL) | 59.8 ± 14.5 | 53.6 ± 10.2 | 0.293 |
| dP/dtmin (mmHg/s) | −4232 ± 524 | −3963 ± 502 | 0.269 |
| EDPVR | |||
| k (mmHg) | 0.179 ± 0.438 | 0.051 ± 0.065 (n = 9) | 0.398 |
| β (µL−1) | 0.086 ± 0.039 | 0.108 ± 0.032 | 0.211 |
ESPVR, end-systolic pressure–volume relationship; Ees, end-systolic elastance; Vo, volume axis intercept; EDPVR, end-diastolic pressure–volume relationship; β, stiffness constant.
Because elevations in the beating frequency can increase diastolic Ca2+ concentrations and induce hypertrophy19 and Iso-induced chronotropic responses were reduced in TRPC1/C4-DKO mice (Figure 6D), we evaluated whether this impaired response may contribute to the protection of hypertrophy development in TRPC1/C4-DKO mice. We used Ivabradine to decrease heart rates before (Figure 6E) and during Iso infusion (Figure 6E and F); however, Ivabradine did not reduce Iso-induced hypertrophy (Figure 6E and F).
Discussion
Here, we identified a novel TRPC1/C4-dependent constitutively active BGCE pathway in cardiomyocytes that fine-tunes Ca2+ cycling and determines diastolic and systolic Ca2+ concentrations during basal electrical pacing and following neurohumoral stimulation. Likewise, TRPC1/C4-DKO mice develop reduced cardiac hypertrophy. Although other cardiac cell types could contribute to this phenotype, the decreased hypertrophic responses in TRPC1/C4-DKO mice can be explained by a reduced activity of the TRPC1/C4-dependent BGCE in adult ventricular myocytes which is not dependent on voltage-gated Ca2+ channels but is increased during neurohumoral-induced cardiac hypertrophy. Moreover, changes of diastolic and peak Ca2+ levels in TRPC1/C4-DKO cardiomyocytes were not brought about by alternative mechanisms, including decreased L-type Ca2+ current,3 SERCA pump activity or by increased Ca2+ extrusion from the cytosol by NCX or plasma membrane Ca2+ pump(s). Nevertheless, the SR-Ca2+ content was reduced resulting in lower amplitudes and frequencies of spontaneous Ca2+ sparks. Their decay time and spatial spread were unaltered, indicating that global as well as local Ca2+ transport systems were unchanged.
In myocytes from TRPC1/C4-DKO mice systolic Ca2+ peaks were reduced after acute stimulation with Iso or AngII. However, during Iso stimulation, the relative amplitude increase was unaltered, indicating that reduced peak Ca2+ solely resulted from the reduced diastolic levels. In contrast, AngII-evoked relative increases of the amplitude were reduced in TRPC1/C4-DKO mice suggesting that the TRPC1/C4-dependent Ca2+ entry pathway was activated by AngII, most likely via Gi/Gq-PLC signalling. Thus, the reduced hypertrophy for both in vivo stimulation regimes can be explained by a reduction in the absolute levels of diastolic and systolic Ca2+ in TRPC1/C4-DKO cardiomyocytes. TRPC3/C6 appear not to be part of BGCE; accordingly, diastolic and systolic Ca2+ levels were unaltered under basal conditions and during neurohumoral stimulation in cardiomyocytes from TRPC3/C6-DKO. Wu et al. used a common protocol to activate store-operated Ca2+ entry in non-excitable cells following SERCA inhibition.9 They found that expression of negative dominant variants of TRPCs suppressed this Ca2+ entry in ventricular cardiomyocytes, but Ca2+ homeostasis was not analysed in beating cardiomyocytes. Our results emphasize the role of TRPC1/TRPC4 but not of TRPC3/TRPC6 for the BGCE pathway described here contributing to Iso- and AngII-induced elevations of diastolic and systolic Ca2+ concentrations in cardiomyocytes and cardiac hypertrophy, and suggest an explanation why in TRPC3/C6-DKO mice development of cardiac hypertrophy is not reduced.
Transgenic models over-expressing TRPC proteins under a cardiomyocyte promotor implicated that TRPCs other than TRPC1/TRPC4 mediate hypertrophy development.9,11,12 Nevertheless, over-expression of TRPC variants may well execute their effects by interaction and functional interference with endogenous TRPC channel proteins in an off-target manner given the considerable amino acid sequence identity of the various TRPC proteins. In our models, the lack of TRPC3/C6 can apparently be compensated by other TRPC proteins and/or TRPC-independent pathways but the relevance of TRPC3/TRPC6 may be more prominent in a model on the C57Bl/6J genetic background8 in which nicotinamide nucleotide transhydrogenase inactivation20 may lead to accumulation of reactive oxygen species that enhance the activity of TRPC6-containing channels.21 Nevertheless, our findings do not preclude a role of TRPC3/C6 for other cardiac remodelling processes, e.g. during atrial fibrillation,22 Klotho23 absence or in scar formation after myocardial infarction.24,25 Moreover, we cannot exclude that acute pharmacological inhibition may be efficient for reducing cardiac hypertrophy as reported for the compound Pyr326; however, Pyr3 also blocks ORAI1-mediated store-operated Ca2+ entry27 which mediates growth of cultured cardiomyocytes.28 Our experiments demonstrate that development of cardiac hypertrophy is not reduced in TRPC1- or TRPC4-single KO mice. Seth et al.10 showed a reduced hypertrophic response induced by pressure overload or AngII administration in TRPC1-KO mice; however, their induction protocols and genetic background of the TRPC1-KO mice were different from ours.
We further addressed the question whether TRPCs in non-cardiac tissues play a role in the development of cardiac hypertrophy. Both, TRPC1 and TRPC4 are expressed in the kidney, suggesting that they may regulate blood pressure and/or renin secretion; however, we ruled out both possibilities. Additionally, the reduced Iso-evoked chronotropic response following inactivation of TRPC1/TRPC4, which may be caused by the lack of their modulatory role on sinoatrial node pacemaking29 is very unlikely to affect hypertrophy development since reduction of positive chronotropy using Ivabradine did not protect from development of cardiac hypertrophy as suggested by in vitro models.19 Additionally, in TRPC1/C4-DKO basal cardiac contractility assessed by echocardiography and working heart showed no compromising differences. In contrast, we demonstrate that TRPC1/TRPC4 inactivation protected against cardiac dysfunction evoked by chronically increased afterload.
Analysis of downstream signalling pathways revealed that the up-regulation of RCAN1-4 and myomaxin expression in the heart is mitigated in the absence of TRPC1/C4 implicating that TRPC1/C4-mediated BGCE contributed to the activation of the calcineurin-NFAT pathway. Myomaxin is a transcriptional target of MEF2a which acts as an integrator of multiple Ca2+-dependent signalling molecules including calcineurin and CaMKII30 in the regulation of transcriptional activity.31 Although the exact signalling cascade activated by BGCE needs to be explored, our results indicate that BGCE contributes to transcriptional reprogramming of the stressed myocardium including up-regulation of hypertrophy-associated foetal and collagen genes and development of interstitial fibrosis emphasizing its relevance for maladaptive cardiac remodelling processes.
In conclusion, TRPC1 and TRPC4 together form a novel BGCE which determines diastolic and systolic Ca2+ as well as their modulation by neurohumoral stimulation in beating cardiomyocytes. It is essential for the development of cardiac hypertrophy and fibrosis in mice. Because the deletion of either TRPC1 or TRPC4 alone appears to be compensated by TRPC4 or TRPC1, respectively, genetic strategies or small molecules specifically targeting the TRPC1/C4-containing Ca2+ entry channel complexes in the heart might represent a promising approach to ameliorate the development of pathological cardiac remodelling in vivo without altering blood pressure or cardiac contractility.
Supplementary Material
Supplementary Material is available at European Heart Journal online.
Authors’ contributions
J.E.C.L., L.K., M.F., and P.L. conceived and designed the research; J.E.C.L., Q.T., K.H., L.S., J.C.L., J.C.R., T.H., M.O., S.M., I.M., S.E.P., W.T., L.K., F.S., and P.L. acquired the data; A.D. and L.B. contributed material; J.E.C.L., J.C.R., I.M., F.S., M.F., and P.L. performed statistical analysis; M.F., V.F., L.K., and P.L. handled funding and supervision; J.E.C.L., M.F., and P.L. drafted the manuscript; and J.E.C.L., J.C.L., U.L., L.B., V.F., M.F., and P.L. made critical revision of the manuscript for key intellectual content.
Funding
This work was supported by the KFO 196 (M.F., P.L., V.F., L.K., and U.L.), GK-1326 (M.F., P.L., V.F., L.K., and S.P.), SFB-530 (M.F., V.F., P.L., and S.E.P.), the HOMFOR Program (P.L., L.K., V.F., and M.F.), the DZHK (German Centre for Cardiovascular Research) and the BMBF (German Ministry of Education and Research) (M.F., P.L., and L.K.), and the Intramural Research Program NIH project Z01-ES-101684 (L.B.).
Conflict of interest: none declared.
Acknowledgement
We are grateful to Sabine Schmidt, Christin Matka, Stefanie Bucholz, Anne Vecerdea, Tanja Volz, Ellen Becker, Manuela Ritzal, and Sabrina Hennig for expert technical assistance.
References
- 1.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest 2013;123:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 2006;7:589–600. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 2008;70:23–49. [DOI] [PubMed] [Google Scholar]
- 4.Goonasekera SA, Molkentin JD. Unraveling the secrets of a double life: contractile versus signaling Ca2+ in a cardiac myocyte. J Mol Cell Cardiol 2012;52:317–322. [DOI] [PubMed] [Google Scholar]
- 5.Beetz N, Hein L, Meszaros J, Gilsbach R, Barreto F, Meissner M, Hoppe UC, Schwartz A, Herzig S, Matthes J. Transgenic simulation of human heart failure-like L-type Ca2+-channels: Implications for fibrosis and heart rate in mice. Cardiovasc Res 2009;84:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, Reiken S, Elrod JW, Correll RN, York AJ, Sargent MA, Hofmann F, Moosmang S, Marks AR, Houser SR, Bers DM, Molkentin JD. Decreased cardiac L-type Ca(2)(+) channel activity induces hypertrophy and heart failure in mice. J Clin Invest 2012;122:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 2009;137:1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo K, Rainer PP, Shalkey Hahn V, Lee DI, Jo SH, Andersen A, Liu T, Xu X, Willette RN, Lepore JJ, Marino JP, Jr, Birnbaumer L, Schnackenberg CG, Kass DA. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc Natl Acad Sci USA 2014;111:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA 2010;107:7000–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L, Rosenberg P. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res 2009;105:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J 2006;20:1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 2006;116:3114–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ Res 2004;95:604–611. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 2002;99:7461–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem 2006;281:33487–33496. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol Cell Biol 2005;25:6980–6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res 2000;87:E61–E68. [DOI] [PubMed] [Google Scholar]
- 18.Huang HT, Brand OM, Mathew M, Ignatiou C, Ewen EP, McCalmon SA, Naya FJ. Myomaxin is a novel transcriptional target of MEF2A that encodes a Xin-related alpha-actinin-interacting protein. J Biol Chem 2006;281:39370–9. [DOI] [PubMed] [Google Scholar]
- 19.Colella M, Grisan F, Robert V, Turner JD, Thomas AP, Pozzan T. Ca2+ oscillation frequency decoding in cardiac cell hypertrophy: role of calcineurin/NFAT as Ca2+ signal integrators. Proc Natl Acad Sci USA 2008;105:2859–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang TT, Naeemuddin M, Elchuri S, Yamaguchi M, Kozy HM, Carlson EJ, Epstein CJ. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet 2006;15:1187–1194. [DOI] [PubMed] [Google Scholar]
- 21.Ding Y, Winters A, Ding M, Graham S, Akopova I, Muallem S, Wang Y, Hong JH, Gryczynski Z, Yang SH, Birnbaumer L, Ma R. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J Biol Chem 2011;286:31799–31809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harada M, Luo X, Qi XY, Tadevosyan A, Maguy A, Ordog B, Ledoux J, Kato T, Naud P, Voigt N, Shi Y, Kamiya K, Murohara T, Kodama I, Tardif JC, Schotten U, Van Wagoner DR, Dobrev D, Nattel S. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 2012;126:2051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 2012;3:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida M, Onohara N, Sato Y, Suda R, Ogushi M, Tanabe S, Inoue R, Mori Y, Kurose H. Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem 2007;282:23117–23128. [DOI] [PubMed] [Google Scholar]
- 25.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell 2012;23:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, Yoshida T, Wakamori M, Mori E, Numata T, Ishii M, Takemoto H, Ojida A, Watanabe K, Uemura A, Kurose H, Morii T, Kobayashi T, Sato Y, Sato C, Hamachi I, Mori Y. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci USA 2009;106:5400–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schleifer H, Doleschal B, Lichtenegger M, Oppenrieder R, Derler I, Frischauf I, Glasnov T, Kappe C, Romanin C, Groschner K. Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca(2+) entry pathways. Br J Pharmacol 2012;167:1712–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, Sussman MA, Most P. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol 2010;48:1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju YK, Chu Y, Chaulet H, Lai D, Gervasio OL, Graham RM, Cannell MB, Allen DG. Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circ Res 2007;100:1605–1614. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem 2007;282:35078–35087. [DOI] [PubMed] [Google Scholar]
- 31.McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci 2002;27:40–47. [DOI] [PubMed] [Google Scholar]