Abstract
Background
Stromal cell-derived factor-1 (SDF-1) promotes tissue repair through mechanisms of cell survival, endogenous stem cell recruitment, and vasculogenesis. Stromal Cell-Derived Factor-1 Plasmid Treatment for Patients with Heart Failure (STOP-HF) is a Phase II, double-blind, randomized, placebo-controlled trial to evaluate safety and efficacy of a single treatment of plasmid stromal cell-derived factor-1 (pSDF-1) delivered via endomyocardial injection to patients with ischaemic heart failure (IHF).
Methods
Ninety-three subjects with IHF on stable guideline-based medical therapy and left ventricular ejection fraction (LVEF) ≤40%, completed Minnesota Living with Heart Failure Questionnaire (MLWHFQ) and 6-min walk distance (6 MWD), were randomized 1 : 1 : 1 to receive a single treatment of either a 15 or 30 mg dose of pSDF-1 or placebo via endomyocardial injections. Safety and efficacy parameters were assessed at 4 and 12 months after injection. Left ventricular functional and structural measures were assessed by contrast echocardiography and quantified by a blinded independent core laboratory. Stromal Cell-Derived Factor-1 Plasmid Treatment for Patients with Heart Failure was powered based on change in 6 MWD and MLWHFQ at 4 months.
Results
Subject profiles at baseline were (mean ± SD): age 65 ± 9 years, LVEF 28 ± 7%, left ventricular end-systolic volume (LVESV) 167 ± 66 mL, N-terminal pro brain natriuretic peptide (BNP) (NTproBNP) 1120 ± 1084 pg/mL, MLWHFQ 50 ± 20 points, and 6 MWD 289 ± 99 m. Patients were 11 ± 9 years post most recent myocardial infarction. Study injections were delivered without serious adverse events in all subjects. Sixty-two patients received drug with no unanticipated serious product-related adverse events. The primary endpoint was a composite of change in 6 MWD and MLWHFQ from baseline to 4 months follow-up. The primary endpoint was not met (P = 0.89). For the patients treated with pSDF-1, there was a trend toward an improvement in LVEF at 12 months (placebo vs. 15 mg vs. 30 mg ΔLVEF: −2 vs. −0.5 vs. 1.5%, P = 0.20). A pre-specified analysis of the effects of pSDF-1 based on tertiles of LVEF at entry revealed improvements in EF and LVESV from lowest-to-highest LVEF. Patients in the first tertile of EF (<26%) that received 30 mg of pSDF-1 demonstrated a 7% increase in EF compared with a 4% decrease in placebo (ΔLVEF = 11%, P = 0.01) at 12 months. There was also a trend towards improvement in LVESV, with treated patients demonstrating an 18.5 mL decrease compared with a 15 mL increase for placebo at 12 months (ΔLVESV = 33.5 mL, P = 0.12). The change in end-diastolic and end-systolic volume equated to a 14 mL increase in stroke volume in the patients treated with 30 mg of pSDF-1 compared with a decrease of −11 mL in the placebo group (ΔSV = 25 mL, P = 0.09). In addition, the 30 mg-treated cohort exhibited a trend towards improvement in NTproBNP compared with placebo at 12 months (−784 pg/mL, P = 0.23).
Conclusions
The blinded placebo-controlled STOP-HF trial demonstrated the safety of a single endocardial administration of pSDF-1 but failed to demonstrate its primary endpoint of improved composite score at 4 months after treatment. Through a pre-specified analysis the STOP-HF trial demonstrates the potential for attenuating LV remodelling and improving EF in high-risk ischaemic cardiomyopathy. The safety profile supports repeat dosing with pSDF-1 and the degree of left ventricular remodelling suggests the potential for improved outcomes in larger future trials.
Keywords: Gene therapy, Stem cell homing, Chronic heart failure, Endogenous tissue repair
See page 2207 for the editorial comment on this article (doi:10.1093/eurheartj/ehv258)
Introduction
Heart failure (HF) from ischaemic heart disease is one of the leading causes of morbidity and mortality in Westernized countries. Because of the growing population there has been significant focus on the development of novel effective approaches that could improve quality of life, stabilize or improve cardiac function, and improve clinical outcomes such as HF-related hospitalizations and mortality.
The stromal cell-derived factor-1 (SDF-1), and its receptor, CXCR4, or SDF-1:CXCR4 axis, has emerged as a key regulator in endogenous tissue repair.1–4 Specifically, the over-expression of SDF-1 has been shown to induce homing of both bone marrow-derived and cardiac stem cells to the site of ischaemic injury,1 inhibits cardiac myocyte cell death,5,6 and leads to prevention and remodelling of myocardial scar.7–9 JVS-100, a non-viral, naked DNA plasmid encoding human SDF-1, was developed to extend the expression of SDF-1 for a sufficient amount of time to induce stem cell homing to the myocardium.7 Pre-clinical studies with JVS-100 demonstrated the potential for direct injection of hSDF-1 plasmid into the border zone of chronically remodelled post-MI myocardium with resultant improvement in cardiac function and cardiac vascular density.7,8 Furthermore, in a porcine model of HF, delivery of JVS-100 with an endomyocardial injection catheter (BioCardia Helical Infusion Catheter (Helix), BioCardia, Inc., San Carlos, CA, USA) demonstrated safety at doses up to 100 mg while improving cardiac function and vasculogenesis up to 90 days post-injection at doses of 7.5 and 30 mg.8 The ability of SDF-1 to positively impact stem cell homing, reduce programmed cell death, and promote revascularization makes non-viral SDF-1 gene transfer a promising candidate for treating HF due to ischaemic heart disease and potentially other forms of cardiomyopathy as well.
The safety and potential efficacy of JVS-100 was initially demonstrated in an open-label Phase I study in patients with ischaemic cardiomyopathy with moderately dilated left ventricular chamber dimensions.10 The findings of this Phase I study led to design of the SDF-1 Plasmid Treatment for Patients with Heart Failure (STOP-HF) study to assess the effects of hSDF-1 over-expression in a randomized, double-blind, placebo-controlled study in which patients were dosed with JVS-100 and were followed for 1 year. The STOP-HF study was powered based on changes in 6-min walk distance (6 MWD) and Minnesota Living with Heart Failure Quality-of-Life Questionnaire (MLWHFQ) findings from the open-label Phase I in moderate risk (Stage C) HF patients. Patients were followed for secondary endpoints, which included changes in cardiac structure, clinical status, and biomarkers at 4 and 12 months after treatment.
Methods
Patient population
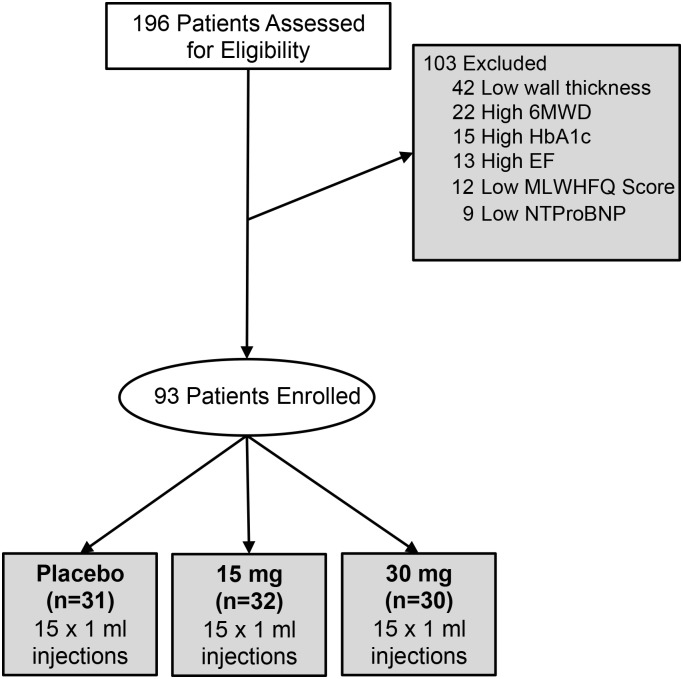
A total of 196 patients with a known history of systolic dysfunction due to prior myocardial infarction and symptomatic but stable HF were screened for participation in the STOP-HF trial (ClinicalTrials.gov—NCT01643590), under approval by the local institutional review boards. Potential study subjects who met all inclusion and exclusion criteria and granted written informed consent to participate were entered in the study. All subjects were on stable optimal medical therapy defined as ACE-I/ARB, β-blocker, aspirin (ASA), statin and diuretic with <50% change in dose over the previous 30 days (unless contraindicated) and had an ICD or cardiac resynchronization therapy-defibrillator present for over 3 months at the start of the trial. Study inclusion criteria also included left ventricular ejection fraction (LVEF) ≤40% (as read by the Cleveland Clinic C5 Research echo core lab), MLWHFQ score ≥20 points, and 6 MWD ≤400 m. Primary exclusion criteria were a minimum wall thickness of <0.3 cm for any segment, estimated glomerular filtration rate <30 mL/min, HgbA-1C > 9 mg/dL, a history of moderate or severe aortic valve regurgitation and aortic stenosis with valve area <1.5 cm2, any history of cancer (excluding curable non-melanoma skin malignancy or resection with no recurrence in 5 years), presence of LV thrombus, or persistent atrial fibrillation). A total of 93 subjects were enrolled and randomized 1 : 1 : 1 to receive a single treatment of either a 15 or 30 mg dose of plasmid stromal cell-derived factor-1 (pSDF-1) or placebo via a series of 15 endomyocardial injections with the BioCardia Helix as previously described.10 Subjects were not considered enrolled until they entered the laboratory in preparation for the injection procedure (Figure 1).
Figure 1.
Consort diagram of patients screened and enrolled between May 2012 and September 2013.
Study design
STOP-HF was a double-blind, randomized, placebo-controlled trial in which subjects were randomized 1 : 1 : 1 to receive investigational product (IP), either JVS-100 (15 or 30 mg) or placebo. Before delivery of IP, the infarct zones were identified using baseline echocardiography. The peri-infarct zones that were ≥0.6 cm in thickness were identified for injection.10 Following study agent delivery, all subjects were monitored overnight for at least 18 h, which included echocardiography within 6 h post-injection procedure to exclude evidence of myocardial perforation. Post-hospital discharge each subject had scheduled visits at 3 and 7 days post-injection to ensure that there were no safety concerns, with subsequent follow-up visits at ∼30 days (1 month), 120 days (4 months), and 360 days (12 months) to assess safety and cardiac function. Cardiac function was assessed by contrast enhanced surface echocardiography with results read by the same independent Cleveland Clinic Echo Core Lab. Contrast and non-contrast images were attempted to be collected in all patients. Images were obtained in three-beat cycles in multiple views and measurements were performed at the core laboratory. All sonographers from C5 reading studies were blinded to subject randomization allocation. Adverse events were tracked for each subject throughout the study. Safety was monitored by an independent Drug Safety Monitoring Committee.
Injection procedure
Cardiovascular interventionists participated in pre-clinical training on the use of the Helix per BioCardia standard operating procedures. Subjects were placed under conscious sedation and access to the femoral artery was obtained using standard techniques to enable insertion of an arterial sheath. An 8 Fr arterial sheath was then introduced and advanced into the artery to enable passage of delivery catheters. Left ventriculography was performed in both the left and right anterior oblique views and the contour of the heart was recorded onto two clear acetate sheets. BioCardia's Helix was used for all endomyocardial injections. For the procedure, unfractionated heparin was administered IV in single or multiple doses as needed to achieve an activated clotting time of ≥250 s. The Helix was positioned within the left ventricle to facilitate 15, 1 mL injections. Each injection lasted 60 s with an additional 15 s dwell, and was targeted to the peri-infarct border zone. Total volume delivered was 15 mL per patient. Post-procedure all subjects were monitored in a clinical post-catheterization hospital unit with telemetry.
Outcomes measures
The primary efficacy endpoint was the difference in change of a composite endpoint consisting of the 6 MWD and MLWHFQ in the treated patients compared with placebo at 4 months. The composite score was calculated by dividing the change in 6 MWD by 30 m11 plus the change in MLWHFQ divided by −10.11,12 These values represent clinically significant changes in the respective measures.11,12 Additional endpoints included changes in left ventricular end-systolic volume (LVESV), left ventricular end-diastolic function (LVEDV), left ventricular ejection fraction (LVEF), and N-terminal pro brain natriuretic peptide (BNP) (NTproBNP) at 4 and 12 months post-injection procedure. For echocardiographic volumes and LVEF, contrast echocardiographic measurements are reported for each patient unless, for a given patient, either a readable baseline contrast echo was not obtained, (n = 9) or an incomplete set (missing time point) of contrast echo was collected and the patient had complete set of non-contrast echocardiograms (n = 4). In these cases, non-contrast echocardiograms were used for all time points.
Safety endpoints included major adverse clinical events (MACE), significant adverse events (SAEs), as well as complete metabolic panel, complete blood count, physical exam, vitals, safety echocardiogram after injection procedure, HF assessment, and anti-nuclear antibody.
Statistical analysis
Stromal Cell-Derived Factor-1 Plasmid Treatment for Patients with Heart Failure was randomized and blinded at the clinical site level 1 : 1 : 1. Descriptive statistics were used to compare continuous efficacy variables between each dose group and the control and across dosing groups. For the complete data set, if the parameter was normally distributed, parametric statistics (mean and SD) were used for comparison; if it was not, non-parametric statistics were used. For subgroup analyses, non-parametric statistics were used for analysis. Safety parameters were collected and assessed qualitatively or summarized quantitatively by descriptive statistics where appropriate. The data from each efficacy parameter was assessed at each time point as either raw values or calculated as change from baseline for each subject. A P-value of 0.05 was considered significant and a P-value of <0.25 was considered evidence of a trend.13
The primary efficacy endpoint was the composite endpoint of 6 MWD and MLWHFQ at 4 months post-dosing. All data were also analysed at the 1 year endpoint.
Baseline characteristics are presented as mean ± SD for continuous variables and percentages for categorical variables. Changes from baseline are presented as median values for each treatment group. Continuous variable difference between the groups was compared with a t-test for two samples with equal variance with a P-value of 0.05 considered significant. For differences in categorical variables, the Wilcoxon rank-sum test was used, with a P-value of 0.05 considered significant. The primary endpoint was analysed using a one-way analysis of variance (ANOVA) model.
Based on previous data from our Phase I study delivering JVS-100 to HF subjects via the same route of administration, we estimated an improvement of at least two points in the primary endpoint with an SD of ∼2.1; enrolment of 93 subjects would be sufficient to detect such a difference with 80% power and α of 0.05. We included in our analyses the complete data set for all randomized subjects who received at least one injection of study drug or placebo, and who had a baseline and at least one post-baseline measurement by intent-to-treat analysis. The endpoint was analysed using all observed data with no imputation for missing data. For safety endpoints, the safety population was used, consisting of all randomized subjects.
There were several pre-specified analyses that included sub-set analysis by LVEF as well as LVESV, with change from baseline by absolute and percentage change from baseline values to results at 4 and 12 months post-delivery of study agent. In addition to comparisons of the entire study population, a pre-specified analysis was undertaken to compare patients in three different groups defined by baseline LVEF. The thresholds were divided by the lowest (LVEF < 26%), mid-range (LVEF 26–32%), and highest tertiles (LVEF > 32%) for all patient baseline LVEF values. Baseline and change from baseline statistics were then compared across treatment groups within each of these sub-groups.
Results
Patient population
Overall, 93 patients were enrolled into the study at 16 investigational sites. Baseline demographic data are shown in Table 1. Importantly, the population as a whole was well treated per guideline directed therapy with >80% receiving ACE-I/ARB, >90% receiving β-blockade and >55% on mineralocorticoid receptor antagonists (MRAs). The three study groups demonstrate fairly well-matched baseline characteristics. Patients were predominantly male, with age in the mid-1960s, with an average duration since myocardial infarction of over 10 years. The median LVEF and LVESV were 29% and 154 mL, respectively, with two-thirds of the population being New York Heart Association (NYHA) Class III. Consistent with NYHA Class III population the average 6 MWD was ≤300 m and the MLWHFQ score averaged 50.8 points.
Table 1.
Baseline demographic data for total population by treatment cohort
| Parameter | Cohort—all patients |
P-valuea | ||
|---|---|---|---|---|
| Placebo | 15 mg | 30 mg | ||
| Patients (n) | 31 | 32 | 30 | |
| Sex (% male) | 90.3 | 87.5 | 90.0 | 1.00 |
| NYHA Class III or IV (%)b | 71.0b | 62.5 | 70.0 | 0.76 |
| Diabetic (%) | 48.4 | 34.4 | 46.7 | 0.47 |
| CRT (%) | 54.8 | 53.1 | 43.3 | 0.64 |
| Age (years) | 68 ± 9 | 65 ± 11 | 64 ± 7 | 0.25 |
| BMI | 30 ± 5 | 29 ± 4 | 31 ± 7 | 0.16 |
| Years since MI | 13 ± 11 | 10 ± 7 | 10 ± 9 | 0.26 |
| LVESV (mL) | 158 ± 64 | 161 ± 63 | 183 ± 71 | 0.28 |
| LVEDV (mL) | 222 ± 76 | 219 ± 69 | 244 ± 79 | 0.37 |
| LVEF (%) | 30 ± 7 | 28 ± 8 | 26 ± 8 | 0.17 |
| NTproBNP (pg/mL) | 1260 ± 1373 | 1144 ± 1005 | 952 ± 802 | 0.54 |
| 6 MWD (m) | 284 ± 98 | 295 ± 96 | 308 ± 73 | 0.58 |
| MLWHFQ | 56 ± 17 | 50 ± 18 | 46 ± 22 | 0.14 |
| ACE-I (%) | 83.9 | 78.1 | 83.3 | 0.84 |
| β-Blocker (%) | 93.5 | 93.8 | 96.7 | 1.00 |
| MRA (%) | 48.4 | 62.5 | 60.0 | 0.51 |
aTwo-sided test comparing all three arms. Fisher's exact for categorical variables and one-way ANOVA for continuous variables.
bOne patient in the safety population had NYHA class IV.
Safety
Pre-specified safety endpoint profile by treatment cohort as shown in Table 2 demonstrated that endomyocardial delivery of JVS-100 was well tolerated. Ninety-six per cent of the patients received the pre-specified 15 endomyocardial injections of 1 mL/injection, total 15 mL. As seen in our pre-clinical porcine studies and Phase I trial,8,10 there was a transient increase troponin I following the injection procedure that decreased over time through 3 days after injection (Table 3). The increase in troponin I was not different between cohorts. Four patients exhibited small pericardial effusions without haemodynamic impairment on the post-dosing safety echocardiogram that were resolved by the following day without intervention. No patients demonstrated any evidence of haemodynamic perturbation or arrhythmia following IP delivery.
Table 2.
Number of major adverse cardiac events by patient and treatment cohort
| MACE component by patient* | Heart failure hospitalization | Death | Treated ventricular tachycardia | Cerebral vascular accident | Acute coronary syndrome |
|---|---|---|---|---|---|
| n (%) (95% CI)a | n (%) (95% CI)a | n (%) (95% CI)a | n (%) (95% CI)a | n (%) (95% CI)a | |
| Placebo total number of patients with MACE component | 2 (6.5) (0.8, 21.4) | 2 (6.5) (0.8, 21.4) | 7 (22.6) (9.6, 41.1) | 0 (0.0) (0.0, 11.2) | 4 (12.9) (3.6, 29.8) |
| 15 mg total number of patients with MACE component | 4 (12.5) (3.5, 29.0) | 1 (3.1) (0.1, 16.2) | 3 (9.4) (2.0, 25.0) | 1 (3.1) (0.1, 16.2) | 4 (12.5) (3.5, 29.0) |
| 30 mg total number of patients with MACE component | 4 (13.3) (3.8, 30.7) | 2 (6.7) (0.8, 22.1) | 8 (26.7) (12.3, 45.9) | 0 (0.0) (0.0, 11.6) | 2 (6.7) (0.8, 22.1) |
aExact two-sided 95% confidence interval for percentage of patents with event.
Table 3.
Change in troponin I levels as a function of time after injection procedure in each cohort relative to pre-injection troponin I level
| Mean Δ from pre-injection as a function of time after injection (ng/mL) |
||||||
|---|---|---|---|---|---|---|
| 6 h | 12 h | 18 h | 3 days | 1 month | 4 months | |
| Placebo | 3.5 ± 4.7 | 3.7 ± 12.1 | 2.8 ± 10.8 | 0.9 ± 3.6 | −0.1 ± 0.2 | 0.0 ± 0.1 |
| 15 mg | 2.1 ± 2.1 | 1.1 ± 1.0 | 0.6 ± 1.0 | 0.13 ± 0.5 | −0.1 ± 0.6 | −0.1 ± 0.5 |
| 30 mg | 2.7 ± 1.9 | 1.5 ± 1.2 | 0.9 ± 0.9 | 0.2 ± 0.4 | −0.1 ± 0.3 | −0.1 ± 0.3 |
Overall results primary endpoint and total population
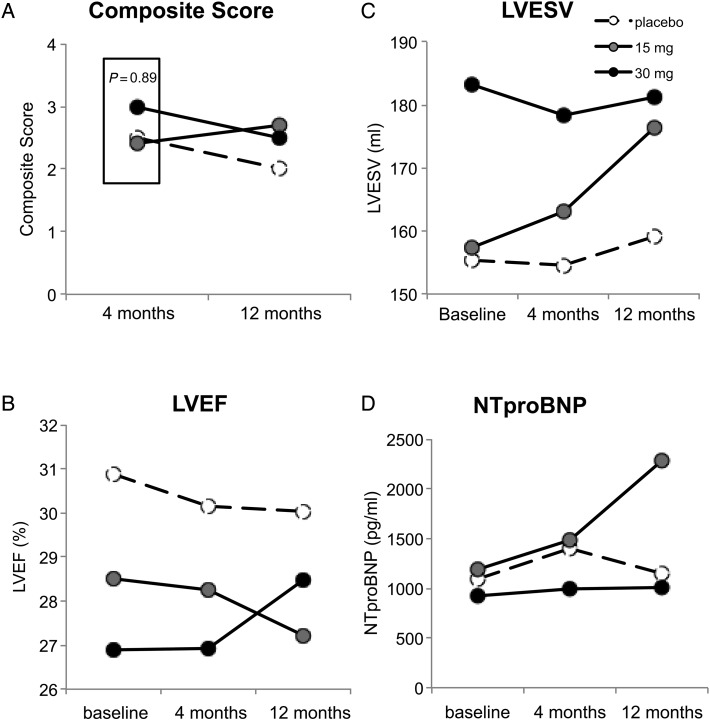
The effects of pSDF injections on the primary endpoint composite score at 4 and 12 months as well as the mean values measured for cardiac function (Figure 2B, LVEF). Cardiac structure (Figure 2C, LVESV) and NTproBNP (Figure 2D) are shown at baseline and 4 and 12 months after injection. Consistent with other contemporary trials testing invasive regenerative strategies in advanced HF patients,14 we observed a significant improvement in the composite score in the treated patients (P ≤ 0.001, Figure 2A); however, there was a significant placebo effect such that the primary endpoint of relative improvement in composite score at 4 months was not achieved. The median changes at 4 and 12 months in 6 MWD and MLWHQ were 46 ± 59, 44 ± 51, 62 ± 77 m and 60 ± 88, 52 ± 76, 50 ± 77 m and −10.7 ± 19.0 points, −9.2 ± 21.0 points, −17.0 ± 20.0 points and −12.5 ± 15.3 points, −9.2 ± 16 points, −13.2 ± 25 points, respectively, for the placebo, 15 and 30 mg cohorts, respectively.
Figure 2.
Effect of stromal cell-derived factor-1 over-expression on structural, functional, and clinical measures in patients with chronic heart failure. Mean values of (A) composite score, (B) left ventricular ejection fraction (%), (C) left ventricular end-systolic volume (mL), (D) N-terminal pro BNP (pg/mL) and (D) N-terminal pro BNP at baseline, 4 and 12 months after treatment with placebo (open circles), 15 (grey circles), or 30 mg (black circles) of JVS-100. Box in (A) represents primary endpoint of trial. P-value represents treated vs. placebo at 4 months.
Left ventricular ejection fraction (Figure 2B) and LVESV (Figure 2C) demonstrated non-significant improvement, with a trend in median change from baseline relative to placebo in LVESV at 4 months (P = 0.24) and a further separation from median changes seen in the placebo cohort in LVEF at 12 months in the 30 mg pSDF cohort (P = 0.20). Median changes in NTproBNP did not demonstrate clinically meaningful changes from baseline.
Effect of plasmid stromal cell-derived factor-1 based on baseline ejection fraction
Based on our understanding of the mechanisms of action,1,2,5,7 we pre-specified an analysis of myocardial response to pSDF-1 as a function of three ranges of baseline EF (low-LVEF <26%, intermediate 26–31%, and high ≥32%). Baseline demographics for each tertile are shown in Table 4. With decreasing LVEF between tertiles, there was an increase in baseline LVESV, and there was no imbalance between treatment cohorts for LVEF and LVESV within each tertile.
Table 4.
Baseline demographic data by tertile of baseline left ventricular ejection fraction
| Tertile of baseline LVEF |
P-value for equipoise among cohorts within tertilea |
|||||
|---|---|---|---|---|---|---|
| EF < 26 | 26 ≤ EF < 32 | EF ≥ 32 | EF < 26 | 26 ≤ EF < 32 | EF ≥ 32 | |
| Patients | 31 | 31 | 31 | |||
| Sex (% male) | 83.9 | 100 | 83.9 | 1.00 | 1.00 | 1.00 |
| NYHA Class (%) | 67.7 | 61.3 | 71.0% | 0.24 | 0.89 | 0.33 |
| Diabetic (%) | 51.6 | 32.3 | 45.2 | 0.32 | 1.00 | 0.66 |
| CRT (%) | 71.0% | 58.1 | 35.5 | 1.00 | 0.39 | 0.81 |
| Age (years) | 65 ± 8 | 65 ± 11 | 66 ± 9 | 0.11 | 0.91 | 0.27 |
| BMI | 29 ± 6 | 30 ± 6 | 30 ± 4 | 0.67 | 0.23 | 0.80 |
| Years since MI | 11 ± 9 | 12 ± 10 | 9 ± 8 | 0.55 | 0.43 | 0.64 |
| LVESV (mL) | 218 ± 71 | 167 ± 47 | 116 ± 28 | 0.88 | 0.049b | 0.70 |
| LVEDV (mL) | 268 ± 85 | 233 ± 63 | 182 ± 44 | 0.86 | 0.046b | 0.62 |
| LVEF (%) | 19 ± 4 | 28 ± 2 | 36 ± 4 | 0.13 | 0.07b | 0.80 |
| NTproBNP (pg/mL) | 1506 ± 1131 | 1130 ± 1308 | 725 ± 543 | 0.31 | 0.39 | 0.37 |
| 6 MWD (m) | 301 ± 97 | 281 ± 84 | 305 ± 88 | 0.43 | 0.26 | 0.97 |
| MLWHFQ (points) | 53 ± 19 | 49 ± 19 | 50 ± 20 | 0.87 | 0.24 | 0.21 |
| ACE-I (%) | 71.0 | 87.2 | 87.1 | |||
| β-Blocker (%) | 93.7 | 100 | 90.2 | |||
| MRA (%) | 70.8 | 57.6 | 41.7 | |||
| 26 ≤ EF < 32 tertile |
||||||
| Placebo | 15 mg | 30 mg | ||||
| LVESV (mL) | 150 ± 41 | 156 ± 50 | 195 ± 43 | |||
| LVEDV (mL) | 211 ± 55 | 215 ± 65 | 270 ± 58 | |||
| LVEF (%) | 29.3 ± 1.4 | 27.6 ± 1.8 | 28.1 ± 1.8 | |||
aTwo-sided test comparing all three arms. Fisher's exact for categorical variables and one-way ANOVA for continuous variables.
bMeans ± SD for significant or borderline significant from 26 ≤ EF < 32 tertile above.
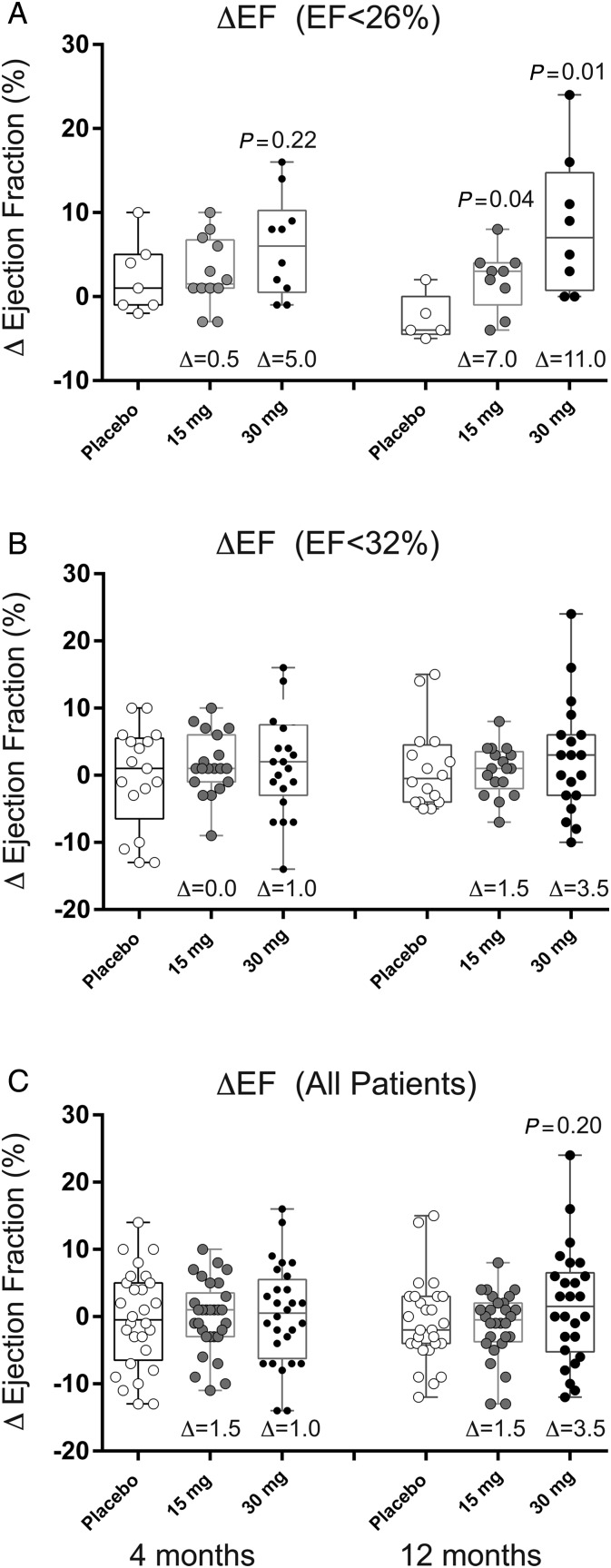
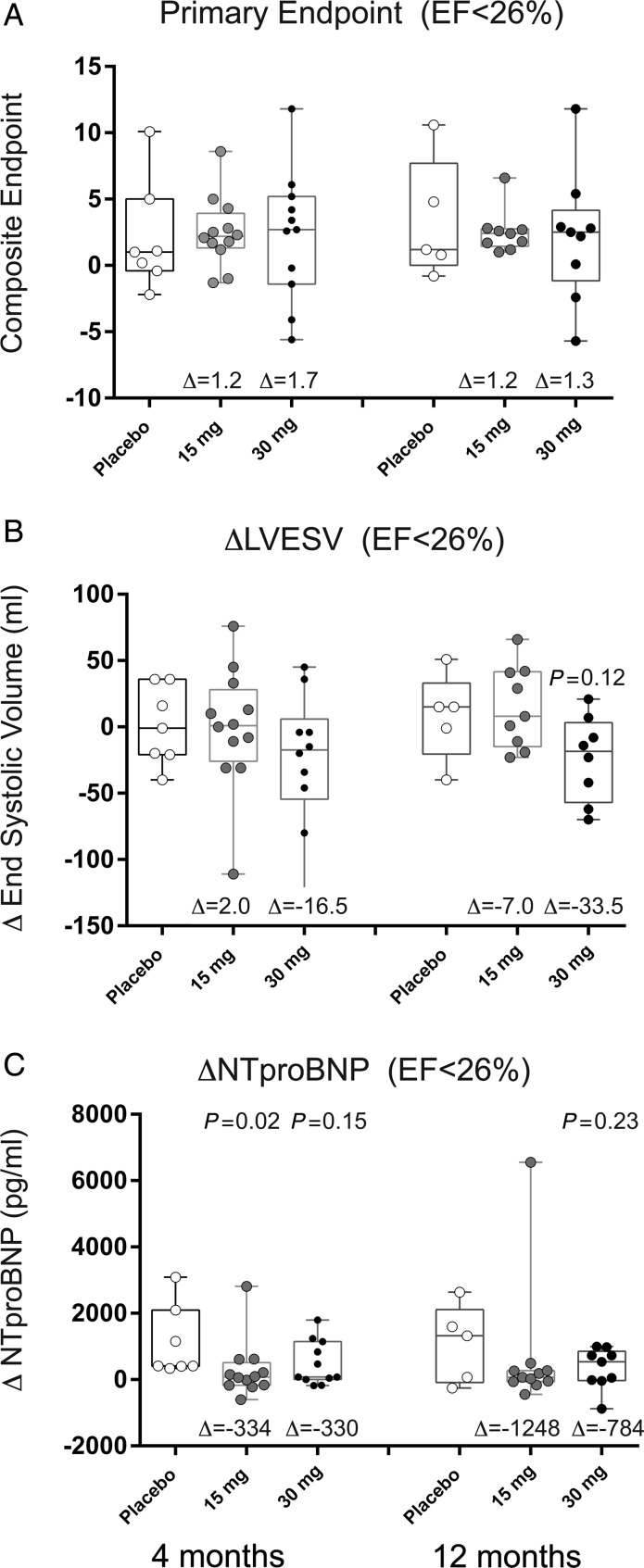
The data in Figure 3 depict change in LVEF by cumulative tertile of EF at 4 and 12 months after treatment. These data demonstrate that in the patients with the greatest degree of LV dilation and systolic dysfunction pSDF-1-induced significant improvement in LVEF. In the lowest tertile of baseline LVEF (<26%), we observed an absolute increase in EF of 5% (P = 0.22) and 11% (P = 0.01) relative to placebo at 4 and 12 months, respectively. The data in Figure 4 quantify the changes in composite endpoint, LVESV, and NTproBNP for the lowest LVEF tertile at 4 and 12 months. In this tertile, there was a decrease in LVESV of 19 mL (P = 0.38) and 34 mL (P = 0.12) relative to placebo at 4 and 12 months, respectively. There was an inverse relationship between LVEF and change in LVESV such that the greatest reduction in LVESV was noted in the group with the lowest LVEF. We observed a trend towards a decrease in NTproBNP in the 30 mg treatment cohort relative to placebo at 4 and 12 months after treatment (4 months: −330 pg/mL, P = 0.15; 12 months: −784 pg/mL, P = 0.23).
Figure 3.
Effect of stromal cell-derived factor-1 over-expression on ejection fraction at 4 and 12 months based on baseline ejection fraction. Data represent change in left ventricular ejection fraction in box-whisker plots with median and interquartile range and minimum and maximum data points. Circles represent individual patient data points for (open circle) placebo, (grey circle) 15, or (black circle) 30 mg treatment cohorts. (A) Patients with EF < 26% at baseline, (B) patients with EF < 32% at baseline, or (C) patients with EF < 40% at baseline. The variable Δ represents the difference between the median in the above-treatment cohort and placebo.
Figure 4.
Effect of stromal cell-derived factor-1 over-expression on parameters of chronic heart failure in high-risk heart failure patients. Data represent change in (A) composite endpoint, (B) left ventricular end-systolic volume, and (C) N-terminal pro BNP in the first tertile (left ventricular ejection fraction <26% at baseline) in box-whisker plots with median and interquartile range and minimum and maximum data points. Circles represent individual patient data points for (open circle) placebo, (grey circle) 15, or (black circle) 30 mg treatment cohorts. The variable Δ represents the difference between the median in the above-treatment cohort and placebo.
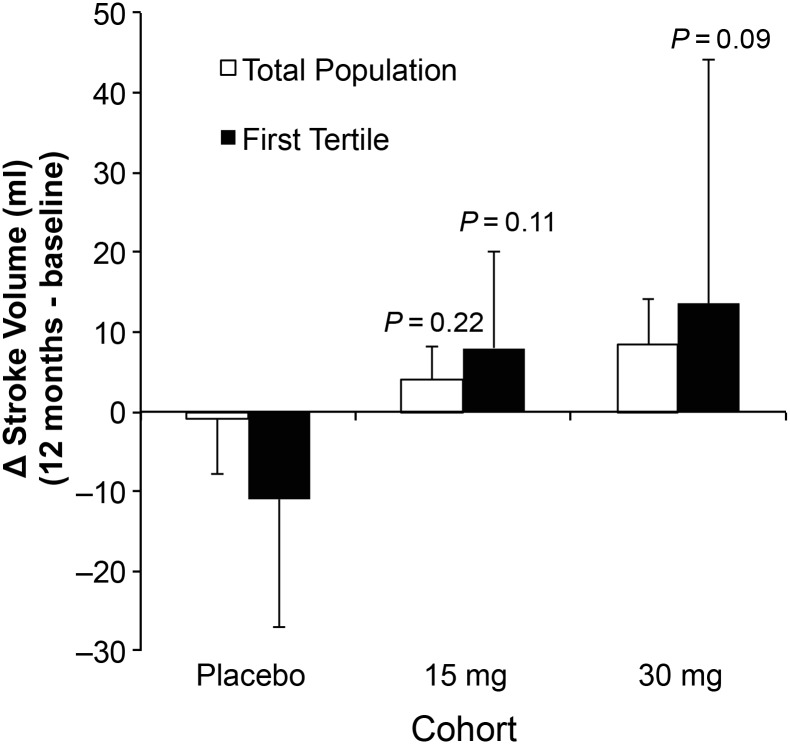
In a post hoc analysis, we calculated stroke volume based on the difference between LVEDV and LVESV obtained by echocardiography. The data in Figure 5 demonstrate the dose-dependent changes in stroke volume (mL) in the total population and the first tertile (EF < 26%) of study subjects. These data demonstrate a non-significant increase in stroke volume in the total population and a trend of a dose-dependent increase in stroke volume in the high-risk cohort relative to placebo (P = 0.09). The findings suggest a differential effect of pSDF depending on baseline LVEF, with the best results noted in those with the lowest baseline LVEF.
Figure 5.
Effect of stromal cell-derived factor-1 over-expression on stroke volume 12 months after treatment. Mean change in stroke volume from baseline to 12 months following administration of placebo or treatment with 15 or 30 mg of JVS-100 in all patients (open bar) or patients in the first tertile of EF (<26%) at baseline. Data represent mean ± SD.
Special populations
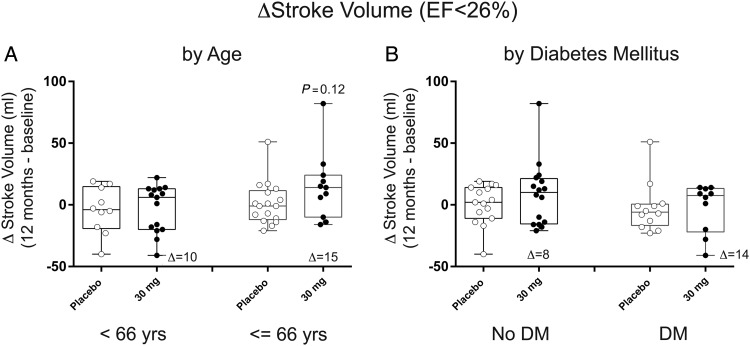
Investigations in diabetic and aged populations suggest that bone marrow-derived stem cells are less functional than non-diabetic or young subjects.15–17 We wanted to begin to determine if there was less of a response to pSDF-1 in diabetic or older patients. Therefore, we compared the change in stroke volume in patients above and below the median age of the trial (66 years) (Figure 6A), as well as non-diabetic and diabetic patients (Figure 6B). This post hoc analysis suggests that aged and diabetic patients appear to be no less responsive to pSDF-1.
Figure 6.
Effect of age and diabetes on change in stroke volume in response to stromal cell-derived factor-1 over-expression in chronic heart failure. Data represent change in left ventricular ejection fraction in patients from the first tertile of baseline left ventricular ejection fraction (<26%) in box-whisker plots with median and interquartile range and minimum and maximum data points. Circles represent individual patient data points for (open circle) placebo or (black circle) 30 mg treatment cohorts. (A) Analysis in patients based on median age and (B) analyses of patients based on presence or absence of diabetes mellitus. The variable Δ represents the difference between the median in the above-treatment cohort and placebo.
Discussion
There has been a significant evolution in identification of specific target genes that have been shown to play an important role in enhancing myocardial function or stem cell mobilization and homing to the heart, as well as the development of novel methods for delivery of these target genes to the heart which are both safe and effective.1,4,6,7,18 The strategy of delivering substantial quantities of target gene as an alternative to stem cells for native cardiac repair is based on the current leading hypothesis that the primary mechanism of stem cell benefit is a paracrine effect.6,19 This hypothesis is derived from the observation that transplanted cells do not remain in situ for more than several days post-delivery, and that the supernatant obtained from cell cultures is as effective as delivery of the cells alone.20,21 Secreted factors from the cells rather than the cells themselves, lead to stimulation of native repair.20,22
One gene and ligand pair that has been shown to be critical to stem cell mobilization and homing is the chemokine SDF-1, and its ligand receptor, CXCR4, or SDF:CXCR4 axis.23–25 Pre-clinical models confirmed the ability to deliver meaningful quantities of a DNA plasmid encoding hSDF-1 without the need for any type of carrier vector. This approach leads to local expression for periods of over 2 weeks,7,8 a duration comparable with reported cell survival after implantation in local tissue. These pre-clinical studies also demonstrated signals of efficacy, with improved cardiac function in models of myocardial infarction.5
The results of the 17 patient pilot STOP-HF trial with this SDF-1 encoding plasmid suggested clinical benefit without SAEs,10 and led to this exploratory Phase II STOP-HF trial in 93 subjects. The current study was powered to assess the safety and efficacy of two different doses of JVS-100 and better define the appropriate target population for subsequent pivotal trials. The intra-myocardial delivery of SDF in the two doses used in this study did not meet the primary endpoint of improvement of clinical status based on a composite endpoint of 6 MWD and MLWHFQ that was designed based on the findings of the small open-label Phase I study.10
Previous data from our pivotal porcine study suggested that the benefit of pSDF-1 was more pronounced in hearts with more advanced cardiac dysfunction as manifested by the lowest baseline LVEF and highest LVESV.8 Based on this understanding of the mechanism of action of the SDF-1:CXCR4 axis and consistent with the exploratory nature of STOP-HF, we pre-specified an analysis of the response to pSDF-1 based on tertiles of baseline LVEF. The results of this analysis suggest a dose-dependent improvement in study endpoints of change in LVEF and LVESV, which appeared to persist between 4 and 12 months post-delivery. This observation is surprising given that the pSDF-1 vector is only expressed for <1 month in pre-clinical models of ischaemic HF.7,8
There are several potential mechanisms that explain the greater response observed in patients with the greatest degree of LV dilation and dysfunction. We have previously demonstrated that the over-expression of SDF-1 in ischaemic cardiomyopathy leads to stabilization and increased perfusion in the infarct border zone. We and others have demonstrated that in chronic ischaemic cardiomyopathy, cardiac myocytes in the infarct border zone are CXCR4 positive.2,26 CXCR4 expression by cardiac myocytes leads to a negative inotropic state.27,28 The transient over-expression of SDF-1 in ischaemic cardiomyopathy has been shown to lead to long-term down-regulation of cardiac myocyte CXCR4 expression, re-recruiting the contractile function of the border zone.2 Improvement in border zone cardiac structure and function has previously been shown to induce global left ventricular remodeling.29 Patients with greater LV dysfunction are likely to have a greater volume of myocardial tissue under stress; therefore, a greater demonstrable response to SDF-1 over-expression as seen in the current study. Regardless of mechanism, these data demonstrate that the cardiac response to biological therapies is impacted by the myocardial substrate being treated.
As stated above, the STOP-HF trial was not powered to observe changes in HF-related hospitalizations or death. The observed MACE events in this study suggest that there may be no increase in MACE with pSDF-1 injections. It is important to note that the degree of LV remodelling we observed has been previously shown to be associated with significant reductions in HF hospitalizations and mortality at 2 years.30 Notably, these observed improvements occurred in patients who were receiving guideline directed therapy including ACE-I/ARB, β-blocker, MRA, and cardiac resynchronization therapy. The fact that patients over 10 years since the last myocardial infarction could be induced to attenuate LV remodelling demonstrates the therapeutic potential of targeting the myocardial substrate including the microvasculature. Of note, older and diabetic patients responded positively to the transient over-expression of SDF-1. These data indicate that while these co-morbidities are associated with decreased stem cell function in in vitro assays,31,32 the stem cells circulating in the blood stream or within the myocardium appear to remain active, or can be activated by SDF-1. We have previously demonstrated that the mechanism of action of mesenchymal stem cells is the release of SDF-1.6 These observations support a possible mechanism for the recent demonstration that the age of mesenchymal stem cells does not alter response to engraftment in chronic HF.33
Limitations
There are limitations of the STOP-HF trial. It is a modest size trial but under powered for HF events including hospitalizations and death. Left ventricular volumes were obtained by contrast echocardiography whenever possible; while cardiac magnetic resonance (cMR) may be a more precise method, the requirement to include implantable cardiac defibrillators ruled out cMR as the primary mode of imaging.
Conclusion
The primary endpoint of the trial was a composite score of 6 MWD and MLWHQ. The composite endpoint was significantly increased in both the control and treated cohorts. As a result, the trial did not achieve statistical significance on the primary endpoint.
Based on pre-specified analyses the STOP-HF study demonstrates the potential for the over-expression of SDF-1 by direct endomyocardial delivery to improve cardiac performance as suggested by the significant improvement in ejection fraction, and the trends in attenuation of LV size and increased stroke volume in the tertile of patients with the lowest LVEF. These data support the need for further investigation of SDF-1 over-expression for the treatment of advanced HF in larger clinical studies to determine if the benefits in cardiac structure and function observed in this trial impact the clinical endpoints of HF hospitalizations and death. Furthermore, the safety and remodelling data from STOP-HF and the fact that JVS-100 is a non-viral DNA plasmid, support investigation of whether there will be greater clinical benefits associated with repeat administration of the myocardium with SDF-1.
Funding
The studies on SDF-1 based gene therapy for the treatment of cardiovascular disease were funded by the Skirball Foundation, New York, NY and the Corbin Foundation, Akron, OH. The STOP-HF clinical trial was funded by Juventas Therapeutics, Inc., Cleveland, OH, USA. Funding to pay the Open Access publication charges for this article was provided by Juventas Therapeutics, Inc., Cleveland, OH, USA.
Conflict of interest: M.P. is named as an inventor on patent applications filed by the Cleveland Clinic for the use of SDF-1 to treat cardiovascular disease that has been licensed by Juventas Therapeutics, Inc. As such he is eligible for royalties from and equity in Juventas Therapeutics. M.P. is the Founder and Chief Medical Officer of Juventas Therapeutics, as such he is a paid consultant. F.P. is a paid consultant by Juventas Therapeutics. A.W., J.M.P., S.J.F., and R.A. are employees of Juventas Therapeutics and have equity in the company.
Acknowledgements
This study was funded by Juventas Therapeutics, Inc. Integrium, Inc. was the coordinating centre for the trial and provided data management, clinical laboratory, and statistical support among other clinical research organization functions. Drug Safety Solutions provided medical monitoring and pharmacovigilance for the study. We thank each of the sites and their investigators. University of Utah: A.N.P.; additional investigators, Anwar Tandar and Rodney Badger. Lindner Center at Christ Hospital, Gene Chung; additional investigators, Joe Choo and Ian Sarembock. University of Florida: Dave Anderson. Center for Therapeutic Angiogenesis, Birmingham, AL, Farrell Mendelsohn. Washington University, Greg Ewald; additional investigator, John Lasala. Johns Hopkins, Peter Johnston; additional investigator, Jeff Brinker. Summa Cardiovascular Institute, Kevin Silver. Pepin Heart Institute, Charles Lambert. Michigan Cardiovascular Institute, Safwan Kassas. Columbia University, Warren Sherman; additional investigator, Mary Jane Farr. Spectrum Health, Michael Dickinson; additional investigator, Ryan Madder. Iowa Heart, Mark Tannenbaum. Hospital at University of Pennsylvania, Saif Anwarruddin; additional investigator, Ken Margulies. Montefiore Einstein Medical Center, Julia Shin; additional investigators, Mark Greenberg and V.S. Srinivas. Minneapolis Heart Institute, Jay Traverse; additional investigator, Tim Henry.
References
- 1.Askari A, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor-1 on stem cell homing and tissue regeneration in ischemic cardiomyopathy. Lancet 2003;362:697–703. [DOI] [PubMed] [Google Scholar]
- 2.Deglurkar I, Mal N, Mills WR, Popovic ZB, McCarthy P, Blackstone EH, Laurita KR, Penn MS. Mechanical and electrical effects of cell-based gene therapy for ischemic cardiomyopathy are independent. Hum Gene Ther 2006;17:1144–1151. [DOI] [PubMed] [Google Scholar]
- 3.Unzek S, Zhang M, Mal N, Mills WR, Laurita KR, Penn MS. SDF-1 recruits cardiac stem cell like cells that depolarize in vivo. Cell Transplant 2007;16:879–886. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003;107:1322–1328. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J 2007;21:3197–3207. [DOI] [PubMed] [Google Scholar]
- 6.Dong F, Harvey J, Finan A, Weber K, Agarwal U, Penn MS. Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation 2012;126:314–324. [DOI] [PubMed] [Google Scholar]
- 7.Sundararaman S, Miller TJ, Pastore JM, Kiedrowski M, Aras R, Penn MS. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Therapy 2011;18:867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penn MS, Pastore J, Miller T, Aras R. SDF-1 in myocardial repair. Gene Therapy 2012;19:583–587. [DOI] [PubMed] [Google Scholar]
- 9.Cheng K, Malliaras K, Smith RR, Shen D, Sun B, Blusztajn A, Xie Y, Ibrahim A, Aminzadeh MA, Liu W, Li TS, De Robertis MA, Marban L, Czer LS, Trento A, Marban E. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in myocardial repair. JACC Heart Failure 2014;2:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penn MS, Mendelsohn FO, Schaer GL, Sherman W, Farr M, Pastore J, Rouy D, Clemens R, Aras R, Losordo DW. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ Res 2013;112:816–825. [DOI] [PubMed] [Google Scholar]
- 11.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J, Evaluation MSGMIRC. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–1853. [DOI] [PubMed] [Google Scholar]
- 12.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease I. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 2011;124:304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desbiens NA. A novel use for the word ‘trend’ in the clinical trial literature. Am J Med Sci 2003;326:61–65. [DOI] [PubMed] [Google Scholar]
- 14.Mathiasen AB, Qayyum AA, Jorgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sorensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J 2015; doi:10.1093/eurheartj/ehv196. [DOI] [PubMed] [Google Scholar]
- 15.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation 2003;108:457–463. [DOI] [PubMed] [Google Scholar]
- 16.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol 2003;42:2073–2080. [DOI] [PubMed] [Google Scholar]
- 17.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res 2006;99:42–52. [DOI] [PubMed] [Google Scholar]
- 18.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells 2007;25:245–251. [DOI] [PubMed] [Google Scholar]
- 19.Van't HW, Mal N, Huang Y, Zhang M, Popovic Z, Forudi F, Deans R, Penn MS. Direct delivery of syngeneic and allogeneic large-scale expanded multipotent adult progenitor cells improves cardiac function after myocardial infarct. Cytotherapy 2007;9:477–487. [DOI] [PubMed] [Google Scholar]
- 20.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11:367–368. [DOI] [PubMed] [Google Scholar]
- 21.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 2006;20:661–669. [DOI] [PubMed] [Google Scholar]
- 22.Menasche P, Vanneaux V, Fabreguettes JR, Bel A, Tosca L, Garcia S, Bellamy V, Farouz Y, Pouly J, Damour O, Perier MC, Desnos M, Hagege A, Agbulut O, Bruneval P, Tachdjian G, Trouvin JH, Larghero J. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: a translational experience. Eur Heart J 2015;36:743–750. [DOI] [PubMed] [Google Scholar]
- 23.Penn MS. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circ Res 2009;104:1133–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355:1210–1221. [DOI] [PubMed] [Google Scholar]
- 25.Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, Lutz A, Khaled W, Klotsche J, Tonn T, Dimmeler S, Zeiher AM. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA 2013;309:1622–1631. [DOI] [PubMed] [Google Scholar]
- 26.Damas JK, Eiken HG, Oie E, Bjerkeli V, Yndestad A, Ueland T, Tonnessen T, Geiran OR, Aass H, Simonsen S, Christensen G, Froland SS, Attramadal H, Gullestad L, Aukrust P. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res 2000;47:778–787. [DOI] [PubMed] [Google Scholar]
- 27.LaRocca TJ, Schwarzkopf M, Altman P, Zhang S, Gupta A, Gomes I, Alvin Z, Champion HC, Haddad G, Hajjar RJ, Devi LA, Schecter AD, Tarzami ST. beta2-Adrenergic receptor signaling in the cardiac myocyte is modulated by interactions with CXCR4. J Cardiovasc Pharmacol 2010;56:548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyo RT, Sui J, Dhume A, Palomeque J, Blaxall BC, Diaz G, Tunstead J, Logothetis DE, Hajjar RJ, Schecter AD. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol 2006;41:834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bian J, Popovic ZB, Benejam C, Kiedrowski M, Rodriguez LL, Penn MS. Effect of cell-based intercellular delivery of transcription factor GATA4 on ischemic cardiomyopathy. Circ Res 2007;100:1626–1633. [DOI] [PubMed] [Google Scholar]
- 30.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol 2010;56:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001;89:E1–E7. [DOI] [PubMed] [Google Scholar]
- 32.DiPersio JF. Diabetic stem-cell ‘mobilopathy’. N Engl J Med 2011;365:2536–2538. [DOI] [PubMed] [Google Scholar]
- 33.Golpanian S, El-Khorazaty J, Mendizabal A, DiFede DL, Suncion VY, Karantalis V, Fishman JE, Ghersin E, Balkan W, Hare JM. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol 2015;65:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]