Abstract
We examined gene expression levels of multiple chemokines and chemokine receptors during Pneumocystis murina infection in wild-type and immunosuppressed mice, using microarrays and qPCR. In wild-type mice, expression of chemokines that are ligands for Ccr2, Cxcr3, Cxcr6, and Cxcr2 increased at days 32 to 41 post-infection, with a return to baseline by day 75 to 150. Concomitant increases were seen in Ccr2 ,Cxcr3, and Cxcr6, but not in Cxcr2 expression. Induction of these same factors also occurred in CD40-ligand and CD40 knockout mice but only at a much later time-point, during uncontrolled Pneumocystis pneumonia (PCP). Expression of CD4 Th1 markers was increased in wild-type mice during clearance of infection. Ccr2 and Cx3cr1 knockout mice cleared Pneumocystis infection with kinetics similar to wild-type mice, and all animals developed anti-Pneumocystis antibodies. Upregulation of Ccr2, Cxcr3, and Cxcr6 and their ligands supports an important role for T helper cells and mononuclear phagocytes in the clearance of Pneumocystis infection. However, based on the current and prior studies, no single chemokine receptor appears to be critical to the clearance of Pneumocystis.
Keywords: Pneumocystis, PCP, chemokines, chemokine receptor, knock-out mice
1. Introduction
Pneumocystis is an opportunistic fungal pathogen that causes severe pneumonia in immunocompromised hosts [1]. Healthy individuals may become infected with Pneumocystis, but infection is rapidly cleared by a robust immune response [2, 3]. A better understanding of the immune response to Pneumocystis in immunocompetent hosts should lead to a better understanding of disease in immunocompromised hosts.
Chemokines and chemokine receptors play an important role in host responses to infections, functioning to coordinate leukocyte trafficking, as well as modulating several other cellular biological processes such as survival, proliferation, differentiation, and anti-microbial activity [4]. The potential role of chemokines in Pneumocystis infection has been examined in a number of in vitro and in vivo studies [5-16]. In vitro studies have demonstrated an increase in Cxcr2 ligands [6, 17], while in vivo studies, which were primarily performed in immunosuppressed mice, have shown increased expression of Ccr2, Ccr5, and Cxcr3 ligands [9-11, 14, 15, 18]. However, no study to date has comprehensively examined chemokine and chemokine receptor responses in immunocompetent hosts.
In a prior microarray study we identified a robust immune response to Pneumocystis infection in immunocompetent mice that was biphasic in nature, the second phase of which coincided with clearance of infection [19]. In contrast, CD40-ligand knockout mice (CD40L-KO), which are highly susceptible to Pneumocystis infection, showed a much more muted response during the same time period. Notably, a large number of chemokines and chemokine receptors were upregulated during the second phase in immunocompetent but not CD40L-KO mice.
The current study was undertaken to better understand the role of chemokines and chemokine receptors in host responses to Pneumocystis in both wild-type and CD40L-KO mice over time, through examination of RNA expression as determined by microarray analysis as well as by real-time quantitative polymerase chain reaction (PCR). To gain insight into cell-specific determinants of anti-Pneumocystis pulmonary immunity, we also used microarray analysis to examine RNA expression in purified lung CD4 cells and alveolar macrophages. We further examined the role in clearance of Pneumocystis infection of two chemokine receptors, Ccr2 and Cx3cr1, which are characteristically expressed on inflammatory and resident monocytes/macrophages, respectively, using Ccr2-KO and Cx3cr1-KO mice [20].
2. Methods
2.1 Animals
Healthy C57 black (C57BL/6) mice were obtained from the National Cancer Institute, CD40 ligand knock-out (CD40L-KO, strain B6, 129S-Tnfsf5tm1lmx) and CD40 knock-out (CD40-KO, strain B6, 129P2-Cd40tm1Kik/J) mice were obtained from Jackson Laboratory. Ccr2 knock-out (B6.129S4-Ccr2tmIIfc/J) mice [21] were kindly provided by Dr. Joshua Farber and Cx3cr1 (B6.129-Cx3cr1tm1Zm) knock-out mice were obtained from Taconic. All mouse strains were subsequently bred at the NIH. Mice were housed in microisolator cages and kept in ventilated racks. All animal work was performed under a Clinical Center Animal Care and Use Committee-approved protocol.
2.2 P. murina infection
Wild-type C57BL/6 and CD40L-KO mice were exposed to P. murina infected seeder mice for 2 weeks, 5 weeks or 6 weeks as described in a previous study; results of immunostaining and limited Western blot analysis of lung homogenates of these wild-type animals have been previously reported [19]. CD40L-KO and CD40-KO mice, and, as controls, CD40L +/−, which were littermates of the CD40L-KO mice, were exposed to P. murina infected seeder mice for 5 months. Lung tissue was collected and placed in RNAlater (Qiagen) for RNA extraction and in PBS for DNA extraction. P. murina infection was confirmed by quantitative real-time PCR as described below.
Ccr2-KO mice were exposed to P. murina infected seeder mice and were sacrificed after 5, 6, 9 and 11 weeks of exposure. Uninfected Ccr2-KO mice were used as controls. Cx3cr1-KO mice and C57BL/6 wild-type mice (as controls in each cage) were exposed to P. murina infected seeder mice in 2 experimental cages. Mice were sacrificed after 5, 9 (Cx3cr1-KO only), and 15 weeks of exposure. Lungs were collected for quantitation of P. murina organisms by quantitative real-time PCR, blood was collected to obtain serum for antibody analysis and spleens were collected at weeks 9 and 11 to measure spleen cell proliferation responses following in vitro stimulation with Pneumocystis antigens [22].
2.3 Microarray studies
Microarray analysis of control and CD40L-KO mice through day 75 after Pneumocystis infection have been previously reported [19]. Additional microarray analysis of control (CD40L +/−), CD40L-KO, and CD40-KO mice at ~5 months following exposure, at a point when they were heavily infected with Pneumocystis, were performed as previously reported [19] using the Mouse Genome 430 2.0 Array (Affymetrix). To examine responses in lung CD4 cells and alveolar macrophages, microarray analysis using the Mouse Gene 2.0 ST Array (Affymetrix) was performed using bead purified CD4 cells (Miltenyi) and alveolar macrophages that were purified by adherence to plastic for ~4 hours. Cells were obtained from whole lung preparations (CD4) or bronchoalveolar lavage (macrophage) from C57BL/6 mice (expressing either CD45.1 or CD45.2) at 35 days following exposure to a seeder mouse, as well as from unexposed mice. Due to the small number of cells recovered, cells from 5 animals were combined in 4 separate pools and each pool was analyzed separately. Because the RNA yield from macrophages was low even after combining cells, RNA was amplified by the Ovation Pico WTA System V2 (NuGen) prior to processing for microarray analysis. The new microarray data discussed in this publication have been deposited in the National Center for Biotechnology Information (NCBI; U.S. National Library of Medicine, Bethesda, MD, USA) Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series Accession Number GSM1654761-92.
2.4 Real-time quantitative PCR of gene expression
RNA was extracted using RNeasy Mini Kit (Qiagen), and quantified using a NanoDrop spectrophotometer. cDNA was synthesized from 2 µg RNA using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCR was performed on an ABI Prism 7900 sequence detection system using Power Sybr Green Master Mix (Applied Biosystems) or iTaq Universal SYBR Green Supermix (Bio-Rad) to measure relative levels of gene expression of the following chemokines and receptors: Cxcl9 (monokine induced by gamma interferon, (MIG)), Cxcl10 (interferon gamma-induced protein 10 (IP-10)), Cx3cl1 (fractalkine), Ccl2 (monocyte chemotactic protein 1 (MCP-1)), Ccl7 (monocyte chemotactic protein 3 (MCP-3)), Ccl17 (thymus and activation regulated chemokine (TARC)), Ccl22 (macrophage-derived chemokine (MDC)), Cxcl16, Ccr2, Ccr4, Ccr5, Ccr6, Cxcr2, Cxcr3, Cxcr6, and Cx3cr1. Ct values were normalized to GAPDH and gene expression is reported as fold change as compared to unexposed animals using the comparative Ct method.
2.5 Quantitation of P. murina Organisms and Preparation of P. murina antigens
DNA was extracted from mouse lung tissue using QIAamp DNA Mini Kit (Qiagen) and Pneumocystis organisms were quantified using a quantitative PCR of the P. murina dhfr gene, a single copy gene, as previously described [3]. Results are reported as dhfr copies per mg lung tissue.
P. murina antigens for cell proliferation assays were prepared from lungs heavily infected with P. murina (as determined by Diff Quik staining) or from uninfected wild-type lungs by homogenization in PBS (0.25 g/mL) using a TissueLyser (Qiagen) or Polytron (Omni International) followed by sonication and centrifugation; the collected supernatant was utilized as the antigen preparation [22]. P. murina antigens for ELISA were similarly prepared from partially purified Pneumocystis organisms.
2.6 Measurement of anti-P. murina antibodies
Anti-P. murina serum antibodies were measured by ELISA using a crude P. murina lysate as previously described [22].
2.7 Splenocyte proliferation
Splenocyte proliferation was measured as previously described [22]. Mouse spleen cells were cultured in 96 well plates (100,000 cells per well) with concanavalin A (2.5 µg/ml) for 4 days as a positive control, and with P. murina antigens (100 µg/ml), normal mouse lung antigens (100 µg/ml) or no antigen for 5 days. Cell proliferation was measured using CellTiter-Glo Luminescent Cell Viability Assay (Promega) according to the manufacturer’s instructions and luminescence was read on a Centro LB 960 luminometer (Berthold Technologies). Results are presented as stimulation index, which represents the fold-change following incubation with P. murina antigens compared to normal mouse lung antigens.
2.8 Statistical analysis
For chemokine expression from the microarray data, P values were determined using Student’s t-test , comparing exposed to unexposed animals. The Q-PCR studies utilized Student’s t-test to compare day 0 results to the various time-points following exposure. Gene selection for the CD4 and macrophage microarray studies utilized ANOVA with two factors (treatment and mouse donor) and thresholds of P≤0.05 and absolute change in expression ≥0.6. Gene Ontology (GO) term enrichment analysis was carried out using DAVID (http://david.abcc.ncifcrf.gov/) [23, 24] with default parameters, default mouse background, and the functional annotation chart report tool.
3. Results
3.1 Lung chemokine gene expression analysis
In a previous microarray study of Pneumocystis infection in wild-type and CD40L-KO mice, we identified a number of immune response genes, including chemokines and chemokine receptors, that were significantly upregulated in the lungs of wild-type but not CD40L-KO mice, at ~days 32 to 41 following Pneumocystis infection that was transmitted by co-housing with infected seeder mice [19]. We utilize this model because it better mimics natural infection and subsequent immune responses than a transtracheal inoculation model which introduces a large number of Pneumocystis organisms, some of which may be non-viable, and which may produce a skewed immune response. Naturally infected wild-type mice typically become infected after 2-3 weeks of exposure, with a peak of infection at ~5 to 6 weeks and clearance after ~7 to 9 weeks [3]. CD40L-KO mice infected with Pneumocystis show similar growth kinetics initially, but are not able to clear the infection and have a progressively increasing organism burden.
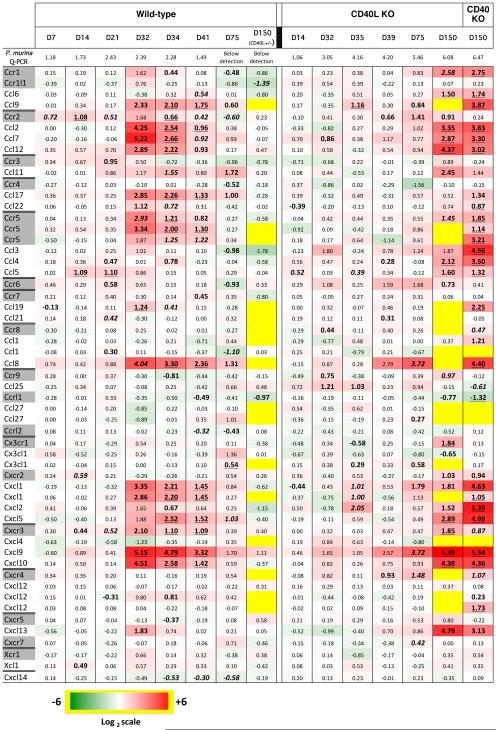
Fig. 1 presents the fold-change in expression of chemokine and chemokine receptor genes from our prior microarray study over time, for both C57BL/6 and CD40L-KO, grouped by chemokine/primary receptor. We have included these data because our prior analysis examined immune responses globally but did not focus specifically on chemokine responses. Also shown are additional microarray data from new experiments that examined expression in CD40L-KO and CD40-KO, both of which are highly susceptible to PCP, at approximately 5 months after infection, together with results for heterozygous (CD40L +/−) littermates with which they were co-housed, and which served as controls. We chose 5 months because we were interested in determining if chemokine networks might be contributing to the severe inflammation that can be seen at this time-point, when both knock-out strains had a very high organism burden (ranging from 105.7 to 106.5 dhfr copies per mg lung tissue), while the controls had all cleared infection.
Fig. 1.
Heat map showing gene expression levels of chemokines and chemokine receptors, as determined by microarray analysis, in the lungs of wild-type, CD40L-KO, and CD40-KO mice following exposure to P. murina at the indicated time-points post-infection (D, day). The geometric mean P. murina organism burden, as determined by Q-PCR using the single copy dhfr gene, is indicated in the top row in log10 copies per mg lung tissue. Red indicates up-regulation, and green, down-regulation, compared to control, uninfected animals. Data were transformed to log2 for the heat-map, and the log-transformed values are indicated within the boxes. Yellow indicates that data are unavailable. Chemokine ligands were grouped with their primary chemokine receptor. When a chemokine or receptor is included in more than one line, the results represent different probe sets for the same gene. Results are shown by mouse strain and day following start of exposure/co-housing, and represent 3 to 10 animals per time-point per strain. Day 150 control mice were CD40L heterozygotes that were littermates of the day 150 CD40L-KO mice; all other controls were wild-type C57BL/6 mice. A subset of the data (days 7, 14, 21, 34, 41, and 75 for controls and days 35, 39, and 75 for CD40L-KO) was included in a previous publication [19]. All values with P<0.05 are bolded; those with P<0.01 are bolded and italicized, and those with P<0.001, bolded and underlined.
For wild-type mice, there is increased expression of multiple chemokines that are ligands for Ccr2, Ccr4, Cxcr2, and Cxcr3 at days 32 to 41, with a return to baseline by day 75. However, while Ccr2 and Cxcr3 expression are also increased at the same time-points, Ccr4 and Cxcr2 expression are notably unchanged. Moreover, Ccr5 expression is also increased, though its ligands are minimally induced. In contrast, CD40L-KO mice do not show similar increases in expression at the same time-points, though individual chemokines such as Ccl8 and Cxcl9 have increased expression at day 39 that further increases at day 75. It is noteworthy that many of the same chemokines that were elevated in healthy mice at ~day 35-40 show increased expression in immunodeficient mice at later time-points (day 150) when they are heavily infected with Pneumocystis.
To explore the chemokine responses further, we performed real-time RT-PCR using RNA from the lungs of an additional set of animals, targeting expression of select chemokine/chemokine receptor families during periods of active infection in immunocompetent animals (Fig. 2). Among wild-type mice, 2 of 6 C57BL/6 mice were infected with a low number of organisms (15 and 13 dhfr copies/mg lung tissue) following 2 weeks exposure to P. murina, while all C57BL/6 mice were infected after 5 (n=6) and 6 (n=6) weeks exposure (<1,000 dhfr copies/mg lung tissue). In parallel we examined CD40L-KO mice from a separate study; 8 of 8 animals were infected at 2 weeks (mean, 399 dhfr copies/mg lung tissue) as well as at 5 weeks (4/4 animals), with a higher organism load (mean, 93,248 dhfr copies/mg lung tissue).
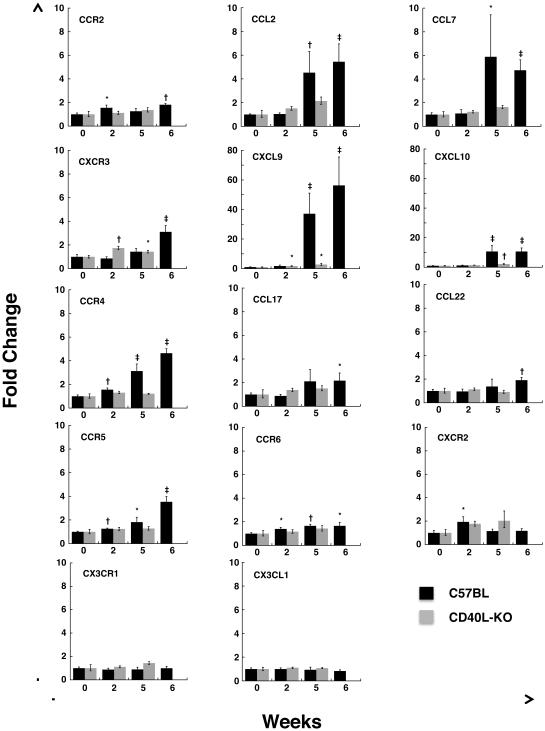
Fig. 2.
Gene expression levels of chemokines and chemokine receptors, as measured by Q-PCR, in the lungs of wild-type and CD40L-KO mice following exposure to P. murina. Wild-type and CD40L-KO mice were cohoused with Pneumocystis-infected seeder mice for 2 weeks (WT n=6, CD40L-KO n=8), 5 weeks (WT n=6, CD40L-KO n=4) and 6 weeks (WT n=6). Chemokine and chemokine receptor levels were measured in the lungs of these mice by real-time PCR and are reported as fold-change compared to unexposed animals (WT n=6, CD40L-KO n=5) using the comparative Ct method. Note the different Y axis range for Cxcl9 and Cxcl10. Error bars indicate the standard error. Statistical significance is indicated by the following symbols: *, p-value ≤ 0.05; †, p-value ≤ 0.01;‡, p-value ≤ 0.001.
Consistent with our initial microarray results, QPCR (Fig. 2) also demonstrated >10-fold increase in expression of Cxcl9 and Cxcl10 in C57BL/6 mice, while their receptor, Cxcr3, showed a low-level increase that reached significance only at 6 weeks. Similarly, Ccl2 and Ccl7 showed an approximately 5-fold increase in expression, while their receptor, Ccr2, showed low-level increases. In contrast, Ccl17 and Ccl22 showed approximately 2-fold increases, while their receptor, Ccr4, increased 3 to 4-fold. Ccr5 and Ccr6 expression were significantly elevated, though in general with low-level increases, at all 3 time-points tested. In contrast, Cxcr2 was significantly induced only at 2 weeks, consistent with microarray results. For Cx3cl1 and its receptor, Cx3cr1, there were no significant changes from baseline. Those chemokines that had increased expression at 2 weeks tended to have substantially greater expression at weeks 5 and 6, while receptors showed more limited increases. Cxcr2 is the only chemokine or receptor that showed a significant increase at week 2 followed by a return to approximately baseline levels at 5 weeks. As previously reported, CD4+ T cells, CD19+ B cells, and CD68+ macrophages were present in increased numbers in the lung tissue of wild-type mice during P. murina infection [19].
In contrast to the generally robust responses seen in immunocompetent mice, CD40L-KO mice showed significant increases only in expression of Cxcr3 and its ligands Cxcl9 and Cxcl10, with levels for the ligands well below those seen in C57BL/6 mice (Figure 2).
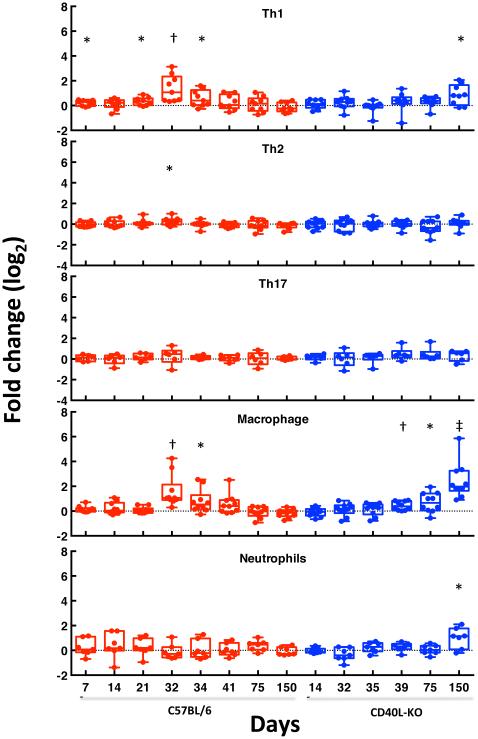
To gain insight into which immune cell populations may be contributing to immune responses to Pneumocystis infection, we utilized the microarray data to examine changes in expression of genes that are markers for CD4 cell subsets as well as macrophages and neutrophils. As shown in Fig. 3, among CD4 cells, Th1 markers were induced in healthy mice, especially at days 32 to 34, while in CD40L-KO mice they were increased only at day 150. Th2 and Th17 cells showed minimal changes during the same time period. Macrophage markers showed a pattern similar to Th1 cells, while neutrophil markers showed an increase only at day 150 in CD40L-KO mice.
Fig. 3.
Changes in expression level of genes that are markers for CD4 cell subsets (Th1, Th2, and Th17), macrophages, and neutrophils following exposure to P. murina. For each cell type, the average level of expression of the selected genes at each time-point, compared to control (uninfected) genes, is shown. Expression of genes encoding the following proteins are included: CD4 Th1: Ccr5, Cxcr3, IFNG, IL12A, IL12B, IL-2, Stat1, Stat4, and IL27RA; CD4 Th2: Ccr3, Ccr4, Ccr8, IL3, IL4, IL5, IL11, IL13, GATA3 and Stat6; CD4 Th17: Ccr6, IL17A, IL17RA, RORC, TGFB1 and Stat3; macrophages: Ccr2, Ccl2, CD14, CD68, CSF1R, CTSK, EMR1, FCGR3, MMP12, and ITGAM; for neutrophils, ELA2, LCN2, LTF, MPO, S100A8, S100A9, and Cxcr2. The whisker-box plots show the median, 25-75% range, and extreme values. Statistical significance is indicated by the following symbols: *, p-value ≤ 0.05; †, p-value ≤ 0.01;‡, p-value ≤ 0.001.
3.2 CD4 and macrophage microarray analysis
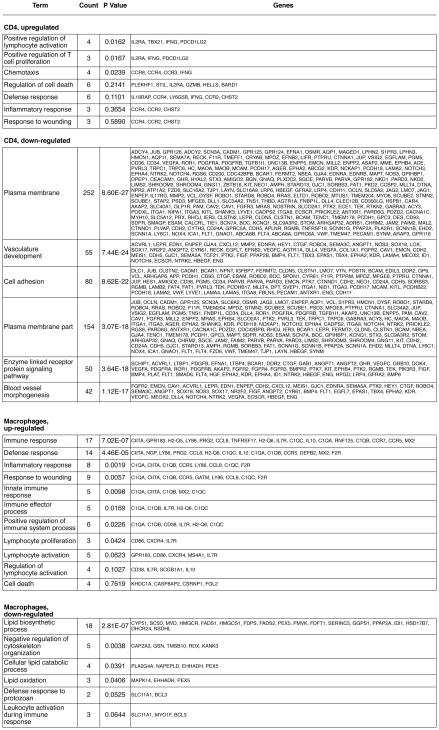
To better understand gene expression changes in individual cell populations, we performed microarray analysis of purified CD4 cells and macrophages from the infected lung at day 35 after exposure to Pneumocystis. All exposed animals were infected with P. murina, as determined by Q-PCR, with an average of ~8,200 dhfr copies/mg lung tissue. Cells from 5 animals were pooled because of the low numbers of cells available from a single animal. For CD4 cells, 987 gene probes were differentially expressed, of which 121 were upregulated and 866 were down-regulated (Appendix 1). For alveolar macrophages, 512 gene probes were differentially expressed, of which 209 were upregulated and 303 were down-regulated (Appendix 2). Fig. 4 lists the gene ontology terms associated with each category. For both cell types, upregulated genes as analyzed by DAVID were primarily related to immune activation and proliferation, while down-regulated genes represented more diverse processes, including cell membrane generation, vasculature development, cell adhesion, and lipid-related metabolic processes.
Fig. 4.
Gene ontology term enrichment analysis of genes that were differentially expressed in CD4 cells and macrophages of wild-type mice at day 35 after exposure to Pneumocystis. CD4 cells were purified from lungs, and macrophages from BAL fluid. All exposed animals were infected with P. murina, as determined by Q-PCR, with an average of 8,200 dhfr copies/mg lung tissue. Analysis was performed using DAVID (http://david.abcc.ncifcrf.gov/).
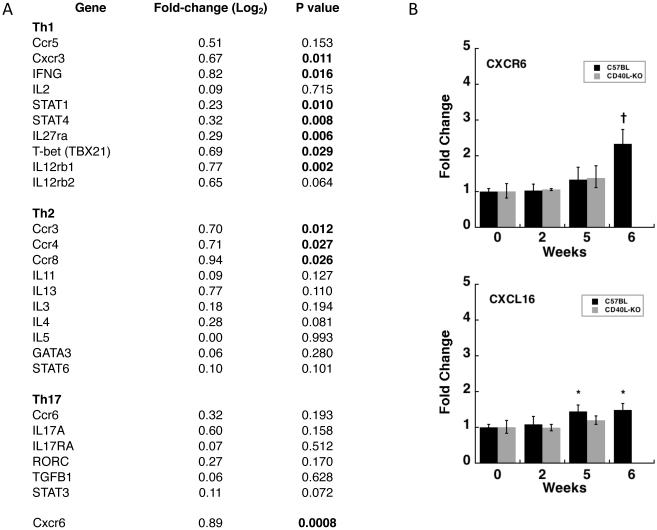
We again examined expression of genes characteristic of CD4 subsets in the CD4-specific microarray results. As seen in Fig. 5A, while a number of Th1-associated genes, including Cxcr3, interferon-gamma, and T-bet were significantly upregulated, only chemokine receptor genes of Th2 cells, and none associated with Th17 cells, were upregulated. Of note, Cxcr6, which was not included in the chip used for the initial microarray studies, was the most significantly upregulated chemotactic factor (p=0.0008). To explore this finding further, we utilized Q-PCR to examine expression of Cxcr6 as well as it’s sole ligand, Cxcl16, in the same animals that were tested in Fig. 2. As seen in Fig. 5B, Cxcr6 was significantly upregulated at week 6, and Cxcl16 at weeks 5 and 6, in C57BL/6 mice. While neither gene was upregulated at early time-points in CD40L-KO mice, both genes were significantly upregulated in CD40L-KO as well as CD40-KO mice at 5 months based on the microarray studies, with fold-changes (log2) of 2.3 (p<0.0007) and 1.4 (p<0.0009) for Cxcr6, and 1.8 (p<2.5x10−5) and 1.9 (p<4.5x10−9) for Cxcl16, respectively.
Fig. 5.
Changes in expression levels of select genes in CD4 cells purified from lung tissue at day 35 after exposure to P. murina. A. Fold-change (log 2), relative to CD4 cells from unexposed animals, in genes expressed by Th1, Th2, and Th17 subsets, as well as Cxcr6, which is expressed by both Th1 and Th17 cells. Indicated P values are based on the microarray analysis as described in the methods; signficiant values (<0.05) are bolded. (B) Gene expression levels of Cxcr6 and Cxcl16, as measured by Q-PCR, in the lungs of wild-type and CD40L-KO mice following exposure to P. murina. Animals for this analysis are the same as in Fig. 2. Error bars indicate the standard error. Statistical significance is indicated by the following symbols: *, p-value ≤ 0.05; †, p-value ≤ 0.01.
3.3 Ccr2 and Cx3cr1 knockout mouse studies
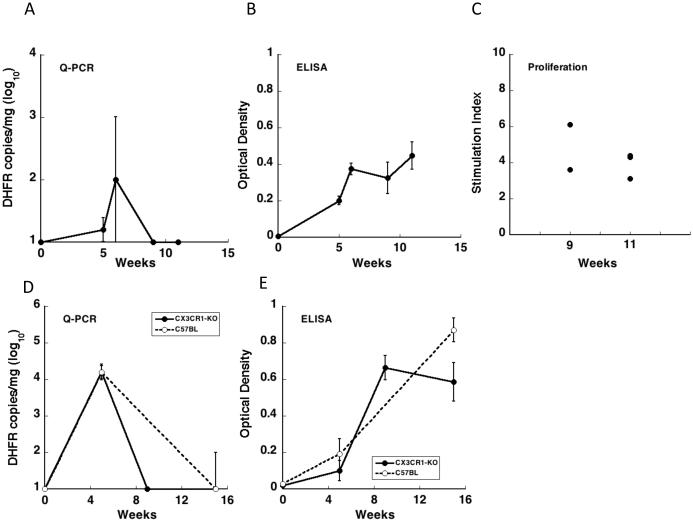
Knockout mice can provide insights into the role of targeted genes in immunity to Pneumocystis. Chemokine receptors in general provide a better target given that there appears to be greater redundancy among ligands than receptors. While the ligands of Ccr2, Cxcr3, and Cxcr2 showed the greatest increases in expression levels, previous studies with Cxcr3-KO mice that were otherwise immunocompetent demonstrated no impairment in clearance of Pneumocystis [9], and studies with Cxcr2-KO mice that were CD4-depleted showed no change in organism burden compared to CD4-depleted control mice [25]. Based on these studies neither Cxcr3 nor Cxcr2 are critical to control of Pneumocystis infection. To determine whether Ccr2 is required, we examined clearance of Pneumocystis infection following exposure of Ccr2-KO mice to P. murina by co-housing with an infected seeder mouse. After 5 to 6 weeks exposure, Pneumocystis organisms were detected in the lungs of Ccr2-KO mice and after 9 to 11 weeks exposure the organisms had been cleared (Fig. 6A). The course of P. murina infection and clearance seen in the Ccr2-KO mice was similar to the course of infection seen in wild-type mice [3]. Following exposure, Ccr2-KO mice produce anti-P. murina antibodies and showed splenocytes proliferation when re-stimulated in vitro with P. murina antigens (Fig. 6B and C), consistent with B and T cell responses to Pneumocystis.
Fig. 6.
Ccr2 and Cx3cr1 KO mice clear P. murina infection. A-C. Ccr2-KO mice were co-housed with Pneumocystis-infected seeder mice and then sacrificed after 5 (n=2), 6 (n=2), 9 (n=2), and 11 (n=3) weeks. A. Pneumocystis organisms were detected in the lungs by quantitative real-time PCR of the dhfr gene of P. murina, and results represent mean log10 dhfr copies per mg lung tissue. The kinetics of P. murina infection and clearance is similar to that seen in wild-type mice. B. Ccr2-KO mice produce anti-P. murina antibodies following exposure to Pneumocystis. Antibody levels were measured by ELISA and results represent mean optical densities. Anti-P. murina antibodies were present in all exposed animals. C. Ccr2-KO mice exposed to Pneumocystis for 9 weeks (n=2) and 11 weeks (n=3) show in vitro spleen cell proliferation in response to P. murina antigen. Results represent the stimulation index (SI) as compared to proliferation of unexposed control animals. D-E. Cx3cr1-KO and wild-type control mice were cohoused with Pneumocystis infected seeder mice in 2 cages. Mice were sacrificed after 5 (Cx3cr1-KO n=4, WT n=2), 9 (Cx3cr1-KO n=6), and 15 (Cx3cr1-KO n=7 WT n=4) weeks exposure. D. Pneumocystis organisms were detected in the lungs by quantitative real-time PCR of the dhfr gene of P. murina and results represent mean log10 dhfr copies per mg lung tissue. The kinetics of P. murina infection and clearance is similar in Cx3cr1-KO mice and wild-type mice. E. Anti-P. murina antibody levels are similar in Cx3cr1-KO mice and wild-type mice following exposure to Pneumocystis. Antibody levels were measured by ELISA and results represent mean optical densities. Anti-P. murina antibodies were present in all exposed animals following 9 and 15 weeks exposure. Error bars indicate the standard error.
Macrophages are felt to be the effector cells that actually kill and clear Pneumocystis from infected lungs [26]. Since inflammatory Ccr2+ mononuclear phagocytes are not essential for clearing Pneumocystis in the healthy host we next examined Pneumocystis infection in Cx3cr1-KO mice. Although Cx3cr1 is expressed at low levels on inflammatory macrophages, it is expressed at much higher levels on resident mononuclear phagocytes [27]. Cx3cr1 was recently shown to mediate killing of another fungal pathogen, Candida albicans, by resident renal macrophages [20]. To determine if Cx3cr1 is required to clear Pneumocystis infection, we exposed Cx3cr1-KO mice to P. murina infected-seeder mice by cohousing for 5, 9, and 15 weeks in 2 experimental cages. As controls, wild-type C57BL/6 mice were cohoused in the same cages. Similar to the Ccr2-KO mice, Cx3cr1-KO mice were infected with Pneumocystis after 5 weeks of exposure, cleared the organisms after 9 weeks and remained clear of infection after 15 weeks (Fig. 6D). Co-housed wild-type mice showed a similar pattern of infection. All exposed animals in both groups had developed anti-P. murina antibodies following 9 and 15 weeks exposure (Fig. 6E).
We examined gene expression levels in both Ccr2-KO and Cx3cr1-KO mice for the same chemokines and receptors as in Fig. 2 using RT-PCR. Although the time-points differed and the number of animals examined during peak infection were limited, since we were focusing on the ability to clear infection in these strains, in general the expression levels were similar to those found in the C57BL/6 mice, with the following exceptions: in Ccr2-KO mice, Ccr2 message levels did not increase (0.8 and 1.3 fold increases above baseline at weeks 5 and 6 respectively for this non-functional message), and Ccl2 levels showed smaller increases (2.3 and 2.8 fold increases at weeks 5 and 6 respectively), while Cx3cr1 levels showed a slight increase (1.5 and 1.9 fold increases at weeks 5 and 6 respectively); in Cx3cr1-KO there was no increase in Ccr6 (0.9 fold increases at week 5).
4. Discussion
This study has demonstrated that a large number of chemokine and chemokine receptor genes, especially Ccr2 and Cxcr3 together with their ligands, have upregulated expression in wild-type, immunocompetent mice during acute infection with Pneumocystis, while similar increases are not seen at the same time-points in CD40L-KO mice, which are highly susceptible to developing PCP. While this suggests that chemokines may play an important role in the innate immune response to the infection, it is noteworthy that studies using knockout mice, including the current study, have found that no single chemokine receptor examined to date (i.e., Cxcr3, Cxcr2, Ccr2, and Cx3cr1) is required for control of Pneumocystis infection [9, 25]. Thus there appears to be redundancy in the signals provided by the chemokine network, or alternatively chemokine/receptor upregulation is a byproduct of the inflammatory/immune response to Pneumocystis but is not critical to clearance of infection.
Upregulation of Cxcr3, Ccr5, Ccr4, and their ligands suggests that T helper cells are important for control of Pneumocystis infection. These data are consistent with a critical role for CD4 cells in clearing this infection, and the increased susceptibility to PCP in HIV-infected patients with low CD4 counts [28]. The microarray studies of lung tissue as well as of purified CD4 cells support this observation, in that primarily Th1-type markers were upregulated in wild-type animals as they were clearing the infection. While previous studies have suggested that Th2 or Th17 responses are predominant in clearance of Pneumocystis infection, these have primarily relied on intratracheal inoculation to transmit infection, or immune reconstitution of heavily infected animals, methodologies which may skew the immune responses [29-31, 12, 32, 33]. In contrast, our model utilizes transmission by natural exposure, which mimics the mode of acquisition of infection in nature.
Our study has identified a potential role for Cxcr6, which was not included in the chips used for the earlier studies, in the host response to Pneumocystis. In our microarray studies with CD4 cells purified from Pneumocystis-infected lungs, Cxcr6 was the most significantly upregulated chemotactic factor, and Q-PCR studies confirmed upregulation of both Cxcr6 and its sole ligand, Cxcl16, in wild-type mice. Cxcr6 has been reported to be a marker of protective antigen-specific T cells in the lungs of a mouse model of tuberculosis [34]. Intriguingly, polymorphisms in the Cxcr6 gene in African Americans have been associated with increased mortality in AIDS-related PCP [35]. Therefore, future studies should examine the in vivo role of the Cxcl16/Cxcr6 axis in anti-Pneumocystis host defense in mice.
Upregulation of Ccr2 and its ligands supports a role for inflammatory mononuclear phagocytes in clearance of infection, which is consistent with a study suggesting that macrophages are effector cells in clearance of Pneumocystis [26], and our prior demonstration of increased macrophages in healthy mice during acute infection [19]. Monocyte chemoattractant proteins (MCPs), which are Ccr2 ligands, are secreted in the lung of mice as well as patients infected with Pneumocystis [22, 13, 36, 37] and from an alveolar epithelial cell line following stimulation with Pneumocystis major surface glycoprotein (MSG) [38]. However, as demonstrated by our experiments in Ccr2-KO mice, this receptor is dispensable for efficient clearance of Pneumocystis in vivo. This is in marked contrast to its role in other fungal infections: Ccr2-KO mice show increased susceptibility to aspergillus, candida, histoplasma, and cryptococcal infection [39-42].
The marked upregulation of Cxcl9 and to a lesser extent Cxcl10 in the setting of only modest elevations in their receptor, Cxcr3, raises the possibility of alternative functions of these chemokines, such as a direct antimicrobial effect. Both chemokines have previously been shown to have such antimicrobial activity against Staphylococcus aureus and Bacillus anthracis [43, 44].
Although neither Cx3cr1 nor its ligand, Cx3cl1 were found to be upregulated in P. murina-infected mice, this pathway is important in clearing certain fungal infections; a recent study showed that survival of Cx3cr1+ renal macrophages is critical to the control of systemic Candida albicans infection in mice [20]. The lack of upregulation of Cx3cr1/Cx3cl1 as well as the absence of any impact on clearance of Pneumocystis in Cx3cr1-KO mice suggests that, unlike for systemic candida infections, this network does not play a role in pulmonary immunity to Pneumocystis.
Neutrophils do not appear to play an important role in clearance of Pneumocystis, although a high neutrophil count in the BAL fluid of HIV patients is a poor prognostic sign [45]. Cxcr2, an important chemokine receptor for neutrophils, was upregulated only transiently in healthy mice, and not during the period when Pneumocystis was being cleared, which temporally was associated with the T helper- and inflammatory monocyte-related responses noted above.
In contrast to C57BL/6 mice, CD40L-KO mice showed very limited changes in expression of the chemokines and receptors at days 32 to 41, when healthy mice began to clear infection. Kinetic studies have shown that Pneumocystis growth continues unchecked in these animals during this period. It is noteworthy that in CD40L-KO as well as CD40-KO mice, both of which are highly susceptible to Pneumocystis infection, there is a late upregulation (day 150) in many of the same chemokines and receptors that are upregulated in C57BL/6 mice at earlier time-points, though in the C57BL/6 mice the levels have returned to baseline by this time-point. However, this response in CD40-KO or CD40L-KO mice is clearly ineffective given the high organism burden seen at that time. This late upregulation, which presumably occurs through pathways independent of CD40-CD40L interaction, may be responsible in part for the inflammatory response that is seen in patients with active PCP, and may contribute to the associated hypoxia that can be life-threatening in those patients.
Many of the prior studies that have examined chemokine networks during Pneumocystis infection have utilized immunodeficient animals with advanced PCP or during immune reconstitution. Consistent with our findings, Cxcl9 and Cxcl10, as well as Ccl2, have markedly increased expression in SIV-infected macaques with PCP [11, 46]. During reconstitution of scid mice, a number of chemokines were induced in conjunction with increased inflammation, including Ccl2, Ccl3, Ccl4, and Ccl5, [14]. Decreases in Cxcl9, Cxcl10, Ccl3, Ccl4, and Ccl5 were seen in IL-23p19−/−animals, which showed transient impaired clearance of Pneumocystis, compared to wild-type animals [12]. In vitro, Pneumocystis can stimulate production of Ccl2 from murine type II alveolar epithelial cells in a JNK-dependent manner [13].
In humans, colonization by Pneumocystis in patients with COPD was associated with an increased expression of the genes for interferon-gamma as well as the interferon-gamma inducible Cxcl9, Cxcl10, and Cxcl11 [7]. Ccl3, Ccl4, and Ccl5 were found to be significantly lower in the BAL of HIV-infected patients with PCP compared to controls without PCP [8].
Thus, chemokines appear to be an integral part of the host immune response to Pneumocystis. They likely play a role in the clearance of this organism in healthy hosts, although the studies with knockout mice to date suggest that there is a functional redundancy in chemokine/chemokine receptor responses. Additional studies are needed to better understand the role that chemokines play in control of Pneumocystis infection as well as the associated inflammation in both immunosuppressed and immunocompetent hosts.
Supplementary Material
Acknowledgements
We thank Rene Costello and Howard Mostowski for providing animal care, Joshua Farber for providing the Ccr2-KO mice and Ester Roffe for methodological assistance.
This research was supported in part by the Intramural Research Programs of the NIH Clinical Center and the National Institute of Allergy and Infectious Diseases, National Institutes of Health. This project has been funded with federal funds from the National Cancer Institute, National Institutes of Health, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health under Contract No. HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicting financial interests.
References
- [1].Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA. 2009;301:2578–85. doi: 10.1001/jama.2009.880. [DOI] [PubMed] [Google Scholar]
- [2].Gigliotti F, Harmsen AG, Wright TW. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect Immun. 2003;71:3852–6. doi: 10.1128/IAI.71.7.3852-3856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vestereng VH, Bishop LR, Hernandez B, Kutty G, Larsen HH, Kovacs JA. Quantitative real-time polymerase chain-reaction assay allows characterization of Pneumocystis infection in immunocompetent mice. J Infect Dis. 2004;189:1540–4. doi: 10.1086/382486. [DOI] [PubMed] [Google Scholar]
- [4].Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International Union of Basic and Clinical Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].An P, Li R, Wang JM, Yoshimura T, Takahashi M, Samudralal R, et al. Role of exonic variation in chemokine receptor genes on AIDS: CCRL2 F167Y association with Pneumocystis pneumonia. PLoS Genet. 2011;7:e1002328. doi: 10.1371/journal.pgen.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am J Respir Cell Mol Biol. 2005;32:490–7. doi: 10.1165/rcmb.2004-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fitzpatrick ME, Tedrow JR, Hillenbrand ME, Lucht L, Richards T, Norris KA, et al. Pneumocystis jirovecii colonization is associated with enhanced Th1 inflammatory gene expression in lungs of humans with chronic obstructive pulmonary disease. Microbiol Immunol. 2014;58:202–11. doi: 10.1111/1348-0421.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Israel-Biet D, Esvant H, Laval AM, Cadranel J. Impairment of beta chemokine and cytokine production in patients with HIV related Pneumocystis jerovici pneumonia. Thorax. 2004;59:247–51. doi: 10.1136/thx.2003.013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McAllister F, Ruan S, Steele C, Zheng M, McKinley L, Ulrich L, et al. CXCR3 and IFN protein-10 in Pneumocystis pneumonia. J Immunol. 2006;177:1846–54. doi: 10.4049/jimmunol.177.3.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nelson MP, Christmann BS, Werner JL, Metz AE, Trevor JL, Lowell CA, et al. IL-33 and M2a alveolar macrophages promote lung defense against the atypical fungal pathogen Pneumocystis murina. J Immunol. 2011;186:2372–81. doi: 10.4049/jimmunol.1002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Qin S, Fallert Junecko BA, Trichel AM, Tarwater PM, Murphey-Corb MA, Kirschner DE, et al. Simian immunodeficiency virus infection alters chemokine networks in lung tissues of cynomolgus macaques: association with Pneumocystis carinii infection. Am J Pathol. 2010;177:1274–85. doi: 10.2353/ajpath.2010.091288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun. 2007;75:3055–61. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang J, Gigliotti F, Bhagwat SP, Maggirwar SB, Wright TW. Pneumocystis stimulates MCP-1 production by alveolar epithelial cells through a JNK-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1495–505. doi: 10.1152/ajplung.00452.2006. [DOI] [PubMed] [Google Scholar]
- [14].Wright TW, Johnston CJ, Harmsen AG, Finkelstein JN. Chemokine gene expression during Pneumocystis carinii-driven pulmonary inflammation. Infect Immun. 1999;67:3452–60. doi: 10.1128/iai.67.7.3452-3460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wright TW, Pryhuber GS, Chess PR, Wang Z, Notter RH, Gigliotti F. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J Immunol. 2004;172:2511–21. doi: 10.4049/jimmunol.172.4.2511. [DOI] [PubMed] [Google Scholar]
- [16].Zhang C, Wang SH, Lasbury ME, Tschang D, Liao CP, Durant PJ, et al. Toll-like receptor 2 mediates alveolar macrophage response to Pneumocystis murina. Infect Immun. 2006;74:1857–64. doi: 10.1128/IAI.74.3.1857-1864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang J, Gigliotti F, Maggirwar S, Johnston C, Finkelstein JN, Wright TW. Pneumocystis carinii activates the NF-kappaB signaling pathway in alveolar epithelial cells. Infect Immun. 2005;73:2766–77. doi: 10.1128/IAI.73.5.2766-2777.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Linke M, Ashbaugh A, Demland J, Koch J, Tanaka R, Walzer P. Resolution of Pneumocystis murina infection following withdrawal of corticosteroid induced immunosuppression. Microb Pathog. 2006;40:15–22. doi: 10.1016/j.micpath.2005.10.002. [DOI] [PubMed] [Google Scholar]
- [19].Hernandez-Novoa B, Bishop L, Logun C, Munson PJ, Elnekave E, Rangel ZG, et al. Immune responses to Pneumocystis murina are robust in healthy mice but largely absent in CD40 ligand-deficient mice. J Leukoc Biol. 2008;84:420–30. doi: 10.1189/jlb.1207816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest. 2013;123:5035–51. doi: 10.1172/JCI71307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci U S A. 1997;94:12053–8. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bishop LR, Helman D, Kovacs JA. Discordant antibody and cellular responses to Pneumocystis major surface glycoprotein variants in mice. BMC Immunol. 2012;13:39. doi: 10.1186/1471-2172-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- [24].Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Swain SD, Wright TW, Degel PM, Gigliotti F, Harmsen AG. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect Immun. 2004;72:5722–32. doi: 10.1128/IAI.72.10.5722-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Limper AH, Hoyte JS, Standing JE. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J Clin Invest. 1997;99:2110–7. doi: 10.1172/JCI119384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- [28].Phair J, Munoz A, Detels R, Kaslow R, Rinaldo C, Saah A. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. Multicenter AIDS Cohort Study Group. N Engl J Med. 1990;322:161–5. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- [29].Garvy BA, Ezekowitz RA, Harmsen AG. Role of gamma interferon in the host immune and inflammatory responses to Pneumocystis carinii infection. Infect Immun. 1997;65:373–9. doi: 10.1128/iai.65.2.373-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect Immun. 1997;65:5052–6. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Myers RC, Dunaway CW, Nelson MP, Trevor JL, Morris A, Steele C. STAT4-dependent and -independent Th2 responses correlate with protective immunity against lung infection with Pneumocystis murina. J Immunol. 2013;190:6287–94. doi: 10.4049/jimmunol.1300431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shellito JE, Tate C, Ruan S, Kolls J. Murine CD4+ T lymphocyte subsets and host defense against Pneumocystis carinii. J Infect Dis. 2000;181:2011–7. doi: 10.1086/315487. [DOI] [PubMed] [Google Scholar]
- [33].Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, et al. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med. 2010;207:2907–19. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee LN, Ronan EO, de Lara C, Franken KL, Ottenhoff TH, Tchilian EZ, et al. CXCR6 is a marker for protective antigen-specific cells in the lungs after intranasal immunization against Mycobacterium tuberculosis. Infect Immun. 2011;79:3328–37. doi: 10.1128/IAI.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Duggal P, An P, Beaty TH, Strathdee SA, Farzadegan H, Markham RB, et al. Genetic influence of CXCR6 chemokine receptor alleles on PCP-mediated AIDS progression among African Americans. Genes Immun. 2003;4:245–50. doi: 10.1038/sj.gene.6363950. [DOI] [PubMed] [Google Scholar]
- [36].Tasaka S, Kobayashi S, Kamata H, Kimizuka Y, Fujiwara H, Funatsu Y, et al. Cytokine profiles of bronchoalveolar lavage fluid in patients with Pneumocystis pneumonia. Microbiol Immunol. 2010;54:425–33. doi: 10.1111/j.1348-0421.2010.00229.x. [DOI] [PubMed] [Google Scholar]
- [37].Chou CW, Lin FC, Tsai HC, Chang SC. The importance of pro-inflammatory and anti-inflammatory cytokines in Pneumocystis jirovecii pneumonia. Med Mycol. 2013;51:704–12. doi: 10.3109/13693786.2013.772689. [DOI] [PubMed] [Google Scholar]
- [38].Benfield TL, Lundgren B, Shelhamer JH, Lundgren JD. Pneumocystis carinii major surface glycoprotein induces interleukin-8 and monocyte chemoattractant protein-1 release from a human alveolar epithelial cell line. Eur J Clin Invest. 1999;29:717–22. doi: 10.1046/j.1365-2362.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- [39].Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis. 2014;209:109–19. doi: 10.1093/infdis/jit413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, et al. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 2014;10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Szymczak WA, Deepe GS., Jr. The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J Immunol. 2009;183:1964–74. doi: 10.4049/jimmunol.0901316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol. 2000;164:2021–7. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- [43].Crawford MA, Zhu Y, Green CS, Burdick MD, Sanz P, Alem F, et al. Antimicrobial effects of interferon-inducible CXC chemokines against Bacillus anthracis spores and bacilli. Infect Immun. 2009;77:1664–78. doi: 10.1128/IAI.01208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yung SC, Parenti D, Murphy PM. Host chemokines bind to Staphylococcus aureus and stimulate protein A release. J Biol Chem. 2011;286:5069–77. doi: 10.1074/jbc.M110.195180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mason GR, Hashimoto CH, Dickman PS, Foutty LF, Cobb CJ. Prognostic implications of bronchoalveolar lavage neutrophilia in patients with Pneumocystis carinii pneumonia and AIDS. Am Rev Respir Dis. 1989;139:1336–42. doi: 10.1164/ajrccm/139.6.1336. [DOI] [PubMed] [Google Scholar]
- [46].Schaefer TM, Fuller CL, Basu S, Fallert BA, Poveda SL, Sanghavi SK, et al. Increased expression of interferon-inducible genes in macaque lung tissues during simian immunodeficiency virus infection. Microbes Infect. 2006;8:1839–50. doi: 10.1016/j.micinf.2006.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.