Abstract
Objectives
Measuring patient-reported outcomes (PROs) has become increasingly important for assessing quality of care and guiding patient management. However, PROs have yet to be integrated with traditional clinical outcomes (such as length of hospital stay) to evaluate perioperative care. This study aimed to utilize longitudinal PRO assessments to define the postoperative symptom-recovery trajectory in patients undergoing thoracic surgery for lung cancer.
Methods
Newly diagnosed patients (N=60) with stage I or II non-small cell lung cancer who underwent either standard open thoracotomy or video-assisted thoracoscopic surgery (VATS) lobectomy reported multiple symptoms from presurgery to 3 months postsurgery using the MD Anderson Symptom Inventory (MDASI). We conducted Kaplan–Meier analyses to determine when symptoms returned to presurgical levels and to mild severity levels during recovery.
Results
The most-severe postoperative symptoms were fatigue, pain, shortness of breath, disturbed sleep, and drowsiness. The median time to return to mild symptom severity for these 5 symptoms was shorter than return to baseline severity, with fatigue taking longer. Pain recovered more quickly for patients who underwent VATS lobectomy vs standard open thoracotomy (8 days vs 18 days, respectively; P = .022). Patients who had poor preoperative performance status or comorbidities reported higher postoperative pain (all P < .05).
Conclusions
Assessing symptoms from the patient's perspective throughout the postoperative recovery period is an effective strategy for evaluating perioperative care. This study demonstrates that the MDASI is a sensitive tool for detecting symptomatic recovery with an expected relationship among surgery type, preoperative performance status, and comorbid conditions.
Keywords: patient-reported outcome (PRO), MDASI, postoperative care, VATS, symptoms, enhanced recovery
INTRODUCTION
Patients with non-small cell lung cancer (NSCLC) who undergo major surgery experience an acute systemic inflammatory, neuroendocrine, and metabolic stress response related to tissue injury and to the medications used during the perioperative period. This response often encompasses a cluster of nonspecific symptoms (eg, fatigue, pain, and disturbed sleep) and organ-specific symptoms (eg, shortness of breath) that together cause considerable functional impairment. In addition, up to 25% of patients who undergo surgery in the United States experience postsurgical complications,1 which may exacerbate symptom severity and functional impairment and prolong convalescence. Postoperative symptoms and their effects on functional recovery are critical determinants of a patient's ability to return to planned cancer treatment, delays in which can negatively impact long-term clinical outcomes.2,3
Enhanced Recovery Programs (ERPs) incorporate Enhanced Recovery after Surgery (ERAS®4) concepts such as multimodal opioid-sparing analgesia and minimally invasive surgical techniques. ERPs generally lead to better postoperative outcomes, which are typically quantified with objective measures (ie, decreased mortality, fewer complications, shorter hospital stays).4-8 Missing from these metrics is the voice of the patient, who is arguably the best source of information about what “recovery” from surgery means.9,10 For example, it is well known that the minimally invasive video-assisted thoracoscopic surgery (VATS) lobectomy is associated with fewer complications and more-rapid recovery than is standard open thoracotomy11,12; however, to our knowledge, no empirical patient-reported outcomes (PRO) data characterize the time course and developmental trajectory of postoperative symptoms, especially during the time frame spanning day of hospital discharge to return of normal functioning.6 Lack of research on how to define and measure symptomatic and functional recovery after major cancer surgery from the patent's perspective is an important gap in comprehensive postoperative care; it also compromises any comparison of ERP innovations against standard care.9,10,13
Capturing the patient's perspective on how well and how quickly he or she is recovering, in terms of symptom severity and persistence, functional impairment, and ability to resume planned oncology treatment, requires a validated PRO measure that is sensitive to differences related to type of surgery and variations in perioperative care. Although PROs have been widely accepted in clinical research,14,15 the use of subjective outcomes in current perioperative practice is relatively novel, despite recent recognition of their potential benefits.9 Inclusion of PROs in an ERP pathway would challenge the current paradigm of standard perioperative care and its reliance on objective metrics that do not reflect the patient's perspective on the effectiveness of cancer therapy and symptom control.9
We therefore conducted a PRO-based longitudinal investigation to (1) examine whether a validated PRO measure, the MD Anderson Symptom Inventory (MDASI),16 could be used to identify the most-severe symptoms experienced by patients with NSCLC beginning before thoracic surgery to 3 months postsurgery, (2) identify risk factors for high postoperative symptoms, and (3) characterize postsurgical recovery in terms of symptom trajectories.
METHODS
Patients
We prospectively recruited newly diagnosed patients with stage I or II NSCLC who were scheduled for thoracic surgery at The University of Texas MD Anderson Cancer Center in Houston, Texas between 2004–2008. Eligible patients were at least 18 years old, naïve to any cancer treatment, able to understand English and the study requirements, and willing and able to respond to a repeated computer/telephone interactive voice response (IVR)-administered symptom rating scale after they were discharged from the hospital. The study was approved by the MD Anderson Institutional Review Board. All participants gave written informed consent.
Study Design
Outcome Measures
Symptoms were assessed using the MDASI, a brief measure of the severity of 13 common cancer-related symptoms over the previous 24 hours. Each symptom is rated on an 11-point scale, with 0 being “not present” and 10 being “as bad as you can imagine.”16
Patients completed a paper and pencil version of the MDASI at the time of enrollment (presurgery baseline) and while in the hospital on day 3 and day 5 postsurgery. Before discharge, patients were given a demonstration of the IVR system and rehearsed using the system until they were comfortable with it. Patients were given options about which days of the week to receive IVR calls and specified their preferred time of day for the call. Patients were called by the IVR system 1 week after discharge and then weekly thereafter until 3 months postsurgery.
Demographic characteristics, postoperative complications, and other clinical variables also were recorded.
Statistical Analysis
All patients included in this analysis provided MDASI data at baseline and on day 3, at a minimum. We used the average of all available scores for each symptom to identify the 5 most-severe symptoms and to construct symptom development trajectories. Cumulative logit mixed-effect models were used for longitudinal analysis. Random intercepts were included in all models. In models comparing symptom scores at specific time points (day 3, day 5, week 1, and month 3 postsurgery) with preoperative symptom levels, we treated time as a categorical variable. In models identifying risk factors for longitudinal symptom burden, we treated time (days from surgery) as a continuous variable, with 2 segments separated by day 3. Age, sex, race, marital status, baseline Eastern Cooperative Oncology group performance status (ECOG PS), comorbid conditions, surgery type, interaction between time and surgery type, length of hospitalization, estimated blood loss, pulmonary complications, and cardiovascular complications were included in the models. SAS 9.3 was used to conduct all analyses. All statistical tests were 2-sided; P-values < 0.05 were considered statistically significant.
We defined “postoperative recovery” as either (1) symptom recovery to baseline level: after surgery, the patient reported 2 contiguous symptom levels at or below the preoperative (baseline) level (patients whose symptoms were >3 at baseline were excluded); or (2) symptom recovery to mild level: after surgery, the patient reported MDASI symptom scores ≤3 (none or mild) at 2 contiguous measurements. Characterizing recovery as the attainment of symptoms rated 0–3 on the 0–10 scale is based on the cutpoints used in multiple clinical practice guidelines, such as National Comprehensive Cancer Network fatigue and pain guidelines.17,18 Empirical work has consistently demonstrated that substantial functional impairment occurs when symptoms reach moderate to severe levels.19,20 Median and mean recovery days and 95% confidence limits (CL) were estimated using Kaplan–Meier analysis.
Funding agency's role in data interpretation
This study was funded by grants from the National Cancer Institute of the National Institutes of Health, including NCI R01 CA026582 (PI: Charles S. Cleeland) and the MD Anderson Cancer Center Support Grant NCI P30 CA016672 (PI: Ronald A. DePinho), and by an ACS Research Scholar Grant from the American Cancer Society (PI: Charles S. Cleeland). None of the sponsors had any role in the study design, data collection, analysis, interpretation, or preparation of the report.
RESULTS
Sample
Of the 119 patients scheduled for thoracic surgery for NSCLC who were screened for participation in the study, 42 were excluded because they did not meet inclusion criteria (34 had stage III or IV cancer and 8 had received previous treatment). Of the 77 patients enrolled, 17 patients did not supply baseline and day 3 data and thus were excluded from analysis, resulting in a final sample size of 60 patients (see Supplemental Figure S1). Among these 60 patients, 5 patients completed 2 postoperative MDASI assessments, and all others completed 3 or more postoperative MDASI assessments. The random missing data rate was 22%.
Table 1 shows baseline demographic and clinical characteristics for the sample. Approximately 48% of patients were treated with a minimally invasive surgical procedure (VATS lobectomy); there was no significant difference in the percentages of patients with stage I cancer vs stage II cancer by type of surgery (P = .152). Other than surgery type, no specific ERP-based management strategies were utilized.4-8
TABLE 1.
Demographic and clinical characteristics
| n | Mean (SD) | Median (range) | |
|---|---|---|---|
| Age, years | 60 | 66.2 (10.5) | 67 (32–89) |
| Length of stay, days | 60 | 6.1 (3.1) | 6 (2–20) |
| Estimated blood loss, mL | 60 | 333.9 (659.2) | 150 (0–3,450) |
| n | % | ||
| Age | |||
| Younger than 60 years | 16 | 26.7 | |
| 60 years and older | 44 | 73.3 | |
| Sex | |||
| Male | 30 | 50.0 | |
| Female | 30 | 50.0 | |
| Race | |||
| Non-Hispanic white | 53 | 88.3 | |
| Other | 7 | 11.7 | |
| Marital status | |||
| Married | 40 | 66.7 | |
| All others | 20 | 33.3 | |
| Type of surgery | |||
| Open thoracotomy | 31 | 51.7 | |
| VATS lobectomy | 29 | 48.3 | |
| Length of hospital stay | |||
| ≤6 days | 41 | 68.3 | |
| 7 days or more | 19 | 31.7 | |
| Estimated blood loss | |||
| ≤150 mL | 34 | 56.7 | |
| >150 mL | 26 | 43.3 | |
| Preoperative ECOG PS | |||
| Good (0–1) | 51 | 85.0 | |
| Poor (2–4) | 9 | 15.0 | |
| Pulmonary complications | |||
| No | 46 | 76.7 | |
| Yes | 14 | 23.3 | |
| Cardiovascular complications | |||
| No | 50 | 83.3 | |
| Yes | 10 | 16.7 | |
| Smoked 100 cigarettes in your lifetime | |||
| Yes | 51 | 85.0 | |
| No | 9 | 15.0 | |
| Comorbid conditions | |||
| No | 5 | 8.3 | |
| Yes | 55 | 91.7 | |
| Day 3 pain rating | |||
| 0–6 | 38 | 63.3 | |
| 7–10 | 22 | 36.7 | |
VATS, video-assisted thoracoscopic surgery; ECOG PS, Eastern Cooperative Oncology Group performance status.
Major Postoperative Symptoms
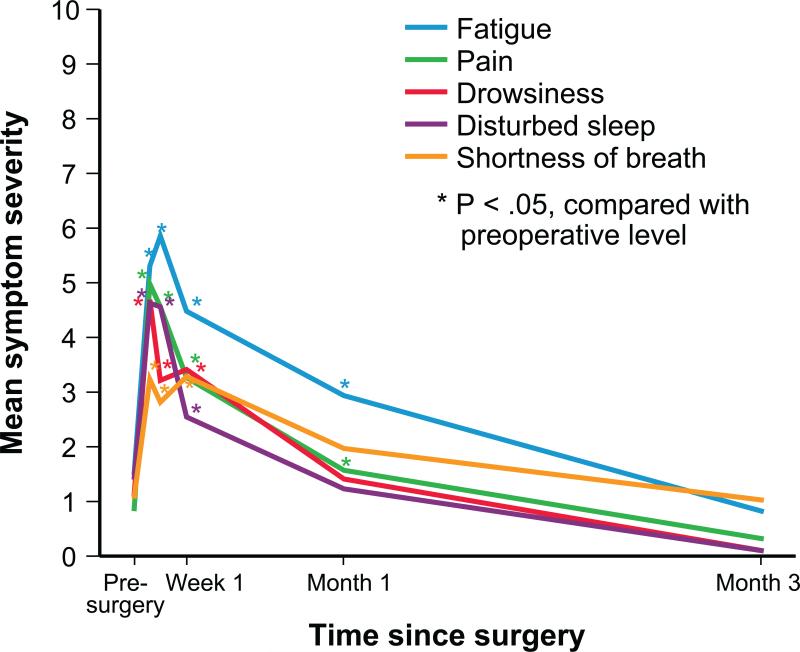
The 5 most-severe postoperative symptoms were pain, fatigue, drowsiness, shortness of breath, and disturbed sleep. Mixed modeling showed that mean fatigue peaked on day 5 postsurgery, whereas the other 4 symptoms had already peaked by day 3 postsurgery (the first postsurgical observation). Before surgery, approximately 13% of patients reported moderate to severe symptoms (≥4 on the 0–10 scale), the most severe of which were fatigue and disturbed sleep. At day 3 after surgery, the prevalence of moderate to severe symptoms was 51.6% for pain, 59.7% for fatigue, 54.8% for drowsiness, 33.9% for shortness of breath, and 56.5% for disturbed sleep. By 4 weeks postsurgery, the only symptoms still more severe than they were at baseline were fatigue (mean 2.92 [SD, 2.45] vs 1.47 [SD, 2.17], respectively; P = .003), and pain (mean 1.55 [SD, 1.97] vs 0.80 [SD, 1.96], respectively; P = .0002), with the other 3 symptoms having returned to preoperative levels. By the end of month 3, all symptoms had improved to better than preoperative levels (see Figure 1 and Supplemental Table S1).
FIGURE 1.
Severity levels over time for the 5 most-severe symptoms after thoracic surgery.
Risk Factors for Higher Postoperative Symptoms
Mixed modeling with ordinal regression analysis was used to examine multiple factors that contributed to the development of the 5 most-severe postoperative symptoms (Table 2). Those with poorer preoperative ECOG PS (2–4 vs 0–1) experienced more-severe postoperative pain (P = .03). Patients reporting any comorbid condition before surgery also reported higher levels of pain (P = .04). Cardiovascular postoperative complications were related to a higher drowsiness score (P = .004).
TABLE 2.
Risk factors for higher symptom burden within 3 months of surgery, by ordinal regression analysis
| Estimate* | SE | P | |
|---|---|---|---|
| Fatigue | |||
| Days from surgery (≤3) | 0.23 | 0.04 | < .0001 |
| Surgery type by days from surgery (≤3) Open thoracotomy vs VATs |
0.22 | 0.09 | .01 |
| Days from surgery (4–100) | −0.28 | 0.04 | < .0001 |
| Surgery type by days from surgery (4–100) Open thoracotomy vs VATs |
−0.19 | 0.09 | .03 |
| Pain | |||
| Days from surgery (≤3) | 0.21 | 0.04 | < .0001 |
| Surgery type by days from surgery (≤3) Open thoracotomy vs. VATs |
0.44 | 0.11 | .0001 |
| Days from surgery (4-100) | −0.26 | 0.05 | <.0001 |
| Surgery type by days from surgery (4–100) Open thoracotomy vs VATs |
−0.43 | 0.12 | .0002 |
| ECOG PS 2–4 vs 0–1 at baseline | 1.14 | 0.52 | .03 |
| Comorbid conditions (yes vs no) | 1.33 | 0.66 | .04 |
| Drowsiness | |||
| Days from surgery (≤3) | 0.14 | 0.04 | .0003 |
| Surgery type by days from surgery (≤3) Open thoracotomy vs VATs |
0.18 | 0.08 | .03 |
| Days from surgery (4–100) | −0.18 | 0.04 | < .0001 |
| Surgery type by days from surgery (4–100) Open thoracotomy vs VATs |
−0.17 | 0.08 | .04 |
| Cardiovascular complications (yes vs no) | 1.33 | 0.46 | .004 |
| Disturbed sleep | |||
| Days from surgery (≤3) | 0.11 | 0.03 | .001 |
| Days from surgery (4–100) | –0.14 | 0.03 | < .0001 |
| Shortness of breath | |||
| Days from surgery (≤3) | 0.27 | 0.05 | < .0001 |
| Surgery type by days from surgery (≤3) Open thoracotomy vs VATs |
0.24 | 0.10 | .02 |
| Days from surgery (4–100) | –0.30 | 0.05 | < .0001 |
| Surgery type by days from surgery (4–100) Open thoracotomy vs VATs |
–0.23 | 0.10 | .03 |
SE, standard error; MDASI, MD Anderson Symptom Inventory; ECOG PS, Eastern Cooperative Oncology Group performance status; VATS, video-assisted thoracoscopic surgery.
Multinomial logit mixed modeling: N=60, 651 observations.
Significant interactions between surgery type and time were found. Compared with patients who underwent VATS lobectomy, patients who underwent standard open thoracotomy reported more rapid increase in the first 3 days postsurgery and slower decrease after 3 days postsurgery for 4 symptoms: fatigue, pain, drowsiness, and shortness of breath.
Defining the Postoperative Recovery Time Course using Symptom Outcomes
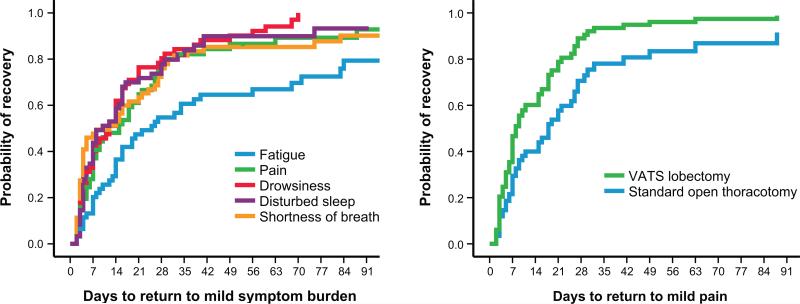
Figure 2A presents Kaplan–Meier curves of recovery to mild severity over time during the 3-month postoperative period for the 5 most-severe symptoms. Patients who underwent VATS lobectomy showed a faster recovery for pain than did patients who underwent standard open thoracotomy (8 days vs 18 days, P = .022) (Figure 2B). Supplemental Tables S2 and S3 show additional data related to Figures 2A and 2B, respectively, on the number of patients with moderate to severe symptoms (≥4 on the 0–10 scale) and the probability of recovery at given time points during the study.
FIGURE 2.
Time to symptom recovery to mild severity after thoracic surgery. A, Kaplan–Meier curves for the 5 most-severe symptoms overall. B, Kaplan–Meier curves for difference in pain recovery between standard open thoracotomy and VATS lobectomy (P = .022). “Return to mild pain” was defined as a report of MDASI pain scores ≤3 (none or mild) at 2 contiguous measurements. VATS, video-assisted thoracoscopic surgery. See Supplemental Tables S2 and S3 for additional data related to Figures 2A and 2B, respectively, on the number of patients with moderate to severe symptoms and the probability of recovery at given time points during the study.
Table 3 presents the median time course of recovery to mild symptom ratings (≤3 on a 0–10 scale) by Kaplan–Meier analysis. Fatigue showed a slower recovery to mild severity than did the other 4 symptoms (median 22 days vs 10–15 days). Generally, symptoms took longer to return to baseline levels than to mild symptom ratings. Median days for recovery to baseline symptom ratings was higher for fatigue than for the other 4 most-severe symptoms (Table 3).
TABLE 3.
Postoperative symptom recovery to mild or baseline severity, by Kaplan–Meier analysis
| N | Recovered, n (%) | Median recovery days (95% CL) | Mean recovery days (95% CL) | |
|---|---|---|---|---|
| Recovery to mild severity (0–3 on the MDASI's 0–10 scale)* | ||||
| Pain | 60 | 49 (81.7) | 15 (7, 23) | 23 (15, 30) |
| Fatigue | 60 | 41 (68.3) | 22 (11, 33) | 39 (29, 48) |
| Drowsiness | 60 | 53 (89.3) | 12 (7, 17) | 18 (13, 23) |
| Shortness of breath | 60 | 51 (85.0) | 12 (2, 22) | 22 (15, 29) |
| Disturbed sleep | 60 | 49 (81.7) | 10 (4, 16) | 20 (13, 26) |
| Recovery to preoperative (baseline) severity† | ||||
| Pain | 60 | 35 (58.3) | 54 (44, 64) | 56 (47, 65) |
| Fatigue | 60 | 29 (48.3) | 75 (61, 89) | 62 (52, 72) |
| Drowsiness | 60 | 42 (70.0) | 27 (17, 37) | 41 (32, 51) |
| Shortness of breath | 60 | 35 (58.3) | 69 (23, 115) | 54 (43, 64) |
| Disturbed sleep | 60 | 46 (76.7) | 16 (5, 34) | 34 (25, 43) |
CL, confidence limit; MDASI, MD Anderson Symptom Inventory.
“Recovery to mild severity” was defined as a report of MDASI symptom scores ≤3 (none or mild) at 2 contiguous measurements.
“Recovery to preoperative (baseline) severity” was defined as a report of MDASI symptom levels at or below the preoperative (baseline) level at 2 contiguous measurements.
DISCUSSION
The current study demonstrates the utility of the MDASI, a PRO assessment tool, for evaluating symptom severity in patients who have undergone surgery for NSCLC and for detecting expected fine differences in symptom report by type of procedure (VATS lobectomy vs standard open thoracotomy) and patient characteristics. To our knowledge, this longitudinal study is the first to use PROs to define a postoperative recovery course.
In this cohort of newly diagnosed, treatment naïve patients with early-stage NSCLC, a cluster of symptoms peaked immediately after surgery, with a third of patients experiencing severe pain on day 3, the first time symptoms were measured postsurgery. Pain, fatigue, and shortness of breath were highly prevalent in the first week after surgery, representing a combined effect from surgical insult and the perioperative care. The mean ratings for most symptoms (except for fatigue) had returned to preoperative levels by the end of the first month. Fatigue remained the most persistent symptom during the 3 month study. Postoperative fatigue had a somewhat different pattern of recovery compared with the other major symptom outcomes in this study, peaking 2 days later (at day 5) than the other symptoms (Figure 1, Supplemental Table S1) and recovering more slowly. The leading role of fatigue in the postoperative setting is similar to that induced by other major cancer therapies, such as chemoradiation or stem-cell transplant,21,22 and reflects the fact that there is currently no effective management strategy for this symptom. Among all MDASI data collected for a total of 13 symptoms, we identified 5 most-severe symptoms that represent a profile of the worst symptom burden for this postsurgery patient cohort.
Our finding of differences in pain severity between VATS lobectomy and standard open thoracotomy mirrors previously reported clinical benefits from thoracoscopic lobectomy for early-stage NSCLC.11,23 This demonstrates that the MDASI is sufficiently sensitive to differentiate postoperative symptoms by type of procedure. The acute pain experienced by patients after open thoracotomy surgery,12,24 which is caused by a combination of insults such as retraction, resection, or fracture of ribs, dislocation of costovertebral joints, injury of intercostal nerves, and further irritation of the pleura by chest tubes,25 is considered as more severe than that produced by VATS lobectomy.
Assessing PROs allows researchers and health professionals to identify who is at greater risk for high postoperative symptoms. Both the Charlson Comorbidities Index26 and the American Society of Anesthesiologists (ASA) physical status classification score, currently used as important routine measures of comorbidity for predicting major complications,27-29 are also predictive of risk for high symptom burden from postoperative pain. This finding is in accord with prior work in other cancer populations demonstrating the detrimental impact of comorbidities on PROs. The impact of comorbidities may be additive or even synergistic–an important area for future investigation.
This work also defined the time course of postoperative recovery after thoracic surgery, using a PRO-based approach. The late phase of recovery has been described as the time “from hospital discharge to return to usual function and activities,”6 yet there is lack of agreement in contemporary clinical practice on how to define and measure recovery after major cancer surgery and on what constitutes optimal care during recovery.30 We defined “recovery” using a 2-pronged approach: symptom return to baseline, or symptom return to a mild severity level. Because two-thirds of the patients in this study sample reported having no symptoms (rated 0 on 0–10 scale) at baseline, it is not surprising that the time to return to mild symptoms (rated 0–3) was much shorter than the time return to baseline symptom levels.
This study had several limitations. First, the sample was a homogenous group of patients with early-stage lung cancer. Future studies with more diversity are warranted in order to identify major symptoms and define the trajectory of postoperative recovery with more generalizability. Second, the MDASI assesses a core set of 13 symptoms that are common to patients with cancer; a MDASI version that is specifically geared to perioperative care after thoracic surgery has not yet been created, tested, and psychometrically validated. Third, we did not collect symptom scores on days 1 and 2 postsurgery, and we did not assess objective measures affecting duration of hospital stay, such as fluid status, pain medicine usage, infection, or chest drainage. The symptomatic differences between VATS lobectomy and open thoracotomy that were observed clinically may have been even more striking if these data had been included in the analysis. Finally, this study occurred before our institution's ERP program was established. The recovery period, symptom profiles, and specific risk factors for impaired recovery could vary after implementation of an ERP pathway for patients undergoing different types of thoracic surgery.9,31
In sum, the current study is among the first to describe the nature of the worst symptoms reported by patients with NSCLC during the 3 months after thoracic surgery. The establishment of an ERP pathway may be an important part of perioperative management after cancer surgery, as ERPs can be expected to provide meaningful improvements in recovery that should hasten a patient's return to planned chemotherapy or other cancer treatment when indicated. Using a straightforward, concise tool like the MDASI to obtain the patient's perspective on how well he or she is recovering is a clinically relevant and user-friendly method for optimizing perioperative care.9 Routine inclusion of an easy method, such as the MDASI, to characterize recovery via symptom report would be a novel PRO application in perioperative care that has the potential to improve standard practice.
Supplementary Material
PERSPECTIVE.
We used the MD Anderson Symptom Inventory (MDASI) to elicit patient report of the worst symptoms experienced after thoracic surgery. Using a validated tool to gain the patient's perspective on symptom burden is a clinically relevant, user-friendly way to optimize perioperative care. Routine inclusion of symptom report to characterize recovery has the potential to improve standard practice.
ACKNOWLEDGMENTS
The authors appreciate Jeanie F. Woodruff, BS, ELS for editorial assistance and Ibrahima Gning, DrPH and Winifred A. Apraku, MS, for data management.
Funding sources: This study was funded by grants from the National Cancer Institute of the National Institutes of Health, including NCI R01 CA026582 (PI: Charles S. Cleeland) and the MD Anderson Cancer Center Support Grant NCI P30 CA016672 (PI: Ronald A. DePinho), and by an ACS Research Scholar Grant from the American Cancer Society (PI: Charles S. Cleeland). None of the sponsors had any role in the study design, data collection, analysis, interpretation, or preparation of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors report no conflicts of interest in this work. Charles Cleeland has received consultant fees from AstraZeneca, Abbott, Genentech, Amgen, Bristol-Myers Squibb, Pfizer, Bayer, Johnson & Johnson, Novartis, and GlaxoSmithKline.
CENTRAL MESSAGE
Using the MDASI to elicit patient-reported symptom burden is a simple, clinically relevant way to optimize care after thoracic surgery.
CENTRAL FIGURE FOR SUMMARY/WEBSITE
Time to recover to mild symptom severity after thoracic surgery (by symptom, surgery type)
REFERENCES
- 1.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–75. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 2.Aloia TA, Lee JE, Vauthey JN, Abdalla EK, Wolff RA, Varadhachary GR, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–55. doi: 10.1016/j.jamcollsurg.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Aloia TA, Zimmitti G, Conrad C, Gottumukalla V, Kopetz S, Vauthey JN. Return to intended oncologic treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol. 2014;110:107–14. doi: 10.1002/jso.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kehlet H, Mythen M. Why is the surgical high-risk patient still at risk? Br J Anaesth. 2011;106:289–91. doi: 10.1093/bja/aeq408. [DOI] [PubMed] [Google Scholar]
- 5.Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–40. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Lee L, Tran T, Mayo NE, Carli F, Feldman LS. What does it really mean to “recover” from an operation? Surgery. 2014;155:211–6. doi: 10.1016/j.surg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Mortensen K, Nilsson M, Slim K, Schafer M, Mariette C, Braga M, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101:1209–29. doi: 10.1002/bjs.9582. [DOI] [PubMed] [Google Scholar]
- 8.Cata JP, Noguera EM, Parke E, Ebrahim Z, Kurz A, Kalfas I, et al. Patient-controlled epidural analgesia (PCEA) for postoperative pain control after lumbar spine surgery. J Neurosurg Anesthesiol. 2008;20:256–60. doi: 10.1097/ANA.0b013e31817ffe90. [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Cella D, Butt Z. Current challenges in using patient-reported outcomes for surgical care and performance measurement: everybody wants to hear from the patient, but are we ready to listen? JAMA Surg. 2014 doi: 10.1001/jamasurg.2013.5285. Epub 2014 May 28. [DOI] [PubMed] [Google Scholar]
- 10.Jaklitsch MT. “How am I doing? Just ask me!” The usefulness of patient self-reported quality of life in thoracic surgery. J Thorac Cardiovasc Surg. 2014 doi: 10.1016/j.jtcvs.2014.11.068. Epub 2014 Dec 02. [DOI] [PubMed] [Google Scholar]
- 11.Stephens N, Rice D, Correa A, Hoffstetter W, Mehran R, Roth J, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg. 2014;46:607–13. doi: 10.1093/ejcts/ezu036. [DOI] [PubMed] [Google Scholar]
- 12.Yan TD, Black D, Bannon PG, McCaughan BC. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2553–62. doi: 10.1200/JCO.2008.18.2733. [DOI] [PubMed] [Google Scholar]
- 13.Moloney R, Conley R, Messner D, Mitchell K, Ganesan N, Tunis S. A multi-stakeholder agenda to advance enhanced recovery for U.S. surgical patients [Internet]. Enhanced Recovery Protocols for Surgical Patients: Challenges and Opportunities. A Multi-Stakeholder Dialogue, Baltimore MD, Jun 10,. 2014. Center for Medical Technology Policy; Baltimore MD: Oct, 2014. [2014 Jun 10]. Available from: http://www.cmtpnet.org/docs/resources/Enhanced_Recovery_White_Paper_9_OCT_2014.pdf. [Google Scholar]
- 14.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 15.Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol. 2012;30:4249–55. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Berger AM, Mooney K, Alvarez-Perez A, Atkinson A, Breitbart WS, Brothers B, et al. NCCN Practice Guidelines in Oncology: Cancer-Related Fatigue [Internet] National Comprehensive Cancer Network; Fort Washington PA: Jan, 2014. [2014 Sep 8]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf. [Google Scholar]
- 18.Swarm RA, Abernethy AP, Anghelescu DL, Benedetti C, Buga S, Cleeland C, et al. Adult cancer pain. J Natl Compr Canc Netw. 2013;11:992–1022. doi: 10.6004/jnccn.2013.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–84. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 20.Wang XS, Zhao F, Fisch MJ, O'Mara AM, Cella D, Mendoza TR, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120:425–32. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang XS, Shi Q, Williams LA, Cleeland CS, Mobley GM, Reuben JM, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113:2102–9. doi: 10.1002/cncr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XS, Shi Q, Shah N, Heijnen CJ, Cohen EN, Reuben JM, et al. Inflammatory markers and development of symptom burden in patients with multiple myeloma during autologous stem cell transplantation. Clin Cancer Res. 2014;20:1366–74. doi: 10.1158/1078-0432.CCR-13-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soukiasian HJ, Hong E, McKenna RJ., Jr. Video-assisted thoracoscopic trisegmentectomy and left upper lobectomy provide equivalent survivals for stage IA and IB lung cancer. J Thorac Cardiovasc Surg. 2012;144:S23–S26. doi: 10.1016/j.jtcvs.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 24.Gerner P. Postthoracotomy pain management problems. Anesthesiol Clin. 2008;26:355–67. doi: 10.1016/j.anclin.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochroch EA, Gottschalk A. Impact of acute pain and its management for thoracic surgical patients. Thorac Surg Clin. 2005;15:105–21. doi: 10.1016/j.thorsurg.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Reid BC, Alberg AJ, Klassen AC, Koch WM, Samet JM. The American Society of Anesthesiologists' class as a comorbidity index in a cohort of head and neck cancer surgical patients. Head Neck. 2001;23:985–94. doi: 10.1002/hed.1143. [DOI] [PubMed] [Google Scholar]
- 28.Froehner M, Koch R, Litz R, Heller A, Oehlschlaeger S, Wirth MP. Comparison of the American Society of Anesthesiologists Physical Status classification with the Charlson score as predictors of survival after radical prostatectomy. Urology. 2003;62:698–701. doi: 10.1016/s0090-4295(03)00570-3. [DOI] [PubMed] [Google Scholar]
- 29.Tzeng CW, Vauthey JN. Postoperative complications and oncologic outcomes after resection of colorectal liver metastases: the importance of staying on track. Ann Surg Oncol. 2013;20:2457–9. doi: 10.1245/s10434-013-2974-x. [DOI] [PubMed] [Google Scholar]
- 30.Fernando HC, Landreneau RJ, Mandrekar SJ, Nichols FC, DiPetrillo TA, Meyers BF, et al. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: Results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg. 2014 doi: 10.1016/j.jtcvs.2014.11.003. Epub 2014 Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee BE, Korst RJ, Kletsman E, Rutledge JR. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg. 2014;147:724–9. doi: 10.1016/j.jtcvs.2013.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.