Abstract
We describe two infants with congenital diaphragmatic hernia (CDH) with severe pulmonary hypertension at 6-weeks. Treprostinil was used with rapid clinical improvement. Repeat cardiac catheterization showed dramatic improvement. Both infants weaned off the drug, representing the first reports of successful short-term treprostinil use in neonates with CDH.
Keywords: pulmonary hypertension, lung hypoplasia, prostanoids, prostacyclin analogues
Congenital diaphragmatic hernia (CDH) occurs 1:4-5,000 live births [1,2]. Despite advances in the care of these infants, pulmonary hypoplasia and resulting pulmonary hypertension (PH) carry significant morbidity and mortality [3,4]. We recently demonstrated that 28% of infants with any PH by echocardiogram at 4 weeks die, and an additional 37% have chronic lung disease [5]. Others have shown 56% mortality among infants with PH at 1-month [6]. Although chronic oral and inhaled drugs from various classes have been used by us and others to treat PH in infants with CDH [7], in chronic, severe PH, these agents may not provide sufficient vasodilation due to dose limitations and/or lack of continuous effect. Thus, when an infant is unable to separate from assisted ventilation, inhaled nitric oxide (iNO), and other adjunctive therapies, parenteral prostanoid therapy may be considered, to allow for withdrawal of these other therapies and transition home.
Case 1
A female infant born at 37 weeks' gestation with pregnancy complicated by late prenatal care was noted at initial referral to have a left-sided CDH with the liver herniated into the thoracic cavity. Fetal echocardiogram showed normal cardiac anatomy and function.
She was intubated at delivery and managed with gentle ventilation, permissive hypercapnia, and iNO, which was initiated for persistently low right upper extremity (pre-ductal) oxygen saturation (SpO2) and pre- and post-ductal SpO2 differential. Initial echocardiogram on day-of-life (DOL) 1 confirmed normal cardiac anatomy, with systemic-to-suprasystemic right ventricular pressure (RVp) estimate and a large patent ductus arteriosus (PDA). On DOL 3 she had an uncomplicated hernia repair with a large Goretex patch required to close the defect. At 1 week, a repeat echocardiogram still showed systemic-to-suprasystemic RVp, while intubated on iNO with pre- and post-ductal SpO2 differential. At 2 weeks, there was persistence of systemic-to-suprasystemic RVp, with decreased function, so prostaglandin E2 (PGE) infusion was initiated to protect the RV. By 3 weeks she was extubated to nasal continuous positive airway pressure (NCPAP) after which an echocardiogram showed improvement in estimated RVp and PGE was discontinued. At 4 weeks, iNO was weaned and a subsequent echocardiogram showed continued improvement, with elevated estimated RVp < 2/3 systemic and a tiny PDA. However, iNO was re-started 2 days later for increasing respiratory distress; she was re-intubated for episodes of hypoxia consistent with pulmonary hypertensive crises. Echocardiogram at that time demonstrated systemic-to-suprasystemic RVp, with decreased systolic and diastolic function with no PDA. Cardiac catheterization at 44 days measured suprasystemic pulmonary pressures with severely elevated pulmonary vascular resistance (PVR) (Table).
Table.
Timing of diagnostic studies and events related to trepostinil therapy.
| Case #1 | Case #2 | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Pre-treatment | 2 weeks treatment |
8 weeks treatment |
7 months of age |
Pre-treatment | 2 weeks treatment |
8 weeks treatment |
8 months of age |
|
| Clinical Characteristics | ||||||||
|
| ||||||||
| Respiratory Support | SIMV, 25/6 | 4 LPM High flow NC | 2 LPM High flow NC | 0.5 LPM NC with sleep | NCPAP 7 cmH20 | NCPAP 6 cmH20 | 0.5LPM | 0.25 LPM NC with sleep |
| FiO2 | 0.5 | 0.4 | 0.3 | 1.0 | 0.4 | 0.25 | 1.0 | 1.0 |
| iNO dose | 20 ppm | off | off | off | 20 ppm | off | off | off |
| BNP (pg/mL) | 4080 | 161 | 25 | - | 143 | 80 | 5 | 7 |
| PH treatment | iNO | Treprostinil 42 ng/kg/min | Treprostinil 35 ng/kg/min, bosentan | None | PGE1, milrinone, iNO | Treprostinil 42ng/kg/min | Treprostinil 44 ng/kg/min | Treprostinil 30 ng/kg/min bosentan |
|
| ||||||||
| Echocardiography | ||||||||
|
| ||||||||
| Estimated RV pressure | Systemic-to- suprasystemic | Systemic-to- suprasystemic | Normal | Normal | Systemic-to- suprasystemic | <2/3 systemic | Normal | Normal |
| Septal position | Flattened | D-shaped | Normal | Normal | D-shaped Moderately | Flattened | Normal | Normal |
| RV systolic function | Low Normal | Normal | Normal | Normal | decreased | Normal | Normal | Normal |
| RV dilation | Moderate | Moderate | Mild | None | Severe | None | None | None |
| RV hypertrophy | Moderate | Moderate | Moderate | None | Moderate | Mild | Mild | None |
|
| ||||||||
| Cardiac Catheterization | ||||||||
|
| ||||||||
| Baseline PAp* | 73/23 (44) | 41/15 (25) | 48/21 (33) | 23/12 (17) | ||||
| PAp with 100% FiO2 + iNO 40ppm | 51/20 (32) | 31/10 (19) | 53/34 (41) | 20/12 (16) | ||||
| Systemic blood pressure | 60/32 (40) | 82/43/62 | 46/28 (36) | 46/28 (35) | ||||
| MCWP | 5 | 6 | 5 | 4 | ||||
| RAp | 5 | 3 | 4 | 4 | ||||
| Qp:Qs | 1 | 1 | 1.7 | 1 | ||||
| Baseline PVRi+ | 13.5 | 3.7 | 7.1 | 4.6 | ||||
| PVRi with 100% FiO2 + iNO 40ppm | 9.9 | 2.8 | 5.8 | 4.3 | ||||
All pressures are reported in mmHg
All resistances are reported as Woods Units
BNP B-type natriuretic peptide, FiO2 Fraction of inspired oxygen concentration, iNO Inhaled nitric oxide, MCWP Mean capillary wedge pressure, NC Nasal cannula, NCPAP Nasal continuous airway pressure, PAp Pulmonary arterial pressure, PH pulmonary hypertension, PVRi Pulmonary vascular resistance index, Qp:Qs Pulmonary to systemic blood flow, RAp Right atrial pressure, SIMV Synchronized intermittent mandatory ventilation
Given her severe condition, the patient was started on continuous intravenous epoprostenol, and transitioned to intravenous treprostinil after 48 hours. Treprostinil was titrated up over the next 4 weeks to a peak dose of 48 ng/kg/min with minimal side effects, initially by 2 ng/kg/min daily, then bi-weekly, then weekly (Figure). She was weaned off iNO, extubated and weaned to nasal cannula (NC) oxygen (Table). At 4 weeks, bosentan was initiated and titrated to goal dose (2 mg/kg twice daily). After 2 weeks, treprostinil was weaned over 30 days, first down to 40 ng/kg/min, then by 5 ng/kg/min bi-weekly until discontinuation (Figure). Echocardiograms continued to show improvement with normal RVp. At 3 months of age, sildenafil was initiated, but then discontinued due to retching and vomiting.
Figure.
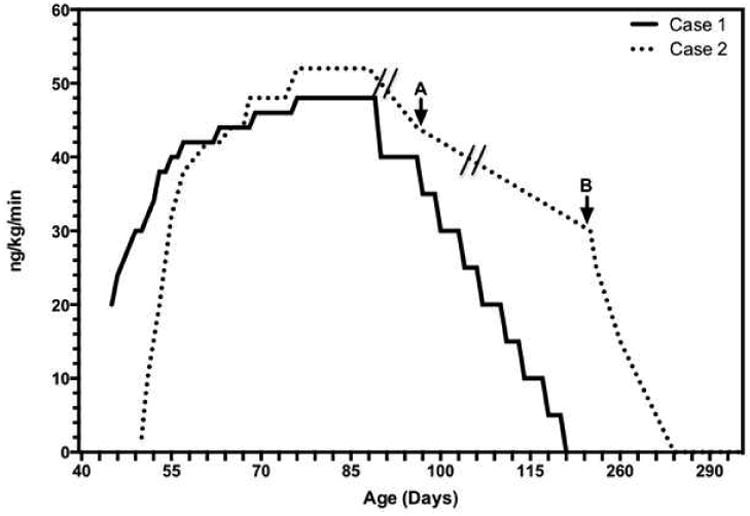
Dose titration for treprostinil, based on infant weight at drug initiation unless otherwise noted. Effective dose, after adjustment for A, weight at discharge and B, weight gain; active wean initiated. Hash marks indicate periods when infant gained weight without active changes in dose or dose adjustment.
The infant was discharged home at 135 days on bosentan and NC 0.5 LPM. Echocardiograms after discharge (at 155, 183, and 344 days of age) continued to be reassuring.
Follow-up cardiac catheterization at 7 months showed near-normal PVR (Table) and bosentan was discontinued. By 1 year, she was weaned from supplemental oxygen and continued to grow and develop well.
Case 2
A female infant was born at 40 weeks gestation with pregnancy complicated by a diagnosis of left-sided CDH with liver herniated into the chest at 16 weeks. Fetal echocardiogram revealed normal cardiac structure. She was intubated and paralyzed at delivery, with initial support by high frequency oscillatory ventilation with FiO2 of 1.0 and 20ppm iNO, due to low pre-ductal SpO2. Echocardiogram on DOL 1 showed evidence of systemic-to-suprasystemic RVp, moderate-severe right heart dilation, and diminished bi-ventricular function. PGE was initiated. On DOL 2, the diaphragamatic defect was closed by internal oblique muscle flap. On DOL 5, an echocardiogram showed continued evidence of systemic-to-suprasystemic RVp, moderate RV dilation and mildly diminished RV function. At 2 weeks, she self-extubated. On NCPAP, iNO and PGE, repeat echocardiograms at 2 and 3 weeks were unchanged. Milrinone was started at 3 weeks for additional pulmonary vasodilation. An echocardiogram at 4 weeks showed transient improvement, with near-systemic RVp. However, at 6 weeks, RVp was systemic-to-suprasystemic with diminished function (Table).
Cardiac catheterization at 48 days of age confirmed normal anatomy and systemic pulmonary pressures with elevated PVR. Subcutaneous treprostinil was initiated and successfully titrated up over the next 5 weeks to a peak dose of 52 ng/kg/min with minimal side effects. The dose was increased by 2 ng/kg/min every 8 hours initially, then daily, then every other day (Figure). She improved rapidly, by clinical and echocardiographic assessment, successfully weaning off PGE, milrinone, and iNO in the first 2 weeks of therapy (Table). The subcutaneous catheter site was changed only once, due to pump occlusion with no other etiology identified. Other indications for catheter site change include leakage, bleeding and signs of local infection, but these did not occur through the inpatient course.
The infant was discharged home at 97 days of age on NC 0.5 LPM and subcutaneous treprostinil. On average, the parents replaced the catheter every 4 weeks, as needed. There was one episode of site infection that resolved with warm compresses. Bosentan was initiated at 6 months of age and increased to full dose (2 mg/kg twice daily). At 8 months, supplemental oxygen was weaned off and repeat cardiac catheterization confirmed normal pulmonary pressures on room air (Table). With excellent growth, her treprostinil dose had effectively decreased to 30 ng/kg/min based on her actual weight. Weaning was initiated, with dose decreases by 5 ng/kg/min every 3 days down to 15 ng/kg/min. An echocardiogram and examination at that time showed no evidence of PH. The dose was then weaned by 5 ng/kg/min every 6 days. An echocardiogram and examination off of treprostinil were normal.
Discussion
These cases demonstrate benefit of short-term treprostinil use initiated at 6-8 weeks of age in neonates with severe PH following CDH repair. These cases are consistent with our prior report demonstrating pulmonary vascular reactivity in infants with CDH at 2-3 months of age [7]. Further, the successful use of subcutaneous treprostinil improves the safety of administration of prostanoids in this vulnerable population, eliminating the need for a central line and its associated risks, and allowing for outpatient therapy in a young infant. Although some practitioners have concerns that appropriate infusion sites will become limited when initiating subcutaneous therapy in infants, this infant had minimal problems with the catheter and she was able to wean off the drug with a limited duration of parenteral therapy.
Acknowledgments
L.L. was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (T32 HD-07162).
Abbreviations and Acronyms
- CDH
Congenital diaphragmatic hernia
- DOL
Day of life
- iNO
Inhaled nitric oxide
- NC
Nasal cannula
- NCPAP
Nasal continuous positive airway pressure
- PDA
Patent ductus arteriosus
- PGE
Prostaglandin E2
- PH
Pulmonary hypertension
- PVR
Pulmonary vascular resistance
- RVp
Right ventricular pressure
- SpO2
Oxygen saturation
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balayla J, Abenhaim HA. Incidence, predictors and outcomes of congenital diaphragmatic hernia: a population-based study of 32 million births in the United States. J Matern Fetal Neonatal Med. 2013;27:1438–44. doi: 10.3109/14767058.2013.858691. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Carmichael SL, Harris JA, Shaw GM. Epidemiologic characteristics of congenital diaphragmatic hernia among 2.5 million California births, 1989-1997. Birth Defects Res A Clin Mol Teratol. 2006;76:170–174. doi: 10.1002/bdra.20230. [DOI] [PubMed] [Google Scholar]
- 3.Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:307–312. doi: 10.1016/j.jpedsurg.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe persistent pulmonary hypertension of the newborn. J Pediatr. 1997;131:55–62. doi: 10.1016/s0022-3476(97)70124-0. [DOI] [PubMed] [Google Scholar]
- 5.Lusk LA, Wai KC, Moon-Grady AJ, Steurer MA, Keller RL. Persistence of Pulmonary Hypertension by Echocardiography Predicts Short-term Outcomes in Congenital Diaphragmatic Hernia. J Pediatr. doi: 10.1016/j.jpeds.2014.10.024. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn J, Krishnan U, Aspelund G, Zhang Y, Duong J, et al. Outcomes of congenital diaphragmatic hernia in the modern era of management. J Pediatr. 2013;163:114–119 e111. doi: 10.1016/j.jpeds.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller RL, Moore P, Teitel D, Hawgood S, McQuitty J, Fineman JR. Abnormal vascular tone in infants and children with lung hypoplasia: Findings from cardiac catheterization and the response to chronic therapy. Pediatr Crit Care Med. 2006;7:589–94. doi: 10.1097/01.PCC.0000244401.53189.CB. [DOI] [PubMed] [Google Scholar]


