Abstract
Background
Using positron emission tomography (PET) it is possible to estimate endogenous dopamine (DA) occupying D2/3 receptors (D2/3R) in the living human brain. Persons with schizophrenia (SZ) (previously medicated and naïve) have increased endogenous DA occupying D2/3R in the caudate. It is unknown whether currently medicated patients demonstrate increased DA levels at D2/3R. Moreover, DA levels have not been estimated in SZ using agonist radiotracers, which may offer a more sensitive quantification over antagonists.
Methods
Using the agonist radiotracer [11C]-(+)-PHNO, DA levels were estimated at D2/3R (ΔBPND) in three patients with SZ (Male, Mage=30±16). Patients were currently being treated long-term with Olanzapine (147±88 nmol/L). Results were compared to ten healthy controls (HC’s).
Results
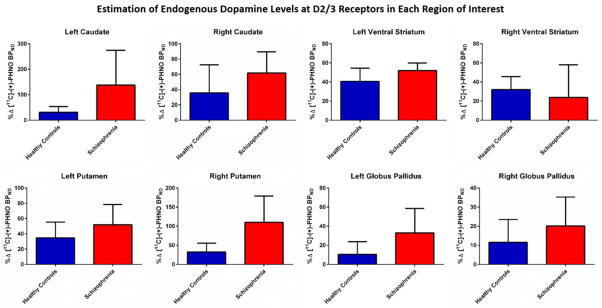
Medicated persons with SZ had greater ΔBPND in the left caudate (U=2, Z=−2.20, p=.03) and right putamen (U=2, Z=−2.20, p=.03). No differences were observed in the ventral striatum or globus pallidus.
Conclusions
It is possible to estimate endogenous DA at D2/3R in SZ patients currently taking antipsychotics. Despite medication, patients continue to have increased endogenous DA at D2/3R. This lends more biological support to the clinical observation that relapses in symptoms can occur in the face of complete antipsychotic discontinuation. Future studies with larger samples are warranted.
Keywords: PET, Dopamine, D2/3R, Schizophrenia
Introduction
Schizophrenia is a severe and chronic mental illness which affects 1% of the population, ranking as one of the leading causes of disability worldwide [1, 2]. While the cause of schizophrenia is currently unknown, the “dopamine (DA) hypothesis of schizophrenia” has been the most enduring idea in psychiatry for the last fifty-years [3]. Thus, long before advanced brain imaging, it was hypothesized that abnormally high levels of the neurochemical DA would be present in the brains of persons with schizophrenia [4].
Positron emission tomography (PET) is an in vivo molecular imaging technique which can allow measurement of protein molecules such as DA receptors. Using PET and radiolabelled DA D2/3 receptor (D2/3R) antagonists, such as [11C]-raclopride, [18F]-fallypride, and [11C]-FLB 457, it has been possible to quantify the availability of D2/3R in the brains of healthy persons and persons with neuropsychiatric disease in vivo [5–7]. Endogenous DA competes with these radiotracers for binding to D2/3R at baseline [8]. Thus, increasing DA levels in the brain can decrease radiotracer binding and vice-versa. Thus changes in radiotracer binding given challenges which alter DA levels can reveal estimates of DA release and endogenous tone [9].
Using PET and a dopamine depletion challenge it is possible to estimate endogenous DA occupying DA D2/3 receptors in the living human brain [10, 11]. Using this method, it has been demonstrated that individuals with schizophrenia (previously medicated and antipsychotic naïve) have increased endogenous DA occupying D2/3R (D2/3R) in the dorsal striatum – in particular the caudate [12, 13]. However, it is unknown whether patients currently taking antipsychotics still demonstrate increased endogenous DA levels at the D2/3R. Moreover, endogenous dopamine levels in patients with schizophrenia have only been estimated using the antagonist radiotracer [11C]-raclopride, which has similar affinity for D2R and D3R.
Our group has developed [11C]-(+)-PHNO [14], the first agonist radiotracer for D2/3R which has preferential affinity for D3 receptors [15–18]. As an agonist, this radiotracer is more sensitive to changes in endogenous DA [19, 20] and may provide a more sensitive and physiologically meaningful estimate of endogenous DA occupying D2/3R in the brains of healthy persons and persons with schizophrenia [21]. Moreover, it can provide estimates of endogenous DA in D3R rich brain regions not previously explored specifically in persons with schizophrenia, such as the globus pallidus [21].
The current investigation sought to address whether patients currently taking antipsychotics have increased endogenous DA in the striatum. This was accomplished using the alpha-methyl-para-tyrosine (AMPT) DA depletion challenge with PET and [11C]-(+)-PHNO to quantify endogenous DA levels
Methods
The radiosynthesis of [11C]-(+)-PHNO, acquisition/analysis of PET images, and the DA depletion paradigm were identical to those described in detail elsewhere [14, 21]. Patients with schizophrenia received two PET scans: one during a baseline state and another during acute DA depletion. Baseline scans occurred before depletion scans and were at least two days apart. Depletion was induced by oral administration of 64 mg of AMPT (metyrosine) per kilogram of body weight over 25 hours.
The estimate of endogenous DA at D2/3R is based on the occupancy model [8, 10, 11]. This model assumes: i) baseline radiotracer binding (BPND) to D2/3R is confounded by endogenous DA - higher DA concentrations result in lower baseline D2/3R BPND, and, ii) the fractional increase in D2/3R BPND after DA depletion [i.e., 100*(Depletion BPND − Baseline BPND)/Baseline BPND = ΔBPND] is linearly proportional to the baseline DA concentration at D2/3R. Thus, the ΔBPND, given appropriate assumptions, is considered a semiquantitative index of endogenous DA levels at D2/3R [11].
We also held the following assumptions. If the amount and timing of antipsychotic dosing remains constant between the baseline and DA depleted PET scans, then any increase in [11C]-(+)-PHNO BPND after depletion should reflect reduced competition by DA. Note that it is assumed that endogenous DA has a higher affinity for D2/3R than antipsychotics in vivo, and will compete more strongly for newly available binding sites given depletion over antipsychotics.
Results
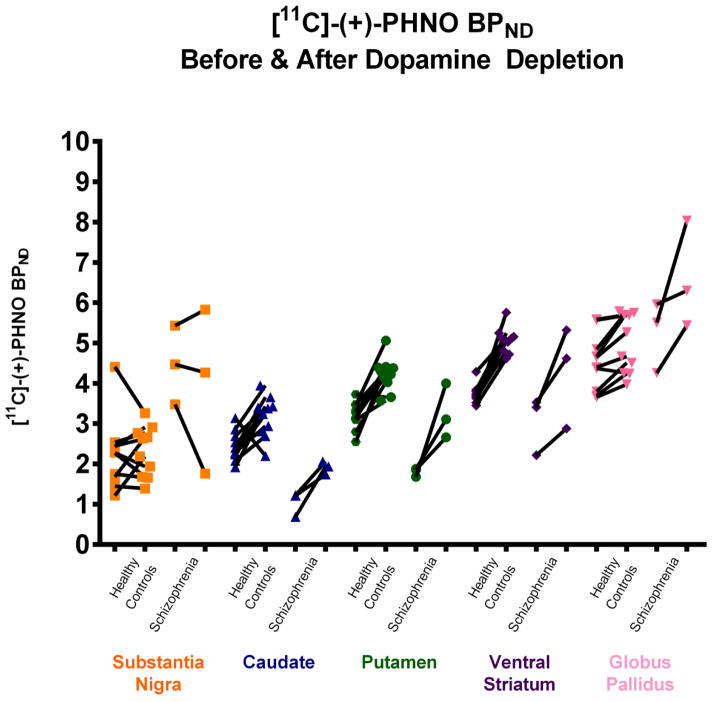
Three patients with schizophrenia (male, mean age=30±16) being treated long-term with olanzapine underwent two [11C]-(+)-PHNO PET scans: one at baseline and one after acute DA depletion. Data from these patients were compared with previously published data from 10 healthy controls (HC) (4 female, mean age: 29.1±8.39) [21]. For one patient with schizophrenia, we were unable to collect plasma samples of AMPT, homovanillic acid (HVA), 3-Methoxy-4-hydroxyphenylglycol (MHPG), prolactin, and olanzapine (See Table 1). The [11C]-(+)-PHNO BPND before and after DA depletion in HC and patients with schizophrenia are presented in Figure 1. The ΔBPND between patients and HC were examined using Mann-Whitney U Tests (p<0.05, two-tailed).
Table 1.
Plasma levels of participants. Ten healthy controls and two patients with schizophrenia.
| Plasma Levels | Group | Baseline | AMPT 5h | AMPT 27h | %Δ27h | ||
|---|---|---|---|---|---|---|---|
| Homovanillic Acid (HVA) nmol/L | Healthy Controls | 81.8 (42.8) | 37.0 (15.0) | 32.4 (27.2) | −60% | ||
| Medicated Patients | 84.2 (35.5) | 43.4 (31.9) | 17.6 (0.8) | −72% | |||
|
| |||||||
| 3-Methoxy-4-hydroxphenyglycol (MHPG) nmol/L | Healthy Controls | 188.3 (99.2) | 160.5 (89.2) | 109.5 (60.3) | −42% | ||
| Medicated Patients | 161.2 (44.9) | 141.3 (31.4) | 104.9 (22.3) | −38% | |||
|
| |||||||
| Prolactin ng/mL | Healthy Controls | 13.0 (9.6) | 36.8 (21.4) | 25.5 (10.4) | 135% | ||
| Medicated Patients | 20.7 (6.8) | 35.7 (8.1) | 32.0 (11.1) | 56.3% | |||
|
| |||||||
| Alpha-methyl-prara-tyrosine (AMPT) umol/L | Healthy Controls | 63.1 (38.2) | 123.0 (53.0) | ||||
| Medicated Patients | 75.7 (23.9) | 57.2 (12.7) | |||||
|
| |||||||
| Olanzapine (nmol/L) | Medicated Patients | 147 (88) | 136 (80) | 144 (84) | −2% | ||
Figure 1.
Compared to HC’s, medicated persons with schizophrenia had greater ΔBPND in the left caudate (U=2, Z=−2.20, p=.03), but not in the right (U=8, Z=−1.18, p=.29). They also showed greater ΔBPND in the right putamen (U=2, Z=−2.20, p=.03), but not the left (U=10, Z=−0.85, p=.47). There was a trend for an increase in ΔBPND for patients in the left ventral striatum (U=4, Z=−1.86, p=.08). No statistically significant differences were observed in the substantia nigra (U=10, Z=−1.69, p=.11), right ventral striatum (U=10, Z=−0.51, p=.69), left globus pallidus (U=7, Z=−1.35, p=.22), or right globus pallidus (U=11, Z=−0.68, p=.57). (See Figure 2).
Figure 2.
Discussion
This preliminary investigation sought to examine whether it is possible to estimate endogenous DA levels in the brains of persons with schizophrenia who are currently taking antipsychotics using PET and an AMPT-challenge. Our data suggest that this is possible, given the assumption of consistent antipsychotic dosing for the baseline and depletion PET scans. It appears that even during chronic treatment with an antipsychotic, patients with schizophrenia demonstrate increased baseline endogenous DA levels in the dorsal striatum at D2/3R. Thus, antipsychotics may not alter endogenous DA levels per se, suggesting that they do not alter disease pathology. This would be in keeping with evidence from clinical practice, where symptomatic relapse can be seen in the face of complete antipsychotic discontinuation. Future PET studies should attempt to estimate endogenous DA levels in medication naïve and chronically treated patients with schizophrenia using [11C]-(+)-PHNO and larger sample sizes.
Highlights.
Dopamine levels were estimated in the brains of medicated schizophrenia patients
Patients with schizophrenia had more dopamine in the left caudate and right putamen
Despite being medicated dopamine levels are still high in patients with schizophrenia
Acknowledgments
This study was funded by Canadian Institutes of Health Research (MOP-114989) and U.S. National Institute of Health (RO1MH084886-01A2). Dr. Graff-Guerrerro currently receives research support from the following external funding agencies: Canadian Institutes of Health Research, the U.S. National Institute of Health, and the Mexico Instituto de Ciencia y Tecnologıa para la Capital del Conocimiento en el Distrito Federal (ICyTDF). He has also received professional services compensation from Abbott Laboratories, Gedeon-Richter Plc, and Lundbeck; grant support from Janssen; and speaker compensation from Eli Lilly.
Footnotes
The other authors report no biomedical financial interests or potential conflicts of interest relevant to the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jablensky A. Epidemiology of schizophrenia: the global burden of disease and disability. Eur Arch Psychiatry Clin Neurosci. 2000;250:274–285. doi: 10.1007/s004060070002. [DOI] [PubMed] [Google Scholar]
- 2.Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:31. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophrenia bulletin. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia. Schizophrenia bulletin. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- 5.Newberg AB, Moss AS, Monti DA, Alavi A. Positron emission tomography in psychiatric disorders. Ann N Y Acad Sci. 2011;25:1749–6632. doi: 10.1111/j.1749-6632.2011.06162.x. [DOI] [PubMed] [Google Scholar]
- 6.Gjedde A, Wong DF, Rosa-Neto P, Cumming P. Mapping neuroreceptors at work: on the definition and interpretation of binding potentials after 20 years of progress. Int Rev Neurobiol. 2005;63:1–20. doi: 10.1016/S0074-7742(05)63001-2. [DOI] [PubMed] [Google Scholar]
- 7.Tatsch K, Poepperl G. Quantitative approaches to dopaminergic brain imaging. Q J Nucl Med Mol Imaging. 2012;56:27–38. [PubMed] [Google Scholar]
- 8.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Laruelle M, Huang Y. Vulnerability of positron emission tomography radiotracers to endogenous competition. New insights. Q J Nucl Med. 2001;45:124–138. [PubMed] [Google Scholar]
- 10.Laruelle M, D’Souza CD, Baldwin RM, et al. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17:162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 11.Verhoeff NP, Kapur S, Hussey D, et al. A simple method to measure baseline occupancy of neostriatal dopamine D2 receptors by dopamine in vivo in healthy subjects. Neuropsychopharmacology. 2001;25:213–223. doi: 10.1016/S0893-133X(01)00231-7. [DOI] [PubMed] [Google Scholar]
- 12.Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 14.Wilson AA, McCormick P, Kapur S, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- 15.Graff-Guerrero A, Redden L, Abi-Saab W, et al. Blockade of [11C](+)-PHNO binding in human subjects by the dopamine D3 receptor antagonist ABT-925. Int J Neuropsychopharmacol. 2010;13:273–287. doi: 10.1017/S1461145709990642. [DOI] [PubMed] [Google Scholar]
- 16.Graff-Guerrero A, Willeit M, Ginovart N, et al. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narendran R, Slifstein M, Guillin O, et al. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- 18.Rabiner EA, Slifstein M, Nobrega J, et al. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- 19.Shotbolt P, Tziortzi AC, Searle GE, et al. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32:127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginovart N, Galineau L, Willeit M, et al. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- 21.Caravaggio F, Nakajima S, Borlido C, et al. Estimating endogenous dopamine levels at D2 and D3 receptors in humans using the agonist radiotracer [(11)C]-(+)-PHNO. Neuropsychopharmacology. 2014;39:2769–2776. doi: 10.1038/npp.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]