Abstract
Herein, we describe the antifungal evaluation of 43 bisamidine compounds, of which 26 are new, having the scaffold [Am]-[HetAr]-[linker]-[HetAr]-[Am], in which [Am] is a cyclic or acyclic amidine group, [linker] is a benzene, pyridine, pyrimidine, pyrazine ring, or an aliphatic chain of two to four carbon, and [HetAr] is a 5,6-bicyclic heterocycle such as indole, benzimidazole, imidazopyridine, benzofuran, or benzothiophene. In the head-to-head series the two [HetAr] units are oriented such that the 5-membered rings are connected through the linker, and in the head-to-tail series, one of the [HetAr] systems is connected through the 6-membered ring; additionally, in some of the head-to-tail compounds, the [linker] is omitted. Many of these compounds exhibited significant antifungal activity against Candida albicans, Candida krusei, Candida glabrata, Candida parapsilosis, and Cryptococcus neoformans (MIC ≤4 μg/ml). The most potent compounds, e.g. P10, P19 and P34, are comparable in antifungal activities to amphotericin B (MIC 0.125 μg/ml). They exhibited rapid fungicidal activity (>3 log10 decrease in cfu/ml in 4 h) at concentrations equivalent to 4× the MIC in time kill experiments. The bisamidines strongly inhibited DNA, RNA and cell wall biosynthesis in C. albicans in macromolecular synthesis assays. However, the half-maximal inhibitory concentration for DNA synthesis was approximately 30-fold lower than those for RNA and cell wall biosynthesis. Fluorescence microscopy of intact cells of C. albicans treated with a bisamidine exhibited enhanced fluorescence in the presence of DNA, demonstrating that the bisamidine was localized to the nucleus. The results of this study show that bisamidines are potent antifungal agents with rapid fungicidal activity that is likely to be the result of their DNA-binding activity. Although it was difficult to obtain a broad-spectrum antifungal compound with low cytotoxicity, some of the compounds (e.g., P9, P14 and P43) exhibited favorable CC50 values against HeLa cells and maintained considerable antifungal activity.
Keywords: bisamidines, antifungal, fungicidal, Candida, Cryptococcus, DNA binding
Graphical Abstract

1. Introduction
Invasive fungal infections are a major clinical problem.1 The mortality rates of infections caused by the three major fungal pathogens, Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans are 20%–40%2, 50%–90%2, and 20–70%3, respectively. The majority of these infections affect patients with compromised immunity. Consequently, the prevalence of these infections has increased as the numbers of people in the population with compromised immunity has increased due to HIV/AIDs, or recipients of solid organ or stem cell transplants.4 Presently, there are only three classes of antifungal agents that are used to treat invasive fungal infections, including the polyenes (e.g. amphotericin B), azoles (e.g. voriconazole), and echinocandins (e.g. caspofungin). Many of these agents have serious side effects, especially amphotericin B and ketoconazole. Because invasive fungal infections affect immune-compromised patients, antifungal compounds that are fungicidal are preferred. However, the echinocandins are fungistatic, and the fungicidal activity of the newer azoles, such as voriconazole, is species-specific. In addition, the emergence of drug resistance has resulted in further limitations on the use of these drugs. It is important, therefore, to explore other classes of compounds for antifungal activity.
Previously, we have described a series of bisamidine-containing compounds that are potent antimicrobial agents with a novel mechanism of action. The bisamidines are broad-spectrum antibacterial compounds, active against Gram-positive (MRSA, MSSA, VRE, MRSE, Group A Strep, B. anthracis, and C. difficile) and Gram-negative (P. aeruginosa, E. coli, Enterobacter spp., Acinetobacter spp., Klebsiella spp., and Burckholderia spp.) pathogens5. The bisamidines are rapidly bactericidal against exponentially growing Gram-positive and Gram-negative bacteria5b and they kill bacteria with a mechanism of action that is unique among antibacterial compounds. We have shown that these compounds bind with high affinity to the minor groove of A-T rich DNA.6 Once bound to DNA, the bisamidines inhibit DNA replication and RNA biosynthesis7, which results in rapid cell death. Because the bisamidines act through a unique mechanism of action, we investigated the antifungal activity of a diverse set of bisamidines. Herein, we report the design, synthesis, and evaluation of a panel of 55 bisamidine compounds for antifungal activity against a diverse panel of pathogenic fungi. In addition, we carried out preliminary mechanism of action studies against Candida albicans. Our results demonstrate that the bisamidines are potent antifungal compounds that are rapidly fungicidal. As in bacteria, this class of compounds kills fungi by binding to DNA and inhibiting DNA replication and RNA synthesis. This non-specific activity of the bisamidines is likely the source of the cytotoxicity of this class of compounds and is an impediment to their further development for systemic applications.
2. Chemistry
In this study, we synthesized the bisamidine compounds in two scaffolds, head-to-head and head-to-tail, as shown in Figure 1. All compounds share the generic structure [Am]-[HetAr]-[linker]-[HetAr]-[Am], in which [Am] is a cyclic or acyclic amidine group, [linker] is a benzene, pyridine, pyrimidine, pyrazine ring, or an aliphatic chain of two to four carbon, and [HetAr] is a 5,6-bicyclic heterocycle such as indole, benzimidazole, imidazopyridine, benzofuran, or benzothiophene. When the two [HetAr] units are oriented such that the link between [HetAr] units is attached through the 5-membered rings, they are “head-to-head”, and when one of the [HetAr] units is connected through the 6-membered ring, they are designated as “head-to-tail” compounds. In some of the “head-to-tail” series, the [linker] unit has been omitted, and the two [HetAr] units are attached directly.
Figure 1.

Generic structures of “head-to-head” and “head-to-tail” bisamidine compounds
The syntheses of the symmetrical bisindole compounds P1, P8, P10, P12-P15, P17, P18, P26, P29-P31; and the unsymmetrical “head-to-head” bisindole analogs P21-P25 have been described elsewhere.5d, 8 The synthesis of new bisindole compounds with various amidine groups is shown in Scheme 1. Treatment of the nitrile 18 with 1,2-diaminopropane in the presence of P2S5 provided P9. Following the Pinner synthesis9, compound 1 was converted to the imidate 210 in the presence of dry HCl in absolute ethanol. The imidate thus formed was treated with various amines to provide the compounds P2-P7, P11 and P16.
Scheme 1.
Synthesis of new bisindole compounds with various amidine groups. Reagents and conditions for (a): P1-P7: R1H, EtOH; for P11: CH3NH(CH2)3NH2, EtOH; for P16: NH2CH2C(CH2OH)2CH2NH2, EtOH.
The syntheses of new unsymmetrical bisindole compounds P19, P20 and P27 and a symmetrical bisbenzofuran compound P28 are shown in Scheme 2. The triaryl cores of these compounds were assembled by Suzuki-Miyaura coupling of the boronic acid/esters 3, 5, 6 and 8 with appropriate aryl bromides. The nitrile functionalities were then converted into the corresponding tetrahydropyrimidines by treatment with P2S5 and 1,3-diaminopropane.
Scheme 2.
Synthesis of unsymmetrical bisindole and symmetrical bisbenzofuran compounds. Reagents and conditions: (a) Pd(Ph3P)4, Na2CO3, DME, H2O, 80 °C; (b) P2S5, 1,3-diaminopropane, 130 °C.
Using a similar strategy, the “head-to-tail” triaryl and diaryl bisamidine compounds were prepared. The Suzuki-Miyaura coupling partners and the final bisamidine products of this compound series are shown in Table 1. Details of the synthesis are given in the experimental section.
Table 1.
Suzuki-Miyaura coupling partners and the final products of the head-to-tail series

|
3. Results and Discussion
Each compound was evaluated for antifungal activity to obtain Minimal Inhibitory Concentration (MIC) values against a panel of fungal strains, and the cytotoxicity (CC50) of each analog against the HeLa mammalian cell line was determined. The results of these assays are shown in Tables 3–5. The strains used in this study, which represent the major pathogens responsible for invasive infections, and their susceptibility profile for several antifungal are shown in Table 2.
Table 3.
The antifungal activity and cytotoxicity of the bis-indoles with different amidine groups
| Structure | MIC (μg/ml)† | ||||||
|---|---|---|---|---|---|---|---|
| # |

|
C. alb | C. kru | C. gla | C. par | C. neo | CC50 (μg/ml) |
| P1 |
|
16 | 2 | 4 | 2 | 3.13 | 7 |
| P2 |
|
0.5 | 0.4 | 0.2 | 0.25 | 1.56 | 3.5 |
| P3 |
|
1 | 1 | 0.63 | 0.5 | 1.97 | 5.5 |
| P4 |
|
0.25 | 1.59 | 0.13 | 0.25 | 1.97 | 14 |
| P5 |
|
0.79 | 1 | 0.5 | 0.79 | 1.1 | 3.5 |
| P6 |
|
2.52 | 2 | 2 | 2 | 2.48 | 24.9 |
| P7 |
|
2.52 | 4 | 4 | 2 | 2.48 | 29.8 |
| P8 |
|
10.08 | 4 | 0.25 | 0.63 | 2 | 32.5 |
| P9 |
|
0.79 | 0.79 | 0.25 | 0.4 | 0.25 | 66.5 |
| P10 |
|
0.25 | 0.13 | 0.06 | 0.1 | 0.08 | 4 |
| P11 |
|
1.59 | 4 | 1.26 | 1 | 1.97 | 4.7 |
| P12 |
|
0.5 | 0.5 | 0.13 | 0.25 | 0.25 | 5.8 |
| P13 |
|
0.5 | 0.25 | 0.13 | 0.25 | 0.25 | 13.9 |
| P14 |
|
0.5 | 12.7 | 0.16 | 0.31 | 0.06 | >80 |
| P15 |
|
4 | 4 | 2 | 8 | 3.13 | 8 |
| P16 |
|
1.26 | 8 | 1 | 2 | 3.13 | 32.4 |
MICs are the geometric mean of three independent assays, MIC values < 1 μg/ml are in blue
Fungal strains: C. alb, Candida albicans (WTBF-50); C. kru, Candida krusei (WTBF-51); C. gla, Candida glabrata (WTBF-86); C. par, Candida parapsilosis (WTBF-88); C. neo, Cryptococcus neoformans Cn18 (WTBF-106).
Table 5.
Antifungal activity and cytotoxicity of the head-to-tail bisindole analogs with varied core structures
| # | Structure | MIC (μg/ml)† | |||||
|---|---|---|---|---|---|---|---|

|
C. alb | C. kru | C. gla | C. par | C. neo | CC50 (μg/ml) | |
| P32 |
|
0.5 | 0.5 | 0.13 | 0.5 | 2 | 34.6 |
| P33 |
|
0.79 | 1 | 0.13 | 0.31 | 0.5 | 13.4 |
| P34 |
|
0.25 | 0.5 | 0.06 | 0.13 | 0.13 | 1.5 |
| P35 |
|
16 | 16 | 32 | 8 | 1 | 20.7 |
| P36 |
|
8 | 8 | 1.59 | 2.52 | 3.94 | 32.5 |
| P37 |
|
2 | 8 | 0.31 | 2 | 2 | 25.5 |
| P38 |
|
4 | 8 | 1 | 0.79 | 1 | 20.7 |
| P39 |

|
4 | 25.4 | 2.52 | 2 | 1 | 40.5 |
| P40 |

|
3.17 | 16 | 5.04 | 2 | 4 | 27.5 |
| P41 |

|
0.31 | 4 | 0.13 | 0.5 | 0.5 | 17.1 |
| P42 |

|
0.4 | 4 | 1 | 0.5 | 1 | 50.5 |
| P43 |

|
1 | 1.26 | 0.16 | 1 | 6.25 | 91.9 |
MICs are the geometric mean of three independent assays, MIC values < 1 μg/ml are in blue.
Fungal strains: C. alb, Candida albicans (WTBF-50); C. kru, Candida krusei (WTBF-51); C. gla, Candida glabrata (WTBF-86); C. par, Candida parapsilosis (WTBF-88); C. neo, Cryptococcus neoformans Cn18 (WTBF-106).
Table 2.
A description of the fungal strains used in this study and their susceptibility to various antifungal compounds.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| MIC (μg/ml)† | |||||||
|
| |||||||
| Strain | Organism | Source | AMB | PAD | 5FC | FLC | KTC |
| WTBF-50 | Candida albicans | Clinical Isolate, Baylor College of Medicine | 0.13 | 25 | 1.25 | ≥32 | 20.2 |
| WTBF-51 | Candida krusei | Clinical Isolate, Baylor College of Medicine | 1 | 19.8 | ≥20 | ≥32 | 4 |
| WTBF-86 | Candida glabrata ATCC 90030 | ATCC* | 0.25 | 3.13 | 0.31 | ≥32 | ≥32 |
| WTBF-88 | Candida parapsilosis ATCC 90018 | ATCC* | 0.31 | 12.5 | 1.25 | 1 | 0.06 |
| WTBF-106 | Cryptococcus neoformans Cn18 (Serotype C) | Clinical Isolate, UMass Medical School | 32 | ≥100 | ≥20 | 32 | 32 |
The values shown for MICs are the geometric mean of the results of three independent assays.
American Type Culture Collection (Manassas, VA)
Abbreviations: AMB, Amphotericin B; PAD, Pentamidine; 5FC, Fluorocytosine; FLC, Fluconazole; KTC, Ketoconazole.
All the compounds shown in Table 3 have the same core structure. Thus, the difference in activities that we observe is caused by the various amidine functionalities. For the acyclic amidines, we noticed that monoalkylated derivatives (P2-P7) had an improved spectrum of activity and potency when compared to the non-alkylated amidine P1. The most active compound in this group was P2, which has the most lipophilic substituent, iso-propyl. The preference for lipophilic amidine substituents also seems to apply to the cyclic amidine compounds (P8-P16). For example, P9 was generally more active than P8. It is interesting to note that P9 could be considered to be a cyclized version of P2, and these two compounds are very similar in their activity against the Candida strains, but P9 is 6-fold more potent than P2 against Cryptococcus neoformans and 16-fold less cytotoxic against HeLa cells. Also noteworthy is the 6-membered cyclic amidine P10, which is the constitutional isomer of P9. It is significantly more potent than P9 against both Candida spp. and C. neoformans, demonstrating the importance of the amidine ring size. Among the derivatives of P10, analogs that carry a lipophilic substituent on the C(5) of the tetrahydropyrimidine ring, e.g. methyl (P12) and fluoro (P13), are more active than the others with more hydrophilic substituents (e.g., P14). Replacing the NH group of the tetrahydropyrimidine ring with an N-methyl group reduced the potency by 6 to 30 fold (see P11 vs. P10). Bis-substitution at C(5) of the tetrahydropyrimidine also reduced the potency of the compounds (see P15 and P16 vs. P12). Compound P14 is unique in that it carries a hydrophilic (OH) substituent at C(5), yet it is very potent against 4 out of 5 fungal strains that we tested, with especially potent activity against C. neoformans, and it also showed low cytotoxicity against HeLa cells.
In Table 4 are shown MIC and CC50 values for compounds having the “head-to-head” scaffold with various core structures and the same tetrahydropyrimidine end group. We noticed that chlorination and bromination at the 3-position of the indole rings led to an increased lipophilicity, but not higher potency against Candida spp. and C. neoformans (see P17 and P18 vs. P10). These analogs, however, exhibit higher cytotoxicity against HeLa cells. Somewhat surprisingly, when one of the indole rings is linked at the 3-position (i.e., P19), the compound remains equally active (as compared to P10) against Candida spp. and is only less potent than P10 against C neoformans. Yet, when one of the tetrahydropyrimidine groups is linked to C(5) of the indole, the resulting compound (P20) is much less active than P10 against both Candida spp. and C. neoformans. Because the topological change is greater in the case of P19, we expected that its antifungal activity would be more different from P10 than that of P20.
Table 4.
Antifungal activity and cytotoxicity of the head-to-head bisindole analogs with varied core structures.
| Structure | MIC (μg/ml)† | ||||||
|---|---|---|---|---|---|---|---|
| # |

|
C. alb | C. kru | C. gla | C. par | C. neo | CC50 (μg/ml) |
| P17 |
|
0.4 | 0.25 | 0.25 | 0.25 | 0.78 | 0.65 |
| P18 |
|
0.63 | 0.5 | 0.5 | 0.5 | 0.78 | 0.5 |
| P19 |

|
0.25 | 0.25 | 0.06 | 0.16 | 0.98 | 2.4 |
| P20 |
|
7.87 | 19.8 | - | - | 3.12 | 13.6 |
| P21 |
|
0.4 | 1 | 0.13 | 0.16 | 0.5 | 14.1 |
| P22 |
|
4 | 6.35 | 4 | 0.5 | 1 | 20.9 |
| P23 |
|
0.63 | 4 | 0.25 | 0.25 | 3.13 | 19.7 |
| P24 |
|
0.5 | 1 | 0.16 | 0.25 | 0.25 | 4.7 |
| P25 |
|
0.5 | 0.5 | 0.06 | 0.13 | 0.25 | 15.6 |
| P26 |
|
0.5 | 0.5 | 0.06 | 0.2 | 3.13 | 42.7 |
| P27 |
|
0.31 | 0.25 | ≤0.03 | 0.13 | 1.24 | 25.5 |
| P28 |
|
20.16 | 20.16 | 16 | 2 | 3.13 | >80 |
| P29 |
|
≥32 | ≥32 | 1 | ≥32 | 1.59 | 14.7 |
| P30 |
|
22.63 | 32 | 12.7 | 32 | 16 | 80.3 |
| P31 |
|
3.17 | 8 | 1 | 2 | 1 | 24.6 |
MICs are the geometric mean of three independent assays, MIC values < 1 μg/ml are in blue.
Fungal strains: C. alb, Candida albicans (WTBF-50); C. kru, Candida krusei (WTBF-51); C. gla, Candida glabrata (WTBF-86); C. par, Candida parapsilosis (WTBF-88); C. neo, Cryptococcus neoformans Cn18 (WTBF-106).
The effects of exchanging one indole ring with a different 5,6-bicyclic heterocycle are reflected in compounds P21-P24. Benzofuran- and benzimidazole-containing compounds (P21, P24) are comparable in their antifungal activities and they are more active than the imidazopyridine-containing compounds (P22, P23). They are all less potent and less cytotoxic (except P24) than P10. Replacing the phenyl linker with an N-containing heterocycle reduces lipophilicity and cytotoxicity of the compounds against HeLa cells (see P25 vs. P24, and P26, P27 vs. P10). Against fungi, the pyridine-linked compound P25 is equally potent as the phenyl-linked P24, while the pyrazine- and pyrimidine-linked compounds P26 and P27 are less active than the corresponding phenyl-linked P10, especially against C. neoformans. Interestingly, replacing both indole rings of P26 with the corresponding benzofuran rings led to a much less active compound (P28) against Candida spp.; its activity against C. neoformans remained the same. Notably, the alkyl-linked bisindoles P29-P31 are significantly less active than the aromatic-linked compounds, and the antifungal activity decreased as the length of the alkyl chain increased. Thus, scaffold rigidity seems to play a significant role in maintaining the high antifungal activity of these compounds.
In Table 5 are shown MIC and CC50 values for the “head-to-tail” bisamidine analogs with varied core structures and the same tetrahydropyrimidine end groups. Within this series there are two groups, one with a phenyl linker, and one without. The general observation is that for ring A, an indole is preferred (see P34, P36 and P37), and for ring B, benzofuran is preferred (see P32, P33 and P34; P39 and P41). Thus, the most potent compound of the triaryl group was P34, and of the diaryl group was P41. We noticed that that switching the position of ring A and ring B could lead to significant changes in MIC values against Candida spp. For example, P33 was >10-fold more potent than P35, and P41 was significantly more potent than P39. However, we did not observe the same effect on P37 and P38. Activity against C. neoformans also did not seem to be affected by this type of change. We observed only small changes in MIC values when the benzofuran is linked at the C(5) or C(6) (see P41 and P42). Overall, these “head-to-tail” compounds showed activity and cytotoxicity that was similar to the “head-to-head compounds”.
To determine whether the most active antifungal bis-amidines are fungicidal, we performed time kill assays using P10, P34, and P19 against C. albicans. The data, shown in Fig. 2, indicate that all three compounds exhibit dose-dependent fungicidal activity that is comparable to, or superior to, that of the positive control amphotericin B (AMB) at 4× the MIC. In addition, all three compounds decreased the numbers of viable cells at a concentration of 1× the MIC. Compound P10 exhibited the most potent fungicidal activity against C. albicans (Fig. 2A). At a concentration equivalent to 1× the MIC, P10 reduced the number of viable cells by 3 log10 within 4 h, an activity comparable to that of the positive control. Compound P10, at concentrations equivalent to 2× the MIC and 4× the MIC, reduced viable cells to levels below the limit of detection within 8 h and 4 h, respectively. P19 exhibited intermediate fungicidal activity, reducing viable cells by 1 log10 and 4 log10 within 24 h at 1× the MIC and 2× the MIC, respectively (Fig. 2C). A concentration of 4× the MIC, P10 reduced viable cells to levels below the limit of detection within 8 h. The fungicidal activity of P34 at 2× the MIC and 4× the MIC was comparable to that of AMB (Fig. 2B) at 4× the MIC. Interestingly, the relative fungicidal activity of these compounds is not correlated with their MICs, as all three compounds exhibit the same MIC against C. albicans (0.25 μg/ml). Because the bisamidines are extremely stable under diverse conditions, including incubation in liver microsomes (data not shown), it is unlikely that the differences in the fungicidal activity of P10, P34, and P19 are due to differences in stability. However, it is possible that the fungicidal activity of P10, P34, and P19 is correlated with their affinity for DNA. Evidence from our laboratory indicates that the bisamidines readily penetrate bacterial cells, where they bind to the minor groove of DNA with high affinity6–7 and inhibit the synthesis of DNA and RNA. The inhibitory activity of these compounds is correlated with their affinity for DNA. Because known inhibitors of bacterial DNA replication (fluoroquinolones) and RNA synthesis (rifampicin) are bactericidal, it is likely that the potent bactericidal activity of the bisamidines is the result of the inhibition of DNA synthesis. Therefore, it is likely that the fungicidal activity of the bisamidines results from the same mechanism of action.
Figure 2.
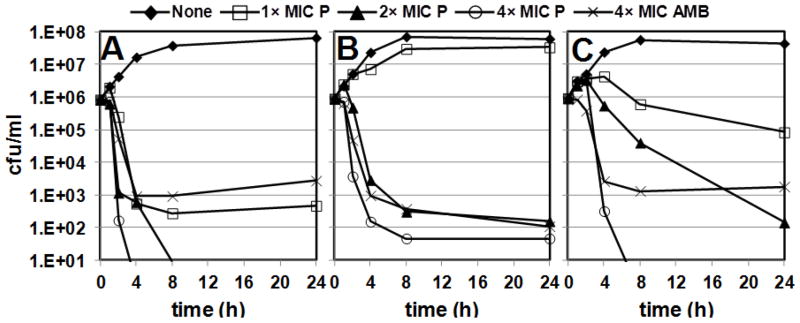
Time kill assays showing the fungicidal activity of the following bisamidines and a positive control compound amphotericin B (AMB) against C. albicans: A) P10, B) P34, and C) P19. Compound concentrations are expressed relative to the MIC for C. albicans.
To determine whether the bisamidines inhibit DNA and RNA synthesis in fungi, we performed macromolecular synthesis assays using C. albicans. In this assay, C. albicans was incubated in the presence of various concentrations of P10 or P19 in media containing a radiolabeled precursor of each of the following macromolecular synthetic pathways: 3H-adenine (RNA and DNA), 3H-uridine (RNA only), 3H-leucine (protein), 3H-glucosamine (cell wall), and 3H-acetate (lipid). The results of these assays are shown in Figures 3A and 3B. We used a curve-fitting algorithm to calculate the half-maximal inhibitory concentrations (IC50) of P10 and P19 against each MMS pathway (Table 6). The results of these assays indicate that P10 and P19 inhibit all MMS pathways except lipid biosynthesis. However, both compounds inhibited the incorporation 3H-adenine into DNA and RNA with the greatest potency, with IC50 values that were ~30-fold lower than those for the incorporation of uridine into RNA (see Table 6). Because C. albicans does not have a mechanism to scavenge the DNA-specific precursor thymine from the medium11, we used 3H-adenine for this experiment, which is incorporated into both DNA and RNA. By comparing the inhibition of adenine vs. uridine incorporation, however, we can estimate the effect of each compound on DNA synthesis. Based on our results, it is evident that P10 and P19 are potent inhibitors of DNA synthesis in C. albicans. The specificity of the bisamidines for DNA synthesis is similar to that of pentamidine (PAD) (see Fig. 3C and Table 3),an aromatic diamidine with potent antifungal activity 12 that binds to the minor groove of DNA13. The similarities between the activity of P10, P19, and PAD demonstrate a strong correlation between the DNA binding activity and inhibition of DNA synthesis. In contrast, Amphotericin B (AMB), a potent antifungal agent that binds to a component of fungal cell membranes (ergosterol) and causes leakage of monovalent ions (K+, Na+, H+, and Cl−)14, inhibited all MMS pathways with equal potency (see Fig. 3D and Table 3), which is consistent with its non-specific mechanism of action. While DNA synthesis is the pathway that is most sensitive to P10, P19, and PAD, these compounds also inhibit RNA, protein, and cell wall synthesis at higher concentrations. The effect of these compounds on other pathways is likely to be the result of either the indirect effects of these compounds on cellular metabolism and energy production, or a direct inhibition of secondary targets.
Figure 3.
Macromolecular synthesis (MMS) assays using radiolabeled precursors for DNA and RNA (adenine), RNA only (uridine), protein (leucine), cell wall (glucosamine) and lipid (acetate) in the presence of various concentrations of the following compounds (cmpds): A) P10, B) P19, C) pentamidine (PAD), and D) amphotericin B (AMB).
Table 6.
The half maximal inhibitory concentrations (IC50) of P10 and P19 against the macromolecular synthetic pathways in C. albicans. The IC50 values were calculated from the curves shown in Figure 2.
|
|
||||
|---|---|---|---|---|
| IC50 (μg/ml)† ± stdev for:
|
||||
| Precursor | P10 | P19 | PAD | AMB |
| 3H-Adenine | 0.01 ± 0.001 | 0.02 ± 0.002 | 0.19 ± 0.046 | 0.038 ± 0.03 |
| 3H-Uridine | 0.32 ± 0.12 | 0.56 ± 0.12 | 12.2 ± 5.39 | 0.014 ± 0.005 |
| 3H-Leucine | 0.59 ± 0.1 | 0.86 ± 0.44 | >25 | 0.052 ± 0.012 |
| 3H-Glucosamine | 0.59 ± 0.07 | 0.87 ± 0.31 | >25 | 0.043 ± 0.019 |
| 3H-Acetate | ≥2 | ≥2 | >25 | 0.036 ± 0.001 |
Average of three trials
Abbreviations: PAD, pentamidine; AMB, amphotericin B.
To determine whether bisamidines bind to DNA in living cells of C. albicans, we used fluorescence microscopy to visualize cells that had been treated with P8 (MBX 1060) and the DNA-intercalating dye Syto9. Because the structure of P8 is similar to that of DAPI (4′,6-diamidino-2-phenylindole), a well-established dye that fluoresces strongly when bound to the minor groove of A-T rich double stranded DNA (λex = 358 nm; λem = 461 nm), it fluoresces strongly in the presence of double stranded DNA. As shown in Fig. 4B, cells treated with P8 exhibit localized fluorescence when viewed with a DAPI-specific filter. The P8 fluorescence is co-localized with that of Syto9 (see Fig. 4C and D), indicating that P8 is binding to nuclear DNA. Taken together, our data strongly suggests that bisamidines bind to the chromosomal DNA of fungi, and inhibits the essential cellular process of DNA replication and RNA synthesis. In addition, it is likely that this non-specific mechanism of action is responsible for the cytotoxicity of this class of antimicrobial agents.
Figure 4.
Micrographs of C. albicans treated with P8 and Syto9. A) Light micrograph, B) P8 fluorescence (DAPI filter), C) Syto9 fluorescence (GFP filter), D) the P8 and Syto9 fluoresence images have been merged. Magnification = 400×.
4. Conclusions
Herein, we report the synthesis of 26 new bisamidine compounds that are based on different “head-to-head” and “head-to-tail” scaffolds. An evaluation of the antifungal activity and cytotoxicity of 26 new bisamidine compounds and 17 previously reported bisamidine compounds identified several compounds (e.g P10, P19 and P34) with very potent antifungal activity. The bisamidines are potent inhibitors of DNA synthesis in C. albicans, which is likely to be the result of the DNA binding activity of this class of compounds. Because of the mechanism of action of the bisamidines, we were unable to obtain a broad-spectrum antifungal compound with low cytotoxicity. However, a few of the bisamidine compounds (e.g., P9, P14 and P43) exhibited favorable CC50 values against HeLa cells and maintained considerable antifungal activity. It is possible that these compounds may prove to be useful for treating non-systemic fungal infections.
5. Experimental
5.1 Fungal strains, media, and reagents
The fungal strains used in this study are listed in Table 1. RPMI 1640 with L-glutamine was purchased from Gibco (Grand Island, NY) and Sabouraud Dextrose Broth (SDB) was purchased from Difco (Franklin Lakes, NJ) in a dehydrated form that was prepared according to the manufacturer’s instructions. The following radiolabeled precursors of macromolecular synthetic pathways were purchased from Perkin Elmer (Waltham, MA): [3H]-adenine (DNA and RNA synthesis), [3H]-uridine (RNA synthesis), [3H]-leucine (protein synthesis), and [3H]-glucosamine (cell wall). [3H]-acetate (lipid synthesis) was purchased from Vitrax (Placentia, CA). The following reagents were purchased from Sigma Aldrich (St. Louis, MO): amphotericin B (AMB), pentamidine (PAD), flucytosine (5FC), fluconazole (FLC), ketoconazole (KTC). Syto9 was purchased from Molecular Probes (Grand Island, NY).
5.2 Antifungal activity assay (MIC)
The antifungal activity of each compound against Candida species were measured as the minimal inhibitory concentration (MIC) using the microbroth dilution method as described in the CLSI protocol15. In the MIC assays using Cryptococcus neoformans, Sabouraud Dextrose Broth (SDB) was used instead of RPMI medium.
5.3. Cytotoxicity (CC50)
The cytotoxicity of compounds against a mammalian cell line (HeLa, ATCC CCL-2) was determined as described16.
5.4. Time kill assays
C. albicans was grown in RPMI medium at 37 °C until the culture reached an optical density at 600 nm (OD600) of approximately 1. The culture was diluted 1:10 in RPMI medium containing various concentrations of test compounds or a control antifungal agent (amphotericin B) and incubated at 37 °C. At various times, samples were removed for serial dilution and plating on SBD agar to measure colony forming units per ml (cfu/ml).
5.5. Macromolecular synthesis (MMS) assays
C. albicans was grown in RPMI medium at 37 °C until the culture reached an optical density at 600 nm (OD600) of approximately 1. Fifty microliters of the culture was transferred to each well of a 96-well assay plates containing a series of 2-fold serial dilutions of test compounds and one of the following radiolabeled macromolecular precursors (10 μCi/ml final concentration) in 50 μl RPMI: 3H-adenine (DNA and RNA), 3H-uridine (RNA), 3H-leucine (protein), 3H-glucosamine (cell wall), 3H-acetate (lipid). The assay plates were incubated at 37 °C for 90 min, which is equivalent to one doubling time, and 100 μl 10% TCA was added to each well, and then the radiolabeled precipitate was collected by filtration and quantified using a scintillation counter. Each compound concentration was tested in triplicate. The fraction of incorporated label for treated samples as compared to the untreated control was calculated and plotted as a function of compound concentration, and a four parameter curve fitting algorithm was used to determine the compound concentration that resulted in half-maximal inhibition (IC50) for each pathway.
5.6. Fluorescence microscopy
An exponential culture of C. albicans was treated with 4x the MIC of MBX 1066 or 33 μM Syto 9 for 30 min and was analyzed using the DAPI and GFP channels, respectively, of a Zeiss fluorescence microscope.
5.7. Compound synthesis
The syntheses and physical properties of the compounds described above are provided in the Supplemental Material.
5.8. Literature preparations
For the synthesis of compounds P1, P8, P10, P12-P15, P17, P18, P26, P29-P31 see Ref. 8. For the synthesis of compounds P21-P25, and the building blocks 4, 8, 10; 2-(4-bromophenyl)indole-6-carbonitrile; 2-(4-bromophenyl) imidazo[1,2-a]pyridine-7-carbonitrile; 2-(4-bromophenyl) imidazo[1,2-a]pyridine-6-carbonitrile see Ref. 5d. The imidate 2 was prepared according to Ref. 10.
5.9. Commercial building blocks
Compounds 3, 5 and 6 were purchased from Combi-Blocks, Inc. (San Diego, CA); 6-Bromoindole-2-carbonitrile was purchased from Matrix Scientific (Columbia, SC); compound 19 was purchased from Ark Pharm, Inc. (Libertyville, IL); compounds 11, 12, 16 and 20 were supplied by Creagen Biosciences (Woburn, MA).
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Rabih Darouiche for providing fungal strains from the Baylor College of Medicine clinical laboratory. We thank Dr. Hwa-Ok Kim and the scientists at Creagen Biosciences (Woburn, MA) for providing the custom-made compounds 11, 12, 16 and 20. This work was supported by the National Institutes of Health (grants 5 U01 AI 082052-03 and 1R43 AI083032).
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20(1):133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai CC, Tan CK, Huang YT, Shao PL, Hsueh PR. Current challenges in the management of invasive fungal infections. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2008;14(2):77–85. doi: 10.1007/s10156-007-0595-7. [DOI] [PubMed] [Google Scholar]
- 3.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23(4):525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 4.Richardson MD. Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother. 2005;56(Suppl 1):i5–i11. doi: 10.1093/jac/dki218. [DOI] [PubMed] [Google Scholar]
- 5.(a) Butler MM, Williams JD, Peet NP, Moir DT, Panchal RG, Bavari S, Shinabarger DL, Bowlin TL. Comparative in vitro activity profiles of novel bis-indole antibacterials against gram-positive and gram-negative clinical isolates. Antimicrob Agents Chemother. 2010;54(9):3974–7. doi: 10.1128/AAC.00484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Panchal RG, Ulrich RL, Lane D, Butler MM, Houseweart C, Opperman T, Williams JD, Peet NP, Moir DT, Nguyen T, Gussio R, Bowlin T, Bavari S. Novel broad-spectrum bis-(imidazolinylindole) derivatives with potent antibacterial activities against antibiotic-resistant strains. Antimicrob Agents Chemother. 2009;53(10):4283–91. doi: 10.1128/AAC.01709-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jacobs MR, Bajaksouzian S, Good CE, Butler MM, Williams JD, Peet NP, Bowlin TL, Endimiani A, Bonomo RA. Novel bis-indole agents active against multidrug-resistant Acinetobacter baumannii. Diagnostic microbiology and infectious disease. 2011;69(1):114–6. doi: 10.1016/j.diagmicrobio.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nguyen ST, Williams JD, Butler MM, Ding X, Mills DM, Tashjian TF, Panchal RG, Weir SK, Moon C, Kim HO, Marsden JA, Peet NP, Bowlin TL. Synthesis and antibacterial evaluation of new, unsymmetrical triaryl bisamidine compounds. Bioorg Med Chem Lett. 2014;24(15):3366–72. doi: 10.1016/j.bmcl.2014.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opperman TJ, Li JB, Lewis MA, Houseweart C, Aiello D, Williams JD, Peet NP, Moir DT, Long EC, Bowlin TL. DNA binding activity of novel bis-indole antibiotics. Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA: American Society; 2009. [Google Scholar]
- 7.Opperman TJ, Houseweart C, Aiello D, Williams JD, Peet NP, Moir DT, Bowlin TL. The Mechanism Of Antibacterial Action Of Novel Bis-indole Antibiotics. Interscience Conference on Antimicrobial Agents and Chemotherapy; Boston, MA: American Society for Microbiology; 2010. [Google Scholar]
- 8.Williams JD, Nguyen ST, Gu S, Ding X, Butler MM, Tashjian TF, Opperman TJ, Panchal RG, Bavari S, Peet NP, Moir DT, Bowlin TL. Potent and broad-spectrum antibacterial activity of indole-based bisamidine antibiotics: Synthesis and SAR of novel analogs of MBX 1066 and MBX 1090. Bioorg Med Chem. 2013;21(24):7790–806. doi: 10.1016/j.bmc.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roger R, Neilson DG. The chemistry of imidates. Chemical Reviews. 1961;61(2):179–211. [Google Scholar]
- 10.Tidwell RR, Geratz JD, Dubovi EJ. Aromatic amidines: comparison of their ability to block respiratory syncytial virus induced cell fusion and to inhibit plasmin, urokinase, thrombin, and trypsin. J Med Chem. 1983;26(2):294–8. doi: 10.1021/jm00356a036. [DOI] [PubMed] [Google Scholar]
- 11.Singh K, Sun S, Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. IV. Mechanism of action. The Journal of antibiotics. 1979;32(6):630–45. doi: 10.7164/antibiotics.32.630. [DOI] [PubMed] [Google Scholar]
- 12.Del Poeta M, Schell WA, Dykstra CC, Jones S, Tidwell RR, Czarny A, Bajic M, Kumar A, Boykin D, Perfect JR. Structure-in vitro activity relationships of pentamidine analogues and dication-substituted bis-benzimidazoles as new antifungal agents. Antimicrob Agents Chemother. 1998;42(10):2495–502. doi: 10.1128/aac.42.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson WD, Tanious FA, Mathis A, Tevis D, Hall JE, Boykin DW. Antiparasitic compounds that target DNA. Biochimie. 2008;90(7):999–1014. doi: 10.1016/j.biochi.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baginski M, Czub J. Amphotericin B and its new derivatives - mode of action. Current drug metabolism. 2009;10(5):459–69. doi: 10.2174/138920009788898019. [DOI] [PubMed] [Google Scholar]
- 15.CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts: Approved Standard-Third Edition. M27-A3 Clinical and Laboratory Standards Institute; Wayne, PA USA: 2008. [Google Scholar]
- 16.Butler MM, Lamarr WA, Foster KA, Barnes MH, Skow DJ, Lyden PT, Kustigian LM, Zhi C, Brown NC, Wright GE, Bowlin TL. Antibacterial activity and mechanism of action of a novel anilinouracil-fluoroquinolone hybrid compound. Antimicrob Agents Chemother. 2007;51(1):119–27. doi: 10.1128/AAC.01311-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






