Abstract
Introduction
While the presence of co-existing psychological stressors has historically been used as a supportive factor in the diagnosis of functional neurological disorders, many patients with functional neurological disorders deny the presence of these stressors. The stress response circuitry in these patients remains largely unexplored.
Methods
We performed an observational study examining biological stress levels in patients with functional movement disorders as compared with matched healthy controls. Specifically, we compared levels of circulating cortisol, the end-product of the hypothalamic-pituitary-adrenal axis. Salivary cortisol samples were collected from patients with “clinically definite” functional movement disorders (n=33) and their age- and sex-matched controls (n=33). Collections were performed at five standardized time points, reflecting participants’ diurnal cortisol cycles. To rule out confounders, participants also underwent extensive psychological assessment including Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Hamilton Anxiety Rating Scale, and Hamilton Rating Scale for Depression.
Results
Patients with functional movement disorders did not differ from matched controls with respect to levels of circulating cortisol.
Conclusion
We demonstrate that current stress levels are not altered in patients with functional movement disorders. Our results warrant careful review of current management of patients with functional neurological symptoms, and suggest that the insistence on heightened stress levels in these patients is unjustified.
Keywords: Functional movement disorders, psychogenic movement disorders, conversion disorder, stress, hypothalamic-pituitary adrenal axis, cortisol
INTRODUCTION
Up to 30% of patients presenting to outpatient neurology clinics have neurological symptoms that are functional, or “psychogenic”, in nature. Historically, functional neurological symptoms have been hypothesized to result from converted psychological stress. The presence of coexisting stressors continues to be used as supportive criteria in the diagnosis of functional movement disorders (FMD)[1]. However, despite this common clinical view, the stress-response circuitry in FMD patients remains unexplored.
The hypothalamic-pituitary-adrenal (HPA) axis is the body’s major stress response system, responding to acute and chronic stress[2]. The impact of psychological stress on the HPA axis is well-established in healthy adults, with several meta-analyses demonstrating increased cortisol levels in the setting of acute stressors involving low predictability, low controllability, novelty and social evaluative threat [3, 4]. Additionally, abnormalities in cortisol, the end-product of the HPA axis, have been demonstrated in a variety of psychiatric disorders, including depression, post-traumatic stress disorder[5], and psychogenic non-epileptic seizures (PNES)[6]. To date, this major stress hormone has not been explored in FMD.
To shed further light on the stress-response circuitry in FMD, we assessed whether FMD patients exhibit abnormalities in the HPA axis by comparing the diurnal cortisol profile of FMD patients with a group of age- and sex-matched healthy controls.
METHODS
Participants
Thirty-three patients with a diagnosis of “clinically definite” FMD[7] were recruited from the Human Motor Control clinic at the National Institutes of Health (NIH) between October 2011 and June 2014. Age- and sex-matched healthy controls were recruited from the NIH Clinical Research Volunteer Program database. Exclusion criteria for all participants included the following: a) comorbid neurological disease; b) comorbid psychotic disorder, bipolar disorder, current substance abuse or current depressive episode; c) history of traumatic brain injury with loss of consciousness; d) active autoimmune disorder; e) current suicidal ideation; f) disease severity requiring inpatient treatment; and g) use of tricyclic antidepressants or antiepileptic medication. Healthy controls were excluded for use of any antidepressant medication within the last six months.
All participants provided written informed consent. The NIH institutional review board approved the study.
Cortisol collection and analysis
Participants were hospitalized overnight at the NIH Clinical Center for collection of salivary cortisol, which reliably reflects unbound serum cortisol[4]. Given diurnal fluctuation in basal cortisol[5], saliva samples were collected by nursing staff at five separate times on two consecutive days: the patient’s bedtime, the subsequent morning upon awakening, 30 minutes after awakening, noon, and 3pm. Participants were instructed not to eat or drink 30 minutes before sampling. Nursing staff was instructed not to awaken participants during the night and to allow participants to wake without use of an alarm clock. Saliva samples were obtained using SalivaBio collection devices. Samples were stored at −80°C and assayed in duplicate to determine cortisol levels using a highly sensitive enzyme radioimmunoassay (Salimetrics, State College, PA) as employed by Raff et al., 1998[8].
Psychological assessment
All participants met with a clinical psychologist, who administered the Hamilton Anxiety Rating Scale (HAM-A)[9] and Hamilton Rating Scale for Depression (HAM-D)[10], and screened subjects for psychiatric diagnoses using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Version IV-TR, Patient Edition (SCID-I/P)[11]. Participants also completed the Beck Depression Inventory (BDI)[12], Childhood Trauma Questionnaire (CTQ)[13], State Trait Anxiety Inventory (STAI)[14] and the Traumatic Life Events Questionnaire (TLEQ)[15]. The TLEQ was used as a screening tool for presence of adult sexual trauma based on results to question 18.
Statistical Analyses
Natural logarithms were used to achieve approximately normal distributions for positively skewed variables. Missing cortisol values were treated as data missing completely at random. To investigate possible group effects on basal diurnal cortisol levels, repeated-measures analysis of variance (ANOVA) was performed. To assess for possible confounders, linear correlations were assessed by calculating Pearson’s correlation coefficients. For variables demonstrating statistically significant differences between groups, Pearson’s correlation coefficients were assessed separately for patients and controls. Two-sided p values < 0.05 were considered statistically significant. Clinical parameters were expressed as the mean and standard deviation.
RESULTS
Patient Characteristics
33 FMD patients and an equal number of age- and sex-matched healthy controls were included in the analysis. Groups did not differ significantly in terms of demographic data, smoking or oral contraceptive use (Table 1). Patients had an increased incidence of mood and anxiety disorders as compared with healthy controls (Table 1). Per HAM-D, 9 of 33 patients exhibited at least moderate depressive symptoms over the last week; per HAM-A, 10 of 33 patients demonstrated at least mild to moderate anxiety over the last week. More than half of patients were taking central nervous system (CNS)-acting medications (Table 1). Patients self-reported a range of involuntary movements, including tremor (n=28), other jerking movements (n=25), abnormal gait and/or balance (n=25), abnormal speech (n=18), abnormal posturing/dystonia (n=16), and paresis (n=13).
Table 1.
Demographic and clinical characteristics of FMD patients and healthy controls.
| FMD | HC | p-valuea | |
|---|---|---|---|
| Number of subjects | 33 | 33 | |
| Age | 43.4 ± 11.6 | 41 ± 10.1 | 0.41 |
| Sex, female/male | 25/8 | 24/9 | 0.78 |
| Females using oral contraception | 4 (16%) | 1 (4%) | 0.18 |
| Cigarette smokers | 3 (9%) | 2 (6%) | 0.65 |
| History of mood disorder | 15 (45%) | 4 (12%) | 0.002 |
| History of anxiety disorder | 21 (64%) | 9 (27%) | 0.002 |
| History of post-traumatic stress disorder | 8 (24%) | 4 (12%) | 0.21 |
| Taking CNS-acting medicationb | 19 (58%) | 0 | < 0.001 |
| Disease duration, years (range) | 4.69 ± 4.97 (0.4 – 19) | - | - |
by 2-tailed student’s t-test;
CNS-acting medications included clonazepam (n=8), lorazepam (n=2), alprazolam (n=1), duloxetine (n=2), gabapentin (n=6), trihexyphenidyl (n=2), carbidopa-levodopa (n=1), ropinirole (n=1), baclofen (n=1), citalopram (n=1), sertraline (n=3), acetazolamide (n=2), desvenlafaxine (n=1), and adderall (n=1).
Basal diurnal cortisol
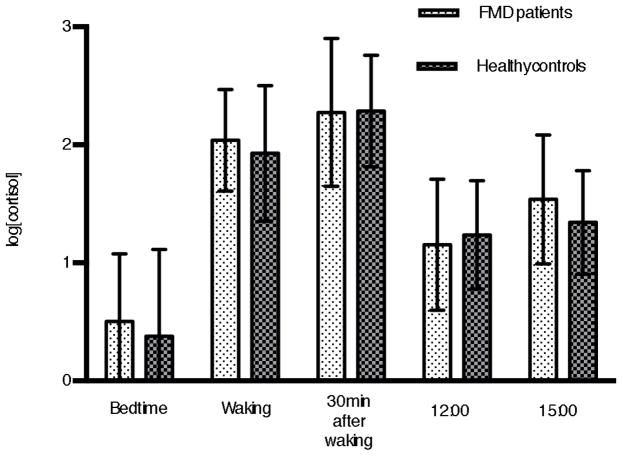
To investigate possible group effects on basal cortisol levels, we performed a repeated-measures ANOVA with group (patients, healthy controls) as between-subject factor, and time (five assessment points from patient’s bedtime on day 1 until 15:00 on day 2) as within-subject factor. Results showed significant effect for time (F(4,237) = 124.9, p < 0.001), but no significant effect for group (F(1,64) = 0.86, p = 0.3566), and no significant time x group interaction (F(4,237) = 0.71, p = 0.5842). Figure 1 illustrates the mean levels of circulating cortisol of FMD patients and their matched controls at the five assessment points. Note that the two groups did not significantly differ from one another at any of these time points.
Figure 1.
Comparison of cortisol values of functional movement disorders (FMD) patients and matched healthy controls. Error bars: standard deviation.
Correlation Analyses
To rule out confounders, linear correlations between cortisol values and history of anxiety disorder, history of mood disorder, and use of CNS-acting medication were assessed. We performed similar assessment with scores on the HAM-A, HAM-D, BDI and STAI scales, as well as scores on various trauma scales. We did not detect a significant linear correlation between cortisol level and any of the psychometric parameters assessed (Table 2, data not shown for healthy volunteers). We also did not detect a significant linear correlation between cortisol level and history of childhood sexual, emotional or physical trauma, or adult sexual trauma (Table 2).
Table 2.
Linear correlations between cortisol values and psychometric variables.
| Cortisol Collection Time | |||||
|---|---|---|---|---|---|
| Bedtime | Waking | Waking + 30minutes | 12:00 | 15:00 | |
| History of mood disordera | 0.001 (0.99) | 0.18 (0.33) | 0.21 (0.25) | −0.12 (0.51) | 0.23 (0.21) |
|
| |||||
| History of anxiety disordera | 0.28 (0.11) | 0.06 (0.76) | 0.06 (0.77) | −0.11 (0.54) | 0.20 (0.28) |
|
| |||||
| Taking CNS-acting medicationa | 0.17 (0.37) | 0.12 (0.52) | −0.02 (0.92) | 0.18 (0.31) | −0.13 (0.46) |
|
| |||||
| HAM-Da | 0.06 (0.76) | 0.01 (0.94) | −0.08 (0.66) | −0.09 (0.60) | −0.09 (0.64) |
|
| |||||
| HAM-Aa | 0.11 (0.53) | −0.002 (0.99) | −0.10 (0.59) | −0.08 (0.67) | −0.09 (0.61) |
|
| |||||
| BDIa | −0.01 (0.95) | −0.15 (0.44) | −0.21 (0.27) | −0.18 (0.32) | −0.15 (0.41) |
|
| |||||
| STAI state | −0.01 (0.94) | −0.098 (0.44) | 0.119 (0.35) | −0.151 (0.23) | −0.212 (0.09) |
|
| |||||
| STAI trait | −0.049 (0.70) | −0.091 (0.48) | 0.173 (0.17) | −0.137 (0.28) | −0.198 (0.11) |
|
| |||||
| Adult sexual trauma | −0.10 (0.43) | 0.004 (0.98) | −0.10 (0.45) | −0.10 (0.45) | −0.02 (0.83) |
|
| |||||
| Childhood sexual trauma | −0.08 (0.52) | −0.01 (0.92) | −0.02 (0.89) | −0.04 (0.75) | 0.05 (0.72) |
|
| |||||
| Childhood emotional trauma | 0.08 (0.56) | −0.12 (0.36) | −0.04 (0.75) | 0.02 (0.86) | 0.14 (0.26) |
|
| |||||
| Childhood physical | −0.03 (0.81) | −0.09 (0.48) | 0.05 (0.71) | 0.06 (0.65) | −0.13 (0.30) |
|
| |||||
| Disease duration | 0.18 (0.32) | −0.20 (0.29) | 0.06 (0.75) | −0.17 (0.35) | −0.21 (0.24) |
Values are expressed as r (p-value).
Given statistically significant differences between groups, correlation coefficients computed separately for patients and controls. Results reported above correspond to patient population.
To explore a possible relationship between disease duration and cortisol levels, linear correlations between disease duration and cortisol levels were assessed. We did not detect a significant linear correlation between disease duration and cortisol level (Table 2).
DISCUSSION
Many neurologists consider heightened stress to be a universal feature of patients with functional neurological disorders[16]. Nevertheless, this is the first study examining objective measures of stress in patients with functional movement disorders. In contrast to the prevalent stereotype, we establish in this study that patients with FMD do not differ from healthy controls with respect to stress level, as measured by basal circulating cortisol.
Data from prior epidemiological studies support our finding. Several studies have demonstrated that patients with conversion disorder do not differ from controls with respect to the number of stressful life events in the year prior to symptom onset[17, 18]. Results from our study contrast with those reported by Bakvis et al., who reported hypercortisolism in PNES patients[6]. That study had a significantly smaller sample size (n=18), and psychometric parameters were based purely on self-report questionnaires.
Our study avoids the self-report bias inherent to prior epidemiological studies by directly measuring the output of the HPA axis. Other strengths of our study include the relatively large sample size, and the study participants’ extensive psychometric characterization. We sought to control for group differences in depression and anxiety, and failed to find correlation between cortisol levels and a wide variety of psychometric parameters. There are several possible explanations for this lack of correlation. First, the number of individuals in our study with current depression and/or anxiety is in fact quite small, and our study is likely not sufficiently powered to address the effect of anxiety and depression on cortisol levels. The elevated morning cortisol seen in patients with anxiety has previously been demonstrated to be driven primarily by patients with panic disorder with agoraphobia[19]. Panic disorder is not highly represented in our population, with only 2 of 33 patients exhibiting evidence of panic disorder on structured psychiatric interview. The lack of correlation between cortisol levels and various psychiatric disorders seen in prior studies has been previously attributed to phenotypic heterogeneity, with different subtypes of depression and anxiety demonstrating different patterns of HPA-axis dysregulation[20].
One major limitation of our study is that patients were on a range of CNS-acting medications. Nevertheless, the use of CNS-acting medication did not linearly correlate with cortisol levels in our study, and therefore was determined not to be a confounder. Another limitation of our study is the reliance on a single parameter for quantifying stress. Despite similar cortisol profiles, patients and controls may differ in other as of yet unidentified downstream targets in the stress response circuitry. Other studies could explore autonomic stress reactivity with heart rate variability or noradrenergic activation with alpha-amylase levels. It is also possible that the abnormality in FMD patients lies not in their stress levels per se, but rather in their downstream response to this stress.
Our patient group is also biased towards those with chronic disease, given that only 24% of patients exhibited disease duration of one year or less. Despite the fact that we did not detect a significant correlation between disease duration and cortisol level in our FMD cohort, it remains possible that patients at earlier stages of the disorder may display an altered stress response. As an additional limitation, our study addresses only current stress levels. Early life stressors may well be important in the predisposition to FMD[17], and may trigger plastic changes in the brain that subsequently persist even in the absence of current stressors. Further studies would be needed to investigate these hypotheses.
CONCLUSIONS
In this study, we demonstrate that stress levels in patients with FMD, as measured by levels of basal circulating cortisol, do not differ from matched healthy controls. Our findings justify careful review of current management of patients with functional neurological symptoms. We acknowledge the importance of psychological assessment in these patients given the increased incidence of comorbid psychiatric disease, and higher rates of prior trauma[17]. We also recognize that some FMD patients appear to have considerable stress. However, our findings demonstrate that the insistence on significant current psychological stress in all FMD patients may be unwarranted. Insisting that all patients have heightened current stress, even when they deny it, seems now to be a mistake that may well negatively influence the doctor-patient relationship.
We examine stress levels in functional movement disorders (FMD) patients
We compare stress levels between patients and matched healthy controls
Salivary cortisol samples were collected, reflecting diurnal cortisol profiles
FMD patients do not differ from healthy controls with respect to cortisol levels
These findings necessitate careful review of current management of FMD patients
Acknowledgments
This study was supported by the NINDS Intramural Program.
Footnotes
AUTHORS’ ROLES:
Dr. Maurer was involved in acquisition, analysis, and interpretation of data, writing the first draft and final version of the paper. Dr. LaFaver was involved in study conception, data analysis and acquisition, and manuscript revision. Dr. Ameli and Mr. Toledo were involved in data acquisition. Dr. Hallett was involved in study conception, data interpretation, and manuscript revision.
All authors have approved the article.
FINANCIAL DISCLOSURES:
Dr. LaFaver reports consulting fees from US World Meds.
Dr. Hallett serves as Chair of the Medical Advisory Board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the Editorial Board of 20 journals, and received royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, John Wiley & Sons, Wolters Kluwer, Springer, and Elsevier. He has received honoraria for lecturing from Columbia University. Dr. Hallett’s research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds have been granted by the Kinetics Foundation for studies of instrumental methods to monitor Parkinson’s disease, BCN Peptides, S.A. for treatment studies of blepharospasm, Medtronics, Inc., for studies of deep brain stimulation, Parkinson Alliance for studies of eye movements in Parkinson’s disease, Merz for treatment studies of focal hand dystonia, and Allergan for studies of methods to inject botulinum toxins.
The remaining authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carine W. Maurer, Email: carine.maurer@nih.gov.
Kathrin LaFaver, Email: kathrin.lafaver@nih.gov.
Rezvan Ameli, Email: amelir@mail.nih.gov.
Ryan Toledo, Email: ryantoledo21@gmail.com.
Mark Hallett, Email: hallettm@ninds.nih.gov.
References
- 1.Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250–60. doi: 10.1016/S1474-4422(11)70310-6. [DOI] [PubMed] [Google Scholar]
- 2.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 4.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–33. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 5.Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:301–15. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakvis P, Spinhoven P, Giltay EJ, Kuyk J, Edelbroek PM, Zitman FG, et al. Basal hypercortisolism and trauma in patients with psychogenic nonepileptic seizures. Epilepsia. 2010;51:752–9. doi: 10.1111/j.1528-1167.2009.02394.x. [DOI] [PubMed] [Google Scholar]
- 7.Williams DT, Ford B, Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Adv Neurol. 1995;65:231–57. [PubMed] [Google Scholar]
- 8.Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83:2681–6. doi: 10.1210/jcem.83.8.4936. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P, 1/2007 revision) Biometrics Research NYSPI; New York: 2002. [Google Scholar]
- 12.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 14.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 15.Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, et al. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol Assess. 2000;12:210–24. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- 16.Kanaan R, Armstrong D, Barnes P, Wessely S. In the psychiatrist’s chair: how neurologists understand conversion disorder. Brain. 2009;132:2889–96. doi: 10.1093/brain/awp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kranick S, Ekanayake V, Martinez V, Ameli R, Hallett M, Voon V. Psychopathology and psychogenic movement disorders. Mov Disord. 2011;26:1844–50. doi: 10.1002/mds.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roelofs K, Spinhoven P, Sandijck P, Moene FC, Hoogduin KA. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508–14. doi: 10.1097/01.nmd.0000172472.60197.4d. [DOI] [PubMed] [Google Scholar]
- 19.Vreeburg SA, Zitman FG, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, et al. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosomatic medicine. 2010;72:340–7. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- 20.Wardenaar KJ, Vreeburg SA, van Veen T, Giltay EJ, Veen G, Penninx BW, et al. Dimensions of depression and anxiety and the hypothalamo-pituitary-adrenal axis. Biological psychiatry. 2011;69:366–73. doi: 10.1016/j.biopsych.2010.09.005. [DOI] [PubMed] [Google Scholar]