Abstract
Background and objectives
Impaired fetal growth is associated with increased cardiovascular morbidity and mortality in adulthood. We sought to determine whether adults born with intrauterine growth restriction have primary maladaptive changes in cardiac structure.
Methods
Study participants were adults (34–49 years) who attended the 31-year follow-up of the Cardiovascular Risk in Young Finns Study (longitudinal cohort). Transthoracic echocardiograms and demographic and cardiovascular risk surveys were completed for 157 adults born small for gestational age (SGA, birth weight <10th population centile) and 627 born average for gestational age (average for gestational age (AGA), birth weight 50th–90th population centile).
Results
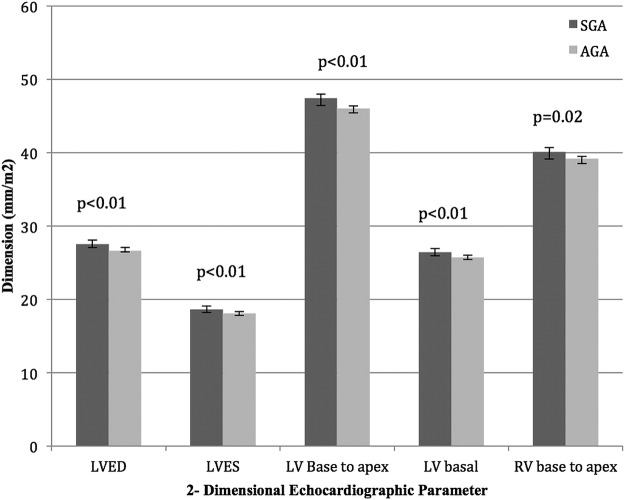
Those born growth restricted had subtly enlarged hearts with indexed left ventricular (LV) end-systolic and end-diastolic diameters slightly greater in the SGA individuals than the AGA group (LVESD 18.7 mm/m2 SGA vs 18.1 mm/m2 AGA, p<0.01; LVEDD 27.5 mm/m2 SGA vs 26.6 mm/m2 AGA, p<0.01); LV base-to-apex length (47.4 mm/m2 SGA vs 46.0 mm/m2 AGA, p<0.01); LV basal diameter (26.4 mm/m2 SGA vs 25.7 mm/m2 AGA, p<0.01); and right ventricular base-to-apex length (40.1 mm/m2 SGA vs 39.2 mm/m2 AGA, p=0.02). LV stroke volume was greater in those born AGA (74.5 mL SGA vs 78.8 mL AGA, p<0.01), with no significant difference in cardiac output (5 L/min SGA vs 5.2 L/min AGA, p=0.06), heart rate, diastolic indices or sphericity index.
Conclusions
Adults born SGA have some statistically significant but subtle changes in cardiac structure and function, which are less marked than have been described in childhood, and are unlikely to play a pathogenic role in their elevated cardiovascular risk.
Keywords: CARDIAC FUNCTION
Key questions.
What is already known about this subject?
Fetal growth restriction has a well-established association with increased cardiovascular morbidity and mortality in adulthood. The underlying pathogenic process is not well understood; however, there is evidence for premature endothelial dysfunction, early progression of atherosclerosis and cardiac structural changes in early life.
What does this study add?
In 784 young adults followed since birth, we found that young adults who had been born small for gestational age have only very subtle changes in cardiac structure and function, compared to those born at normal weight.
How might this impact on clinical practice?
This study will help to focus the investigations into the elevated cardiovascular risk in those born small for gestational age on other possible mechanisms such as endothelial dysfunction and premature atherosclerotic progression. Despite the predominantly neutral results in this study, it is extremely valuable in terms of shedding light on the mechanisms contributing to this cohort’s cardiovascular risk profile.
Introduction
Impaired fetal growth (birth weight<10th population centile) has well-established epidemiological links with increased cardiovascular morbidity and mortality in adulthood.1 2 The mechanisms responsible for this link are not well defined; however, low birthweight babies have early onset and early increased progression of subclinical atherosclerosis.3–6 There is also evidence in the literature that primary maladaptive structural cardiac changes occur in utero; with dilated cardiomyopathy-like changes and subsequently a more globular heart, with increased transverse diameters (by approximately 20%) in childhood.7 This is accompanied by subtly reduced stroke volume, which appears to be compensated for by an increased heart rate (by approximately 10%). These changes have been shown to extend into adulthood in experimental animal models of fetal growth restriction; however, whether these changes in cardiac structure and function persist into adulthood in low birthweight humans remains unknown.7 8 Recent studies do, however, indicate that low birth weight does significantly correlate with left ventricular (LV) mass, an independent predictor of long-term cardiovascular morbidity and mortality, in adolescence.9 If these structural changes do persist into adulthood, the long-term clinical ramifications of a reduced stroke volume and relative sinus tachycardia have not been clearly defined.
Accordingly, we sought to determine whether cardiac structure and function are altered in adults who were born with fetal growth restriction, when compared to those born with healthy birth weight. Specifically, we hypothesised that the changes in LV and right ventricular (RV) structure and function seen in childhood, notably a globular or less elongated heart,8 persist into adulthood.
Methods
Population
The Cardiovascular Risk in Young Finns Study is an ongoing longitudinal community-based study, initiated in 1980, that enrolled 3596 children and adolescents aged 3–18 years.10 It encompasses five Finnish University cities with medical schools and the surrounding area, with participants invited at random from the National Population Registry. The participants attended follow-up visits at three yearly intervals between 1980 and 1992 with comprehensive data collection performed at each time point. This included completion of a questionnaire regarding their birth weight, gestation at birth, and psychosocial data; physical examination, blood tests and cardiovascular diagnostic studies. They were asked to bring their records from the well-baby clinics, which were reviewed by the study nurses.6 10
The 31-year follow-up visits were completed in 2011 at age 34–49 years. Cardiac data were obtained on a subset of individuals (1680; figure 1). All participants were born at term, with small for gestational age (SGA) prospectively defined as <10th cohort-specific percentile for birth weight stratified by gender, and appropriate weight for gestational age (AGA) defined as 50th–90th population centile. At this visit, the prospectively defined participants of interest (n=157 born SGA and n=627 born AGA consented to participate) underwent a transthoracic echocardiogram with standard views obtained. Other relevant follow-up data obtained at this visit included socioeconomic, lifestyle and cardiovascular risk profiles.10–12 This study complies with the Declaration of Helsinki and was approved by local ethics committees. Participating individuals gave written informed consent.
Figure 1.
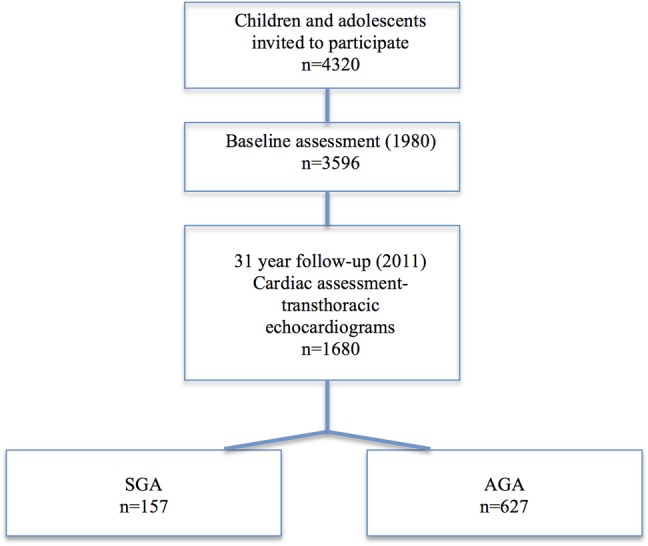
Study population (AGA, appropriate birth weight for gestational age; SGA, small for gestational age).
Transthoracic echocardiograms
The examinations were performed according to American and European guidelines.13 14 Sonographers from different locations were trained for cardiac echocardiography and study protocol. Transthoracic echocardiograms were performed with Acuson Sequoia 512 (Acuson Mountain View, California, USA) ultrasonography, using a 3.5 MHz scanning frequency phased-array transducer. Analysis of the echo images were performed by a single observer using the ComPACS 10.7.8 analysis program (MediMatic Solutions, Genova, Italy). Both the sonographer and the observer were blinded to the individual's birth weight category. Standard echocardiographic views were obtained in all participants; parasternal long and short axis, and apical four-chamber. Complete two-dimentional, M mode, continuous and pulsed-wave Doppler, and tissue velocities of the cardiac chambers were performed.
All dimensions were indexed to body surface area (BSA) during analysis, as per the European Society of Cardiology guidelines. The formula used to calculate BSA was DuBois and DuBois ((weight)kg to the power of 0.425×(height)m to the power of 0.725×0.007184). Dilated left ventricular end-diastolic (LVED) diameter indexed to BSA was defined as >32 mm/m2 in women and >31 mm/m2 in men.13 15 16
Assessment of demographics and cardiovascular risk factors
Blood pressure was measured using a random zero sphygmomanometer (Hawksley & Sons), with an average of three measurements recorded. Heart rate was recorded manually by trial nurses. A self-administered questionnaire was used to determine current health status (medications, medical conditions), smoking status, employment and marital status.
Statistical analysis
On the basis of the changes noted in previous studies in the low birth weight children, our prospectively defined primary outcomes were end-diastolic ventricular dimensions (LVED diameter and volume, RV end-diastolic diameter and volume), LV stroke volume, heart rate, cardiac output and sphericity indices for both ventricles.7
Normal distribution of data was assessed using a visual assessment of histograms and confirmed by the Kolmogorov-Smirnov test. Skewed data was log transformed for analysis. Descriptive data are shown as the mean (SD) for normally distributed data and as the median (IQR) for non-normally distributed data, with χ2 tests for categorical data. The associations of birth weight category with cardiac measures were analysed by analysis of covariance, adjusting for age, sex, blood pressure, physical activity and socioeconomic status. Intraobserver variability was assessed in 50 randomly selected participants (from all birth weight categories) for left and RV chamber size and mass using intraclass correlation coefficient (ICC) with 5th and 95th centile CIs and coefficient of variance (mean±SE). This demonstrated excellent reproducibility (ICC 0.77–0.94, table not included). A two-tailed value of p<0.05 was considered statistically significant. Statistical analysis was carried out using SPSS V.22.0.
Results
Study population
The perinatal characteristics of the study population are outlined in table 1. As expected, the SGA cohort had a lower mean birth weight of 2816 g (SD 220.9), compared with 3844 g (SD 193.2) in the AGA group (p<0.01). Birth length in the two groups was also significantly different, with a mean of 48.2 cm (SD 1.7) in SGA and 51.4 cm (SD 1.3) in AGA (p<0.01). The mean ponderal index was 2.5 (SD 0.3) for those born SGA, and 2.8 (SD 0.2) in the AGA group (p<0.01).
Table 1.
Characteristics of study population
| Participant characteristics | SGA (n=157) | AGA (n=627) | p Value |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 41.7 (4.91) | 41.2 (4.87) | 0.39 |
| Median (IQR) | 43.0 (9.00) | 40.0 (9.00) | |
| Gender (%) | 49.7 M, 50.3 F | 45.0 M, 55.0 F | 0.29 |
| Birth characteristics | |||
| Birth weight (g) | |||
| Mean (SD) | 2815.7 (220.9) | 3844.0 (193.2) | <0.01 |
| Median (IQR) | 2860.0 (223.0) | 3825.0 (280.0) | |
| Birth length (cm) | |||
| Mean (SD) | 48.2 (1.7) | 51.4 (1.3) | <0.01 |
| Median (IQR) | 48.0 (2.0) | 51.0 (2.0) | |
| Ponderal Index | |||
| Mean (SD) | 2.5 (0.3) | 2.8 (0.2) | <0.01 |
| Median (IQR) | 2.5 (0.3) | 2.8 (0.3) | |
| Current characteristics | |||
| Current height (cm) | |||
| Mean (SD) | 169.2 (8.5) | 173.8 (9.7) | 0.01 |
| Median (IQR) | 169.0 (14.0) | 173.0 (15.0) | |
| Current weight (kg) | |||
| Mean (SD) | 76.5 (16.0) | 80.9 (18.2) | 0.48 |
| Median (IQR) | 74.0 (22.0) | 79.0 (23.0) | |
| Current BMI | |||
| Mean (SD) | 26.6 (4.7) | 26.7 (5.2) | 0.49 |
| Median (IQR) | 26.2 (6.4) | 25.8 (6.0) | |
| Current BSA | |||
| Mean (SD) | 1.9 (0.2) | 2.0 (0.2) | 0.50 |
| Median (IQR) | 1.9 (0.3) | 1.9 (0.3) | |
| Current systolic BP (mm Hg) | |||
| Mean (SD) | 119.1 (14.0) | 118.1 (14.1) | 0.05 |
| Median (IQR) | 117.3 (19.3) | 116.5 (17.3) | |
| Current diastolic BP (mm Hg) | |||
| Mean (SD) | 74.8 (10.3) | 74.5 (10.3) | 0.07 |
| Median (IQR) | 73.7 (14.0) | 73.3 (13.3) | |
| Current exercise levels | |||
| Exercise at least once/week (%) | 83.9 | 84.7 | 0.80 |
Data presented as means (SD) and medians (IQR).
AGA, appropriate weight for gestational age; BMI, body mass index; BP, blood pressure; BSA, body surface area; F, female; M, male; SGA, small for gestational age.
In adult life, there was no significant difference in mean weight between SGA (76.5 kg, SD 16.0) and AGA (80.9 kg, SD 18.2); the SGA cohort was, however, noted to be significantly shorter in stature (169.2 cm SGA, SD 8.5 compared to 173.8 cm AGA, SD 9.7; p=0.01). There was a 1 mm Hg difference in mean systolic blood pressure between the two cohorts (119.1 mm Hg systolic SGA, SD 14.0 compared to 118.1 mm Hg systolic AGA, SD 14.1; p=0.05); however, there was no statistically significant difference in body mass index or BSA. Current exercise levels were similar between the two cohorts with 83.9% born SGA and 84.7% born AGA engaging in physical activity at least once per week (p=0.80; table 1).
Transthoracic echocardiogram
Geometry
Left ventricular end-systolic (LVESD) and LVEDD were subtly but significantly greater in the SGA individuals than the AGA group (LVESD 18.7 mm/m2 SGA vs 18.1 mm/m2, p<0.01; LVEDD 27.5 mm/m2 SGA vs 26.6 mm/m2, p<0.01). This increase in dimensions relative to body size in those born SGA was also seen in the indexed four-chamber LV base-to-apex length and basal diameters. There was a non-significant increase in indexed LVED volumes in the SGA group (62.4 mL/m2 SGA vs 60.5 mL/m2, p=0.06). In females, the OR for having abnormally increased LVEDD (>32 mm/m2) was 3.1 (95% CI 1.2 to 7.9, p=0.02) in those born SGA relative to AGA adults. (tables 2 and 3)
Table 2.
Cardiac volumes and dimensions (non-indexed)
| Cardiac morphometry | SGA (95% CI) | AGA (95% CI) | p Value |
|---|---|---|---|
| Left atrium | |||
| Left atrial area (cm2) | 15.8 (15.1 to 16.4) | 16.6 (16.1 to 17.0) | 0.01 |
| Left atrial volume (mL) | 41.1 (38.5 to 43.8) | 44.4 (42.6 to 46.2) | 0.01 |
| Left ventricle | |||
| Sphericity Index | 1.8 (1.8 to 1.8) | 1.8 (1.8 to 1.8) | 0.94 |
| End-diastolic diameter (mm) | 50.5 (49.6 to 51.4) | 50.8 (50.2 to 51.4) | 0.51 |
| End-systolic diameter (mm) | 34.6 (33.8 to 35.4) | 34.9 (34.4 to 35.5) | 0.40 |
| Base-to-apex length (mm) | 88.2 (86.9 to 89.5) | 89.4 (88.5 to 90.3) | 0.06 |
| Basal diameter (mm) | 49.3 (48.5 to 50.1) | 50.0 (49.5 to 50.6) | 0.07 |
| Interventricular septum (mm) | 6.9 (6.7 to 7.0) | 7.0 (6.9 to 7.1) | 0.07 |
| Posterior wall (mm) | 7.0 (6.8 to 7.1) | 7.2 (7.1 to 7.3) | 0.02 |
| End-diastolic volume (mL) | 115.0 (110.8 to 119.2) | 116.3 (113.5 to 119.2) | 0.52 |
| End-systolic volume (mL) | 44.6 (42.6 to 46.8) | 45.4 (44.0 to 46.9) | 0.42 |
| Mass (g) | 129.7 (124.1 to 135.2) | 134.7 (130.9 to 138.4) | 0.06 |
| Right atrium | |||
| Right atrial area (cm2) | 16.0 (15.4 to 16.6) | 17.1 (16.7 to 17.5) | <0.01 |
| Right atrial volume (mL) | 25.3 (23.5 to 27.1) | 26.5 (25.3 to 27.8) | 0.16 |
| Right ventricle | |||
| Sphericity Index | 2.2 (2.1 to 2.2) | 2.2 (2.2 to 2.2) | 0.61 |
| Base-to-apex length (mm) | 74.6 (73.2 to 76.1) | 76.1 (75.1 to 77.1) | 0.05 |
| Basal diameter (mm) | 34.5 (33.6 to 35.4) | 35.4 (34.7 to 36.0) | 0.04 |
| End-diastolic volume (mL) | 42.7 (40.0 to 45.6) | 46.0 (44.0 to 48.1) | 0.02 |
| End-systolic volume (mL) | 17.5 (16.4 to 18.8) | 18.7 (17.9 to 19.6) | 0.05 |
All data shown as means (95% CI) and adjusted for age, sex, blood pressure, physical activity levels and socioeconomic status.
AGA, appropriate birth weight for gestational age; SGA, small for gestational age. LV volumes calculated using Z-derived method; RV volumes using Simpson Single-Plane 4 chamber; LV mass using short-axis area-length method.
Table 3.
TTE indices adjusted for BSA
| TTE characteristic indexed to BSA | SGA (95% CI) | AGA (95% CI) | p Value |
|---|---|---|---|
| LAA (cm2/m2) | 8.2 (7.9 to 8.6) | 8.3 (8.1 to 8.5) | 0.79 |
| LA volume (mL/m2) | 22.3 (20.9 to 23.7) | 23.0 (22.1 to 24.0) | 0.29 |
| RAA (cm2/m2) | 8.6 (8.3 to 8.9) | 8.8 (8.6 to 9.0) | 0.22 |
| RA volume (mL/m2) | 14.5 (13.5 to 15.6) | 14.7 (14.0 to 15.4) | 0.75 |
| LV end-diastolic diameter (mm/m2) | 27.5 (26.9 to 28.0) | 26.6 (26.3 to 27.0) | <0.01 |
| LV end-systolic diameter (mm/m2) | 18.7 (18.2 to 19.1) | 18.1 (17.8 to 18.4) | <0.01 |
| LV base-to-apex length (mm/m2) | 47.4 (46.5 to 48.2) | 46.0 (45.5 to 46.6) | <0.01 |
| LV basal diameter (mm/m2) | 26.4 (25.9 to 26.9) | 25.7 (25.3 to 26.0) | <0.01 |
| LV end-systolic volume (mL/m2) | 24.1 (23.1 to 25.2) | 23.6 (22.9 to 24.2) | 0.31 |
| LV end-diastolic volume (mL/m2) | 62.4 (60.3 to 64.5) | 60.5 (59.1 to 61.9) | 0.06 |
| LV mass (g/m2) | 68.7 (66.2 to 71.3) | 68.5 (66.8 to 70.3) | 0.86 |
| LV stroke volume (mL/m2) | 39.5 (38.1 to 41.0) | 40.0 (39.0 to 41.0) | 0.51 |
| RV base-to-apex (mm/m2) | 40.1 (39.3 to 41.0) | 39.2 (38.7 to 39.8) | 0.02 |
| RV basal diameter (mm/m2) | 18.6 (18.1 to 19.1) | 18.3 (18.0 to 18.7) | 0.25 |
| RV end-systolic volume (mL/m2) | 9.9 (9.3 to 10.6) | 10.2 (9.7 to 10.7) | 0.44 |
| RV end-diastolic volume (mL/m2) | 24.4 (22.8 to 26.1) | 25.1 (23.9 to 26.2) | 0.44 |
All data shown as means (95% CI) and adjusted for age, sex, blood pressure, physical activity levels and socioeconomic status.
AGA, appropriate birth weight for gestational age; BSA, body surface area; LA, left atrial; LAA, LA area; LV, left ventricular; RA, right atrial; RAA, RA area; RV, right ventricular; SGA, small for gestational age; TTE, transthoracic echocardiograms.
After indexing for BSA, indexed RV longitudinal base-to-apex length was significantly greater for the SGA versus AGA adults (40.1 mm/m2 SGA vs 39.2 mm/m2 AGA, p=0.02), with no difference noted in the indexed basal diameter or volumes. Despite the differences in indexed dimensions between the two groups, there was no difference in LV or RV sphericity index (tables 2 and 3; figure 2).
Figure 2.
Dimensions indexed to body surface area (AGA, appropriate birth weight for gestational age; SGA, small for gestational age; LVED, left ventricular end-diastolic; LVES, left ventricular end-systolic; RV, right ventricular). All data shown as means (95% CI) and adjusted for age, sex, blood pressure, physical activity levels and socioeconomic status.
Absolute left atrial areas (LAA) ad right atrial areas (RAA) and volumes, not indexed to BSA, were smaller in the SGA individuals as compared to the AGA cohort; however these values were not significantly different when indexed to BSA (tables 2 and 3).
A sensitivity analysis was performed where echocardiography parameters were indexed to height rather than BSA, given the significant difference in current height between the two cohorts. Indexed LAA was similar between both groups (9.3 cm2/m SGA vs 9.5 cm2/m AGA, p=0.25), while RAA was smaller in the SGA group (9.7 cm2/m SGA vs 10.1 cm2/m AGA, p=0.02). LVEDD remained larger in the SGA cohort (30 mm/m SGA vs 29 mm/m AGA, p=0.01). LVESD and base-to-apex length, while slightly larger in those born SGA, were no longer statistically significant when indexed for height (LVEDD: 21 mm/m SGA vs 20 mm/m AGA, p=0.10. Base to apex: 52 mm/m SGA vs 51 mm/m AGA, p=0.06). LV basal diameter, RV base-to-apex length and RV basal diameter were not significantly different between the two groups when indexed to height. Stroke volume indexed to height was smaller in the SGA group, but this was not statistically significant (44 mL/m SGA vs 45 mL AGA, p=0.14).
Ventricular systolic and diastolic function
LV stroke volume was lower in those born SGA (74.5 mL SGA vs 78.8 mL AGA, p<0.01);however, this was not statistically significant when indexed to BSA (p=0.51) or height (p=0.14). There was some evidence for slightly lower cardiac output in the SGA group (5 L/min SGA vs 5.2 L/min AGA, p=0.06). Mean heart rate did not differ between the two groups (63.4 bpm SGA vs 62.9 bpm AGA, p=0.51), and there was no significant difference in LV ejection fraction, LV fractional area change or RV fractional area change (table 4).
Table 4.
Ventricular systolic and diastolic function (non-indexed)
| Cardiac function | SGA (95% CI) | AGA (95% CI) | p Value |
|---|---|---|---|
| Systolic function | |||
| Heart rate (bpm) | 63.4 (61.6 to 65.4) | 62.9 (61.6 to 64.1) | 0.51 |
| MV S′ lateral (cm/s) | 13.0 (12.3 to 13.7) | 13.6 (13.2 to 14.0) | 0.06 |
| LV stroke volume (mL) | 74.5 (71.5 to 77.6) | 78.8 (76.7 to 80.9) | <0.01 |
| LV ejection fraction (Simpson's) | 57.6 (56.9 to 58.2) | 58.0 (57.5 to 58.5) | 0.18 |
| Cardiac output (L/min) | 5.0 (4.7 to 5.2) | 5.2 (5.0 to 5.3) | 0.06 |
| LV fractional area change (%) | 42.2 (41.6 to 42.8) | 42.6 (42.1 to 43.0) | 0.22 |
| RV fractional area change (%) | 42.8 (41.1 to 44.6) | 43.7 (42.5 to 44.9) | 0.30 |
| Diastolic function | |||
| MV E wave (m/s) | 0.8 (0.7 to 0.8) | 0.7 (0.7 to 0.8) | 0.09 |
| MV A wave (m/s) | 0.5 (0.5 to 0.5) | 0.5 (0.5 to 0.5) | 0.19 |
| MV E′ medial (cm/s) | 14.3 (13.9 to 14.7) | 14.4 (14.2 to 14.7) | 0.45 |
| MV E′ lateral (cm/s) | 17.7 (17.1 to 18.3) | 17.6 (17.2 to 18.0) | 0.69 |
| MV E/E’ | 4.3 (4.1 to 4.5) | 4.2 (4.1 to 4.4) | 0.41 |
| MV deceleration time (s) | 207.9 (200.7 to 215.3) | 211.9 (206.6 to 217.0) | 0.28 |
All data shown as means (95% CI) and adjusted for age, sex, blood pressure, physical activity levels and socioeconomic status.
AGA, appropriate birth weight for gestational age; MV, mitral valve; LV, left ventricular; RV, right ventricular; SGA, small for gestational age.
LV diastolic function was assessed using mitral valve (MV) E and A wave velocities, MV E prime velocities, MV deceleration time and E to E prime ratio. No significant difference was noted between the two groups in any of these parameters (table 4).
Discussion
This study indicates that some of the changes in cardiac geometry and function previously described in children and adolescents born SGA are present in adults. When indexed to BSA, those in the SGA cohort consistently exhibited slightly greater LV systolic and diastolic dimensions and RV base-to-apex length. This indicates that SGA adults have slightly larger hearts relative to their BSA, as compared with adults born AGA. Indeed, in females born SGA, their OR for having a dilated indexed LVED dimension was 3.1 (95% CI 1.2 to 7.9). Our data were indexed to BSA as there is extensive evidence that cardiac dimensions are dependent on age, sex and BSA.13 15 16 A sensitivity analysis indexing to height rather than BSA also demonstrated subtly increased LV dimension in those born SGA; however, several parameters did not reach statistical significance.
Furthermore, SGA participants had a trend towards lower LV stroke volumes (74.5 mL vs 78.8 mL, p<0.01; indexed 39.5 mL/m2 SGA vs 40 mL/m2 AGA, p=0.51) as compared to their average birth size contemporaries. This reduction in stroke volume has been noted in childhood, but this is the first time there is some evidence that it may persist, albeit to a smaller extent, into adulthood.7 It is accompanied by no significant change in heart rate, but a trend towards reduced overall cardiac output (5 L/min SGA vs 5.2 L/min AGA, p=0.06). Importantly, while significant differences in both cardiac geometry and systolic function were noted in this study between those born SGA and those born AGA, all mean values were still within normal adult ranges.13
While these changes were statistically significant, they are unlikely to be clinically relevant, given their very small magnitude. This is particularly true when we note that there are considerably less marked differences than were seen in childhood (approximately 2% vs 10% mean differences).7 The lesser differences in cardiac parameters in adulthood between the SGA and AGA groups, compared with the differences seen in childhood, might suggest progressive adaptation over time. Interestingly, those born SGA demonstrated a systolic blood pressure 1 mm Hg greater than those born AGA (119.1 mm Hg vs 118.1 mm Hg, p=0.05). Whilst this result was statistically significant, we are not able to quantify the clinical relevance or implications of this difference due to our small sample size.
Unfortunately, this study was not sufficiently powered to determine if there was a subgroup of severe SGA in whom there were more marked changes in cardiac structure and function. An analysis of IQR for all echocardiographic parameters, however, did not demonstrate significantly larger IQRs in the SGA group than was seen in the AGA group (see online supplementary tables S1–S3).
The association between low birth weight and increased risk of cardiovascular disease in adulthood has been well established over decades. Barker et al2 reviewed birth weights from men born during 1911–1930 and documented that those with the lowest weights at birth and 1 year had the highest death rates from ischaemic heart disease. Despite knowledge of this correlation, the underlying pathological processes have been more difficult to elucidate.17
There are numerous theories that have been identified as potential mechanisms for this association. ‘Small baby syndrome’, where low birth weight/fetal malnutrition is associated with endothelial dysfunction and subsequent increased risk of premature hypertension, stroke and coronary artery disease, is supported by numerous international studies, including the Cardiovascular Risk in Young Finns Study, based on carotid intima-media thickness, brachial flow-mediated dilation and cardiovascular risk factors.3 5 6 17 18
Our study sought to probe one of the other main theories that have been hypothesised to be mechanistic in this process, that growth restriction in utero might be associated with persistent significant changes in cardiac structure into adult life.7 Indeed, there is good evidence that intrauterine growth restriction and hypoxia results in a dilated cardiomyopathic process in utero in the developing fetus, both in humans and in animal models.8 19 There are also haemodynamic data in the newborn period that SGA babies have relative cardiac hypertrophy and elevated brain natriuretic peptide levels, possibly indicative of increased cardiac workload and ventricular dysfunction.20 Population studies have also indicated that birth weight independently correlates with LV mass in adolescence.9 Our data, however, indicated that, in proportion to body size, the left (and possibly right) ventricle was very subtly dilated in adults who were born SGA, as distinct from previous studies in childhood that showed a more globular heart with increased transverse diameters and reduced longitudinal lengths.7 This is supported by the lack of difference in the sphericity index, a marker of a globular heart, between the SGA and AGA groups in our study. A proposed mechanism for this change in cardiac structure is altered loading conditions in utero. Increased placental vascular resistance leads to hypoxia and undernutrition, with a subsequent increase in afterload, decrease in arterial compliance, and resultant increased wall stress.7 Supporting this, fetal growth restriction has been proven to be associated with higher placental resistance indices in vivo.19 21 These maladaptive changes may lead to a more inefficient heart in childhood, with reduced stroke volumes and resultant reliance on relative sinus tachycardia in order to maintain cardiac output. These changes, however, attenuate over time. We demonstrated that by adulthood there was only an approximate 5% decrease in LV stroke volume (compared to approximately 20% in childhood),7 with no significant change in the heart rate, cardiac output or LV diastolic function.
In our study, we defined AGA as the 50th–90th centile of birth weight. A sensitivity analysis comparing SGA babies with AGA babies, where that was defined as 10th–90th centile birth weight, resulted in no statistically significant changes in the results obtained. Further, we performed another sensitivity analysis where echocardiographic measures were indexed to current height (significantly different between the 2 cohorts) rather than BSA. This demonstrated slightly greater LV dimensions in the SGA group (although only LVEDD reached statistical significance), but no difference in RV dimensions.
The strengths of this study include the large sample size, comprehensive information on birth weight and birth length, and the extensive available information on potential confounding factors such as socioeconomic status and physical activity levels. The standardised method used for obtaining transthoracic data is also noteworthy, reducing potential variations in results depending on individual sonographer techniques.
Our study also has limitations. As distinct from previous studies,7 we did not use prenatal Doppler to classify SGA into severe and mild. Thus, it is possible that a milder SGA cohort could be partially responsible for the attenuation in cardiac structural and functional changes that we had noted in adulthood, as compared to other studies performed in childhood such as that by Crispi et al7 Furthermore, cardiac MRI may be a more sensitive way to measure ventricular volumes and mass than transthoracic echocardiography.22 Lewandowski et al have shown (using cardiac MRI) that individuals born preterm exhibit unique LV geometry and function in adulthood, specifically increased LV and RV mass, smaller LV and RV volumes, and reduced LV and RV systolic and diastolic function. They have not, however, been able to determine if there are any structural and functional changes in those born at term but SGA, due to the study size and power.23 24
Conclusion
This study indicates that adults born SGA have some statistically significant but subtle changes in cardiac structure and function, although these are less marked than have been described in childhood. These structural and functional changes are unlikely to be clinically significant or contribute to the pathogenesis of the increased cardiovascular risk profile seen in individuals born SGA.
Footnotes
Contributors: CA analysed and interpreted the data, drafted the article, edited and finalised the document and is accountable for all aspects of the work's accuracy and integrity. MRS assisted with analysis and interpretation of data, edited and finalised the document and is accountable for all aspects of the work's accuracy and integrity. SR contributed to the conceptual design and data acquisition, interpretation of data, revision of the article, final approval of the document and is accountable for all aspects of the work's accuracy and integrity. MJ contributed to the conceptual design and data acquisition, interpretation of data, revision of the article, final approval of the document and is accountable for all aspects of the work's accuracy and integrity. JSAV contributed to the conceptual design and data acquisition, interpretation of data, revision of the article, final approval of the document and is accountable for all aspects of the work's accuracy and integrity. MK contributed to the conceptual design and data acquisition, interpretation of data, revision of the article, final approval of the document and is accountable for all aspects of the work's accuracy and integrity. TLe contributed to the conceptual design and data acquisition, interpretation of data, revision of the article, final approval of the document and is accountable for all aspects of the work's accuracy and integrity. TLa contributed to the conceptual design and data acquisition, interpretation of data, revision of the article, final approval of the document and is accountable for all aspects of the work's accuracy and integrity. DSC analysed and interpreted the data, drafted the article, edited and finalised the document and is accountable for all aspects of the work's accuracy and integrity. OTR contributed to the conceptual design, data aquisition, data analysis and interpretation, document editing and finalising, and is accountable for all aspects of the work's accuracy and integrity.
Funding: The Young Finns Study has been financially supported by the Academy of Finland: grants 134309 (Eye), 126925, 121584, 124282, 129378 (Salve), 117797 (Gendi), and 41071 (Skidi), the Social Insurance Institution of Finland, Kuopio, Tampere and Turku University Hospital Medical Funds, Juho Vainio Foundation, Sigrid Juselius Foundation, Paavo Nurmi Foundation, Finnish Foundation of Cardiovascular Research and Finnish Cultural Foundation, Tampere Tuberculosis Foundation and Emil Aaltonen Foundation. Dr Skilton is supported by a National Health and Medical Research Council of Australia fellowship (grant number 1004474).
Competing interests: None declared.
Ethics approval: This study complies with the Declaration of Helsinki and was approved by local ethics committees, Finland.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Huxley R, Owen CG, Whincup PH et al. . Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr 2007;85:1244–50. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Winter PD, Osmond C et al. . Weight in infancy and death from ischaemic heart disease. Lancet 1989;2:577–80. 10.1016/S0140-6736(89)90710-1 [DOI] [PubMed] [Google Scholar]
- 3.Goodfellow J, Bellamy MF, Gorman ST et al. . Endothelial function is impaired in fit young adults of low birth weight. Cardiovasc Res 1998;40:600–6. 10.1016/S0008-6363(98)00197-7 [DOI] [PubMed] [Google Scholar]
- 4.Norman M. Low birth weight and the developing vascular tree: a systematic review. Acta Paediatrica 2008;97:1165–72. 10.1111/j.1651-2227.2008.00904.x [DOI] [PubMed] [Google Scholar]
- 5.Skilton MR, Evans N, Griffiths KA et al. . Aortic wall thickness in newborns with intrauterine growth restriction. Lancet 2005;365:1484–6. 10.1016/S0140-6736(05)66419-7 [DOI] [PubMed] [Google Scholar]
- 6.Skilton MR, Viikari JS, Juonala M et al. . Fetal growth and preterm birth influence cardiovascular risk factors and arterial health in young adults: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol 2011;31:2975–81. 10.1161/ATVBAHA.111.234757 [DOI] [PubMed] [Google Scholar]
- 7.Crispi F, Bijnens B, Figueras F et al. . Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 2010;121:2427–36. 10.1161/CIRCULATIONAHA.110.937995 [DOI] [PubMed] [Google Scholar]
- 8.Tintu A, Rouwet E, Verlohren S et al. . Hypoxia induces dilated cardiomyopathy in the chick embryo: mechanism, intervention, and long-term consequences. PLoS ONE 2009;4:e5155 10.1371/journal.pone.0005155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hietalampi H, Pahkala K, Jokinen E et al. . Left ventricular mass and geometry in adolescence: early childhood determinants. Hypertension 2012;60:1266–72. 10.1161/HYPERTENSIONAHA.112.194290 [DOI] [PubMed] [Google Scholar]
- 10.Raitakari OT, Juonala M, Ronnemaa T et al. . Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol 2008;37:1220–6. 10.1093/ije/dym225 [DOI] [PubMed] [Google Scholar]
- 11.Juonala M, Viikari JS, Raitakari OT. Main findings from the prospective Cardiovascular Risk in Young Finns Study. Curr Opin Lipidol 2013;24:57–64. 10.1097/MOL.0b013e32835a7ed4 [DOI] [PubMed] [Google Scholar]
- 12.Raiko JR, Viikari JS, Ilmanen A et al. . Follow-ups of the Cardiovascular Risk in Young Finns Study in 2001 and 2007: levels and 6-year changes in risk factors. J Intern Med 2010;267:370–84. 10.1111/j.1365-2796.2009.02148.x [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB et al. . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Appleton CP, Gillebert TC et al. . Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107–33. 10.1016/j.echo.2008.11.023 [DOI] [PubMed] [Google Scholar]
- 15.Maceira AM, Prasad SK, Khan M et al. . Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J 2006;27:2879–88. 10.1093/eurheartj/ehl336 [DOI] [PubMed] [Google Scholar]
- 16.Maceira AM, Prasad SK, Khan M et al. . Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:417–26. 10.1080/10976640600572889 [DOI] [PubMed] [Google Scholar]
- 17.Kaijser M, Bonamy AK, Akre O et al. . Perinatal risk factors for ischemic heart disease: disentangling the roles of birth weight and preterm birth. Circulation 2008;117:405–10. 10.1161/CIRCULATIONAHA.107.710715 [DOI] [PubMed] [Google Scholar]
- 18.Gale CR, Ashurst HE, Hall NF et al. . Size at birth and carotid atherosclerosis in later life. Atherosclerosis 2002;163:141–7. 10.1016/S0021-9150(01)00760-2 [DOI] [PubMed] [Google Scholar]
- 19.Verburg BO, Jaddoe VW, Wladimiroff JW et al. . Fetal hemodynamic adaptive changes related to intrauterine growth: the Generation R Study. Circulation 2008;117:649–59. 10.1161/CIRCULATIONAHA.107.709717 [DOI] [PubMed] [Google Scholar]
- 20.Pinedo M, Villacorta E, Tapia C et al. . Inter- and intra-observer variability in the echocardiographic evaluation of right ventricular function. Rev Esp Cardiol 2010;63:802–9. 10.1016/S0300-8932(10)70183-4 [DOI] [PubMed] [Google Scholar]
- 21.Gardiner H, Brodszki J, Marsal K. Ventriculovascular physiology of the growth-restricted fetus. Ultrasound Obstet Gynecol 2001;18:47–53. 10.1046/j.1469-0705.2001.00436.x [DOI] [PubMed] [Google Scholar]
- 22.Grothues F, Smith GC, Moon JC et al. . Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29–34. 10.1016/S0002-9149(02)02381-0 [DOI] [PubMed] [Google Scholar]
- 23.Lewandowski AJ, Augustine D, Lamata P et al. . Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013;127:197–206. 10.1161/CIRCULATIONAHA.112.126920 [DOI] [PubMed] [Google Scholar]
- 24.Lewandowski AJ, Bradlow WM, Augustine D et al. . Right ventricular systolic dysfunction in young adults born preterm. Circulation 2013;128:713–20. 10.1161/CIRCULATIONAHA.113.002583 [DOI] [PubMed] [Google Scholar]