Abstract
While direct ethanol metabolites, including ethylglucuronide (EtG), play an important role for the confirmation of prenatal alcohol exposure (PAE), their utility is often limited by their short half-lives in blood and urine. Maternal hair might allow for a retrospective measure of PAE for up to several months. This study examined the validity of hair EtG (hEtG) relative to self-reporting and five other biomarkers (gamma glutamyltranspeptidase [GGT], carbohydrate-deficient transferrin [%dCDT], urine ethyl glucuronide [uEtG], urine ethyl sulfate [uEtS], and phosphatidylethanol [PEth]) in 85 pregnant women. Patients were recruited from a University of New Mexico prenatal clinic, which provides care to women with substance abuse and addiction disorders, and followed until early postpartum. The composite index, which was based on self-reported measures of alcohol use and allowed us to classify subjects into PAE (n = 42) and control (n = 43) groups, was the criterion measure used to estimate the sensitivity and specificity of hEtG and other biomarkers. Proximal segments of hair were collected at enrollment (average 22.0 gestational weeks) and analyzed by liquid chromatography–tandem mass spectrometry (LC-MS/MS). At the same visit, maternal blood and urine specimens were collected for analysis of GGT, %dCDT, PEth, uEtG, and uEtS. The study population included mostly opioiddependent (80%) patients, a large proportion of ethnic minorities (75.3% Hispanic/Latina, 8.2% American Indian, 4.7% African-American), and patients with low education (48.2% < high school). The mean maternal age at enrollment was 26.7 ± 4.8 years. Hair EtG demonstrated 19% sensitivity and 86% specificity. The sensitivities of other biomarkers were comparable (5–20%) to hEtG in this cohort, but specificities were higher (98–100%). Hair EtG sensitivity improved when combined with other biomarkers, especially with GGT (32.5%) and PEth (27.5%). In addition, validity of hEtG improved in patients with less frequent shampooing and those who did not use hair dyes/chemical treatments. Mothers of two children with Fetal Alcohol Syndrome had hEtG levels of 158 and 58 pg/mg. These data suggest that hEtG alone, as measured in maternal hair, is not a sufficiently sensitive or specific biomarker to be used separately for the identification of PAE, but might be useful in a battery along with other maternal biomarkers.
Keywords: pregnancy, biomarkers, alcohol, hair, ethylglucuronide, prenatal alcohol exposure
Introduction
Alcohol has been recognized as a human teratogen since the late 1960s to early 1970s. The term Fetal Alcohol Syndrome (FAS) was introduced (Jones, Smith, Streissguth, & Myrianthopoulos, 1974; Jones, Smith, Ulleland, & Streissguth, 1973; Lemoine, Harousseau, Borteyru, & Menuet, 2003) when a specific pattern of structural and neurobehavioral abnormalities was identified. Despite extensive research in this field during the past 40 years, concerted efforts continue in the primary and secondary prevention of Fetal Alcohol Spectrum Disorder (FASD). Recent findings from an active case ascertainment study demonstrated that as many as 2.4–4.8% of a representative middle-class community of young schoolchildren in the Midwestern United States were affected by FASD (May et al., 2014). Early and accurate recognition of alcohol consumption during pregnancy followed by targeted harm reduction strategies, such as brief intervention which can be administered by health professionals, are recognized tools to reduce the number of children affected by prenatal alcohol exposure (PAE) (Jones, Bailey, & Sokol, 2013). However, social stigma associated with alcohol use in pregnancy often leads to substantial maternal under-reporting.
The utility of supplementing a potentially unreliable maternal self-report with ethanol biomarkers to more comprehensively capture PAE has long been recognized. Hair ethylglucuronide (hEtG) is a promising biomarker due to its high sensitivity and specificity for the identification of heavy chronic alcohol use. A meta-analysis conducted by Boscolo-Berto and colleagues (2014) found an overall sensitivity of 96% and specificity of 99% for hEtG in chronic alcohol users from eight studies (Boscolo-Berto et al., 2014). Furthermore, hEtG has a broader detection window (up to several months) compared to biomarkers measured in more traditional biological matrices such as blood and urine (Bakhireva & Savage, 2011). That is, urine or blood screening for alcohol biomarkers at the first prenatal visit will be negative in many pregnant women who discontinue drinking upon pregnancy recognition, while hair analysis can identify risky drinking in the periconceptional period – the most crucial period of organogenesis. In addition, hEtG, as a direct ethanol metabolite, has a higher specificity than traditional alcohol biomarkers, such as gamma glutamyltranspeptidase (GGT) and disialo carbohydrate-deficient transferrin (%dCDT), which are known to be affected by a number of maternal conditions and physiological changes in pregnancy. Specifically, advanced gestational age, iron deficiency, hypertension, and liver conditions have been reported to affect %dCDT in addition to excessive alcohol use. (Bakhireva et al., 2012; Bianchi, Ivaldi, Raspagni, Arfini, & Vidali, 2011; De Feo et al., 1999; Kenan, Larsson, Axelsson, & Helander, 2011). One biological matrix with a comparable detection window to hair is meconium – a newborn’s first stool. While ethanol biomarkers measured in meconium can capture PAE from approximately 20 gestational weeks onward, a number of challenges have been described with meconium analyses, including logistical difficulties with collection and storage, variations on laboratory analysis methods (Lange, Shield, Koren, Rehm, & Popova, 2014), and the possibility of increased false positive rates (Zelner, Hutson, Kapur, Feig, & Koren, 2012). While analysis of alcohol biomarkers in neonatal hair might present some cultural and aesthetic challenges, obtaining a sample of maternal hair appears more feasible and acceptable.
Prior studies have examined the validity of hEtG in pregnant (Morini et al., 2013; Morini, Marchei, et al., 2010; Wurst et al., 2008) and non-pregnant women (Kronstrand, Brinkhagen, & Nyström, 2012; Politi, Morini, Leone, & Polettini, 2006) with mixed results. In two studies by Morini et al. (Morini, Marchei, et al., 2010; Morini et al., 2013), maternal alcohol consumption information was obtained from medical records rather than an in-depth face-to-face interview, which may be limiting in validating hEtG as a reliable biomarker. Differences in alcohol consumption patterns, the methods used to ascertain alcohol exposure, and timing of assessment might contribute to heterogeneity of findings. To our knowledge, none of the previous studies has compared the validity of hEtG to a battery of direct and indirect alcohol biomarkers. In this report, we examine the sensitivity and specificity of hEtG among 85 pregnant women and compare it with the performance of maternal biomarkers measured in more traditional media – maternal urine and blood.
Materials and methods
Study design and participant recruitment
Study participants were enrolled in the Biomarkers in Pregnancy Study (BIPS) (P.I.: Bakhireva) approved by the University of New Mexico (UNM) Human Research Review Committee. This study utilized a prospective cohort design with a baseline visit during one of the patient’s first prenatal care visits and a follow-up visit during the hospital stay after delivery. The detailed study methodology has been described elsewhere (Bakhireva et al., 2012). Patients were recruited from a UNM prenatal clinic providing medical care to women with substance abuse and addiction disorders and had to meet the following initial eligibility criteria: (1) a singleton pregnancy; (2) plan to deliver at UNM Hospital; (3) be less than 32 weeks gestation; and (4) be able to give written consent. After enrollment, a semi-structured maternal interview was conducted which captured detailed information on alcohol and substance use in the periconceptional period and during pregnancy. Demographic (i.e., age, marital status, race/ethnicity, education, current employment, and health insurance status), medical, and reproductive health characteristics (i.e., any chronic conditions, complications during pregnancy, use of medications and vitamins, gravidity, parity, pregnancy dates) were also ascertained. Participants were also queried about the use of hair products (i.e., use of hair dyes and chemical treatment during the past 6 months and frequency of shampooing per week). Within 24 hours after delivery, a follow-up interview was administered to capture changes in alcohol and substance use since the enrollment interview as well as alcohol use in the 2 weeks before delivery. Information was abstracted from patients’ electronic medical records regarding pregnancy and newborn outcomes, including any maternal and newborn complications and any diagnosis of major structural anomalies for the newborn.
Self-reported alcohol use and group allocation
The timeline follow-back (TLFB) interviewing technique (Sobell & Sobell, 1992) captured alcohol consumption in the periconceptional period (2 weeks before and 2 weeks after the first day of their last menstrual period [LMP]) and the 2 weeks before enrollment. Participants were asked to recall and report the specific types and number of drinks containing alcohol they consumed during those time periods. The reported quantities were converted into standard drink units (SDUs) based on the percentage of alcohol and quantity consumed. Reported quantity and frequency of alcohol use were then converted into absolute ounces of alcohol per day and per drinking day in periconceptional period (AAD0 and AADD0, respectively) and the 2-week period before enrollment (AAD1 and AADD1, respectively) as previously described (Bakhireva et al., 2012; Jacobson, Chiodo, Sokol, & Jacobson, 2002). Study participants were also administered a standard 10-question Alcohol Use Disorders Identification Test (AUDIT) questionnaire and were asked to report the maximum number of drinks consumed during 24 hours any time after LMP.
A total of 102 patients were enrolled into the study and were preliminarily classified into PAE (n = 46) or control (n = 56) groups based on the self-reported measures. Initial allocation in the control group required that participants meet all of the following criteria (1) no binge drinking episodes (≥ 4 drinks/occasion) in the periconceptional period, (2) no more than two drinks per week in the periconceptional period (AAD0 ≤ 0.14), and (3) abstinence during the 2 weeks prior to enrollment per TLFB calendar (AAD1 and AADD1 = 0). The control group was further restricted to 44 participants after disqualifying patients who either admitted some alcohol use after the LMP but prior to the 2-week window immediately before enrollment (n = 7), had an AUDIT score ≥ 8 (n = 3), or both (n = 2). Allocation in the PAE group required that participants report either ≥ 3 drinks per week on average at enrollment (average AAD1 ≥ 0.21) or report at least one binge drinking episode at enrollment (AADD1 ≥ 2.0). Given the broad detectability window of hair biomarkers, patients recruited before 10 weeks of gestation (4 PAE and 1 control) were excluded from the analysis to avoid a potential confounding by alcohol use prior to the periconceptional period. Thus, the final sample size for this study was limited to 85 patients (43 controls and 42 PAE). Moreover, a subgroup analysis was conducted after further restricting the sample size to 52 patients (29 controls and 23 PAE) who reported no chemical hair treatment or hair dyeing in the past 6 months, to avoid potential confounding by hair products.
Biospecimens and laboratory measures
After completion of the maternal baseline interview, maternal biospecimens (1 mL serum, 1 mL whole blood, 10 mL urine, and hair) were collected. From each participant, a section of approximately 80 strands of hair was cut with scissors from the posterior vertex immediately above the scalp. Participants were explained that the hair sample would be cut from an area that would not be visible nor would affect the cosmetic appearance of their hairstyle. After collection, a 6-cm proximal section of the hair specimen was measured, cut, and separated from the distal section, weighed, wrapped in foil and packaging according to the United States Drug Testing Labortory (USDTL; Des Plaines, IL) specimen preparation instructions, and stored at room temperature prior to shipment for hEtG analysis. The remaining distal section beyond 6 cm was discarded. Given that the rate of hair growth is approximately 1.0 cm per month (Harkey, 1993; Hayashi, Miyamoto, & Takeda, 1991) and the fact that most participants were enrolled around 22 gestational weeks, hEtG analysis in the collected specimen approximates exposure in the periconceptional period and during pregnancy.
Analysis of hEtG was performed by USDTL by liquid chromatography–tandem mass spectrometry (LC-MS/MS) as previously described (Jones et al., 2012). Briefly, hair specimens were decontaminated using hexane, methylene chloride, and methanol. After drying, specimens were placed in a high-energy cell disruptor (BioSpec Products, Bartlesville, OK) until powdered, then extracted with water. Solid phase extraction with methanol and deionized (DI) water was used followed by vacuum drying. EtG was extracted using 2% formic acid in methanol, evaporated under nitrogen, and reconstituted with DI water. The extracted EtG was then analyzed using a LC-MS/MS Agilent Technologies 1200 system. The limit of detection (LOD) was 2.0 pg/mg and the limit of quantitation was 8.0 pg/mg. Hair EtG values greater than the 8.0 pg/mg cut-off (Kerekes, Yegles, Grimm, & Wennig, 2009; Stewart, Koch, Willner, Randall, & Reuben, 2013) were considered a ‘positive’ test.
Maternal urine and whole blood specimens were sent frozen in dry-ice packaging to the USDTL for urine EtG (uEtG), urine ethyl sulfate (uEtS), and phosphatidylethanol (PEth) analysis using LC-MS/MS (Jones, 2011). Maternal serum specimens for GGT analysis were sent to the TriCore Reference Laboratories (Albuquerque, NM) and analyzed as part of a liver panel by the enzymatic rate method for GGT activity on a Beckman Synchron LX analyzer (Beckman Coulter Inc., Brea, CA). Another aliquot of serum was sent to the Medical University of South Carolina (Charleston, SC) for %dCDT analysis by an internationally validated HPLC and spectrophotometric detection method (Anton & Youngblood, 2006; Bakhireva et al., 2012). The following established cut-offs were used to classify tests as ‘positive’: uEtG ≥ 25 ng/mL, uEtS ≥ 7 ng/mL (Bakhireva et al., 2014), GGT > 40 U/L (Hietala, Puukka, Koivisto, Anttila, & Niemelä, 2005), %dCDT > 2.0 (Bakhireva et al., 2012; Bianchi et al., 2011; Kenan et al., 2011), and PEth ≥ 8 ng/mL (Bakhireva et al., 2014; Viel et al., 2012).
Statistical analysis
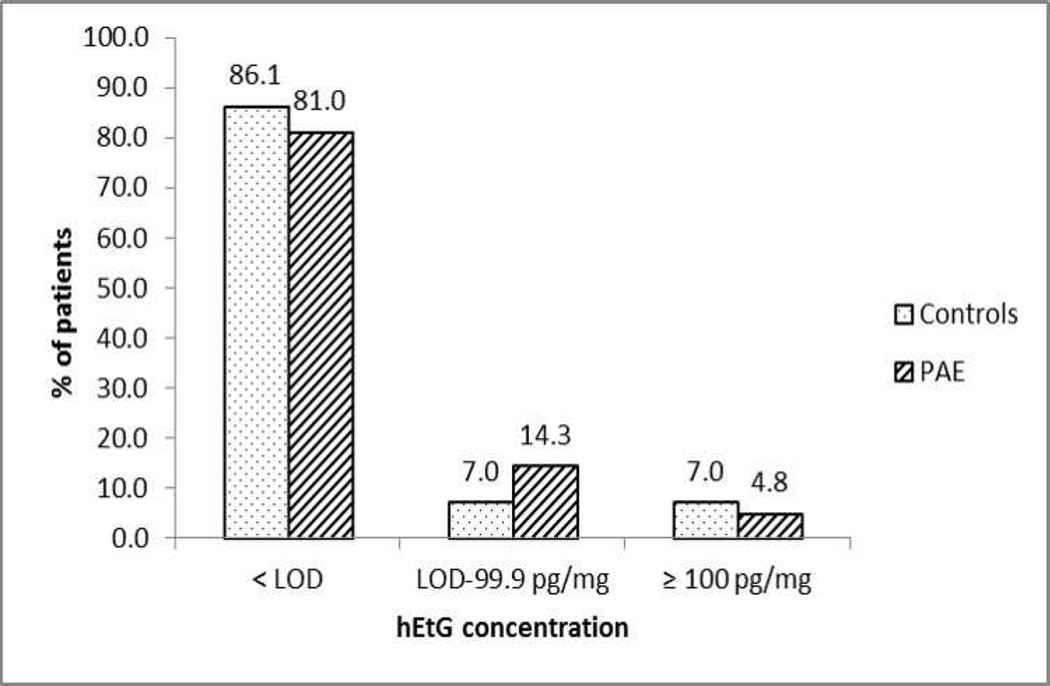
To describe the study sample, the mean ± standard deviation for continuous variables (mother’s age and gestational age at baseline visit) and frequencies and percentages for categorical variables (marital status, ethnicity, race, education, insurance, primigravida, and unplanned pregnancy) were estimated. The distribution of continuous and categorical variables between PAE and control groups was compared by a 2-sample t test with unequal variances and a chi-square test, respectively. Given that more than 80% of hEtG values were below the LOD, hEtG was treated as a categorical variable to demonstrate its distribution in the study sample. That is, the proportion of patients who fall under the three categories of hEtG (<LOD, LOD-99.9 pg/mg, ≥100 pg/mg) was presented among PAE and control groups. Summary statistics describing various measures of alcohol use during the periconceptional period and at enrollment were also presented.
For each biomarker, the sensitivity was defined as the percent of the exposed individuals who were positive for the biomarker; and the specificity was defined as the percent of the controls who were negative for the biomarker. The positive and negative predictive values (PPV, NPV) were defined as the percent of true positive and the percent of true negative tests from the total number of positive and negative tests, respectively. The 95% confidence intervals for sensitivity and specificity were calculated using the conservative exact binomial distribution. In addition to estimating sensitivity and specificity for each individual biomarker, validity indices were estimated for a combination of hEtG with each of the other maternal biomarkers (uEtG, uEtS, GGT, %CDT, PEth) as well as 3-biomarker batteries (hEtG+GGT+%CDT, hEtG+GGT+PEth, hEtG+PEth+%CDT). For a combination of biomarkers, a battery was considered ‘positive’ if at least one biomarker was above the established cut-off, and was considered ‘negative’ if all biomarkers were below the cut-off.
Given that sensitivity and specificity of hEtG may be impacted by hair product use, we created an additional hair product usage indicator, defined as no reported chemical hair treatment or hair coloring in the past 6 months. We also created a frequent shampooing indicator, defined as shampooing hair at least once per day. The sensitivity and specificity of hEtG were estimated after stratification by the hair product usage and the shampooing frequency indicators. To compare validity of hEtG to other maternal biomarkers, the percent agreement and the kappa statistic between hEtG and each other maternal biomarker was estimated.
There were no missing values for hEtG. A complete case analysis was conducted for the remaining biomarkers. Specifically, missing values were omitted as needed for uEtG (2), uEtS (2), GGT (3), CDT (3), and PEth (2). Of the 85 women included in this study, there were 80 individuals with complete data for all biomarkers. All hypothesis tests were 2-sided and conducted at the 0.05 significance level, and all confidence intervals were at the 95% level. Data were analyzed in Stata version 13 (StataCorp, College Station, TX).
Results
The study population included a large proportion of ethnic minorities (75.3% Hispanic/Latina, 8.2% American Indian, 4.7% African-American), single or separated/divorced women (61.2%), and women with less than a high school (48.2%) or high school/equivalent (28.2%) level of education attainment. The vast majority of pregnancies were unplanned (85.9%). The mean maternal age was 26.7 ± 4.8 (SD) years, and the mean gestational age at enrollment was 22.0 ± 7.6 (SD) weeks. As is evident from Table 1, patients in the PAE group were slightly older (p = 0.04) and more likely to have no medical insurance (p = 0.04) than controls. However, no other statistically significant differences in socio-demographic characteristics between the study groups were observed (all p > 0.05). Two children born to study participants in the PAE group were diagnosed with full Fetal Alcohol Syndrome (FAS), as evident by growth retardation and two cardinal facial features.
Table 1.
Demographic Characteristics of the Study Sample (N=85)
| Characteristics | Controls (N=43) |
PAE (N=42) |
p value |
|---|---|---|---|
| Mean ± s.d. | Mean ± s.d | ||
| Maternal age (years) | 25.7 ± 3.9 | 27.8 ± 5.4 | 0.04 |
| Gestational age at enrollment (weeks) | 21.3 ± 7.7 | 22.7 ± 7.5 | 0.41 |
| % | % | ||
| Ethnicity: Hispanic/Latina | 79.1 | 71.4 | 0.41 |
| Race: | |||
| White | 90.7 | 85.0 | 0.63 |
| American Indian | 7.3 | 10.8 | 0.59 |
| African-American | 2.4 | 8.3 | 0.25 |
| Marital status: | |||
| Single/separated/divorced | 53.5 | 69.1 | |
| Married/cohabitating | 46.5 | 30.9 | 0.14 |
| Health insurance: | |||
| No insurance | 0 | 9.5 | |
| Any other insurance | 100 | 90.5 | 0.04 |
| Education | |||
| Less than high school | 44.2 | 52.4 | |
| High school or equivalent | 34.9 | 21.4 | |
| Some college, vocational school or higher | 20.9 | 26.2 | 0.39 |
| Primigravida | 23.3 | 11.9 | 0.17 |
| Unplanned pregnancy | 79.1 | 92.9 | 0.07 |
| Hair product usage | |||
| Hair coloring in the past 6 months | 32.6 | 45.2 | 0.23 |
| Chemical treatments in the past 6 months | 2.3 | 2.4 | 0.99 |
| Shampoo hair at least once per day | 55.8 | 57.1 | 0.90 |
PAE = prenatal alcohol exposure
Alcohol- and substance-use patterns by study group are presented in Table 2. None of the controls and 76.2% of PAE subjects had an AUDIT score ≥ 8 (p < 0.001). Consistent with the eligibility criteria, none of the controls had any binge drinking episodes in the periconceptional period or after LMP. All controls abstained from alcohol use after LMP (AAD1 = 0 and AADD1 = 0) and had very light levels of alcohol consumption in the periconceptional period (AAD0 = 0.002 ± 0.001; AADD0 = 1.00 ± .50). The vast majority of patients in the PAE group reported binge drinking in the periconceptional period with 26.2% reporting binge episodes ≥ 5 times a week. However, despite a very high alcohol consumption pattern during the periconceptional period (AAD0 = 2.23 ± 0.44 and AADD0 = 3.94 ±0.45) in that group, only a few patients disclosed alcohol use 2 weeks prior to the enrollment interview that resulted in a relatively low level of consumption (AAD1 = 0.091 ± 0.24; AADD1 = 3.83 ± 3.09).
Table 2.
Use of Alcohol & Other Substances by Study Group (N=85)
| Controls N=43 |
PAE N=42 |
p value | ||
|---|---|---|---|---|
| Alcohol Use | ||||
| Past 12 months | ||||
| AUDIT (Mean ± s.d.) | 1.49 ± .28 | 16.12 ± 1.44 | <.001 | |
| AUDIT ≥ 8 (%) | 0.0 | 76.2 | <.001 | |
| Alcohol use in periconceptional period | ||||
| Frequency of binge (≥4 drinks) | ||||
| No binge episodes (%) | 100.0 | 4.8 | ||
| Once/week or less (%) | 0.0 | 40.5 | ||
| 2–4 times a week (%) | 0.0 | 28.6 | ||
| ≥5 times/week (%) | 0.0 | 26.2 | <.001 | |
| Average AA per day (AAD0; Mean ± s.d.) | 0.002 ± 0.001 | 2.23 ± 0.44 | <.001 | |
| Average AA per drinking day (AADD0; Mean ± s.d.)a | 1.00 ± .50 | 3.94 ± 0.45 | 0.02 | |
| Alcohol use at enrollment | ||||
| At least one binge episode during pregnancy (%) | 0 | 100.0 | <.001 | |
| Average AA per day (AAD1; Mean ± s.d.) | 0 | .091 ± .24 | – | |
| Average AA per drinking day (AADD1; Mean ± s.d.) b | 0 | 3.83 ± 3.09 | – | |
| Illicit Drugs and Tobacco | ||||
| Marijuana (%) | 32.6 | 54.8 | 0.04 | |
| Cocaine/crack-cocaine (%) | 30.2 | 31.0 | 0.94 | |
| Opioids/opioid-maintenance therapy (%) | 93.0 | 66.7 | <0.01 | |
| Benzodiazepines (%) | 18.6 | 21.4 | 0.75 | |
| Amphetamines (%) | 14.0 | 26.2 | 0.16 | |
| Tobacco (%) | 81.4 | 78.6 | 0.75 | |
Only among patients who reported any drinking in periconceptional period (2 controls and 41 exposed).
Only among patients who reported any drinking 2 weeks before enrollment (5 exposed).
AA = ounces of absolute alcohol.
Given that the study population was recruited from a substance-abuse specialty clinic, the majority of patients in both groups were opioid-dependent (93.0% of controls; 66.7% of PAE patients; p < 0.01), as shown in Table 2. Despite the high prevalence of drug and tobacco use in the study population, no significant differences were observed between the study groups (all p > 0.05) except for marijuana use, which was more prevalent among PAE subjects (54.8%) compared to controls (32.6%; p = 0.04).
Hair specimen collection was well received by the study population and a 100% collection rate was achieved. The distribution of hEtG among PAE and control subjects is presented in Fig. 1. As demonstrated in Table 3, hEtG reached 19.1% (95% CI: 8.6%; 34.1%) sensitivity and 86.1% (95% CI: 72.1%; 94.7%) specificity. The estimated sensitivity of hEtG was comparable to that of GGT (20.0%) and PEth (17.5%). The estimated sensitivity of uEtG and uEtS was much lower (4.9% and 7.3%, respectively), possibly reflecting the shorter detection window of these urine biomarkers. The estimated specificity of hEtG (86.1%) was lower compared to all other biomarkers evaluated in the study (all ≥ 97.6%).
Figure 1.
Categorical Distribution of hEtG by Study Group
Table 3.
The Validity of hEtG and other Maternal Biomarkers
| Biomarkers | Sensitivity (%) (95% CI) |
Specificity (%) (95% CI) |
PPV (%) | NPV (%) |
|---|---|---|---|---|
| hEtG ≥ 8 pg/mg | 19.1 (8.6; 34.1) | 86.1 (72.1; 94.7) | 57.1 | 52.1 |
| GGT > 40 U/L | 20.0 (9.1; 35.7) | 97.6 (87.4; 99.9) | 88.9 | 56.1 |
| %dCDT > 2.0%a | 5.0 (0.6; 16.9) | 100 (91.6; 100) | 100 | 52.5 |
| %dCDT > 1.7%a | 22.5 (10.8; 38.5) | 71.4 (55.4; 84.3) | 42.8 | 49.2 |
| uEtG ≥ 25 ng/mL | 4.9 (0.6, 16.5) | 97.6 (87.4, 99.9) | 66.7 | 51.2 |
| uEtS ≥ 7 ng/mL | 7.3 (1.5, 19.9) | 97.6 (87.4; 99.9) | 75.0 | 51.9 |
| PEth > 8 ng/mL | 17.5 (7.34; 32.8) | 100 (91.8, 100) | 100 | 56.6 |
| Combinations | ||||
| hEtG + uEtG | 22.0 (10.6; 37.6) | 83.3 (68.6; 93.0) | 56.2 | 52.2 |
| hEtG + uEtS | 24.3 (12.4; 40.3) | 83.3 (68.6; 93.0) | 58.8 | 53.0 |
| hEtG + GGT | 32.5 (18.6; 49.1) | 83.3 (68.6; 93.0) | 65.0 | 56.4 |
| hEtG + %dCDT > 2.0% | 22.5 (10.8; 38.5) | 85.7 (71.5; 94.6) | 60.0 | 53.7 |
| hEtG + %dCDT > 1.7% | 37.5 (22.7; 54.2) | 59.5 (43.3; 74.4) | 46.9 | 50.0 |
| hEtG + PEth | 27.5 (14.6; 43.9) | 86.0 (72.1; 94.7) | 64.7 | 56.1 |
| hEtG+%dCDTb+GGT | 50.0 (33.8; 66.2) | 56.1 (39.7; 71.5) | 52.6 | 53.5 |
| hEtG+GGT+PEth | 37.5 (22.7; 54.2) | 83.4 (68.6; 93.0) | 68.2 | 58.3 |
| hEtG+PEth+%dCDTb | 42.5 (27.0; 59.1) | 59.5 (43.3; 74.4) | 50.0 | 52.1 |
PPV = positive predictive value; NPV = negative predictive value
Both a traditional cut-off for %dCDT > 1.7% and a recently proposed cutoff for pregnant women of >2.0% are presented.
%d CDT > 1.7% was used in this battery.
After hEtG was combined with other biomarkers, the sensitivity of the battery improved without a substantial drop in specificity, i.e., estimated sensitivity reached 32.5% for the hEtG/GGT combination with 83.3% specificity. Both PPV (57.1%) and NPV (52.1%) for hEtG were low and only marginally improved when hEtG was combined with other biomarkers. Among 3-biomarker batteries, it appears that hEtG+GGT+PEth only slightly improved sensitivity (37.5%) without a substantial decrease in specificity (83.4%). Other 3-biomarker batteries increased sensitivity at the expense of lower specificity.
Interestingly, mothers of two children with FAS identified in the study tested positive for hEtG at enrollment (levels of 158 pg/mg and 57.5 pg/mg). Both patients were in the PAE group. Of these, one subject tested positive for two additional maternal biomarkers: GGT (43 U/L) and PEth (78.3 ng/mL) at enrollment. The other subject tested positive for three additional maternal biomarkers: GGT (71 U/L), CDT (2.1%), and PEth (218 ng/mL).
To explore whether lower estimated specificity of hEtG could be due to the use of hair products, the validity of hEtG was presented after stratification by the use of hair products and frequency of shampooing (Table 4). After restricting the sample to a subgroup of patients with no hair chemical treatment or hair dye application in the previous 6 months prior to specimen collection, the estimated sensitivity of the hEtG improved to 26.1% (95% CI: 10.2%; 48.4%), while it was lower among patients with hair treatment/dye (10.5%; 95% CI: 1.3%; 33.1%). Similarly, the estimated specificity of hEtG was higher among patients with no hair treatment/dye use in the previous 6 months (89.7%; 95% CI: 72.7; 97.8%) compared to those patients with hair treatment/dye (78.6%; 95% CI: 49.2; 95.3%). A similar pattern was observed with the frequency of shampooing with higher sensitivity and specificity of hEtG among patients with less frequency of shampooing (Table 4).
Table 4.
The Validity of hEtG Stratified by the Use of Hair Products
| Hair products | Sensitivity of hEtG (%) (95% CI) |
Specificity of hEtG (%) (95% CI) |
|---|---|---|
| Hair product usage | ||
| None | 26.1 (10.2, 48.4) | 89.7 (72.7, 97.8) |
| Hair coloring and/or chemicals | 10.5 (1.3, 33.1) | 78.6 (49.2, 95.3) |
| Shampooing frequency | ||
| < once per day | 22.2 (6.4, 47.6) | 89.5 (66.9, 98.7) |
| ≥ once per day | 16.7 (4.7, 37.4) | 83.3 (62.6, 95.3) |
Discussion
In this study of moderate-to-light chronic or intermittent binge drinkers, it does not appear that hEtG can be used as a single measure of PAE in a clinical setting. These results are in accord with a randomized controlled trial by Kronstrand et al. (2012) that examined whether hEtG could be used to differentiate low-to-moderate drinking over a 3-month period versus complete abstinence in 43 non-pregnant patients. Among the 7 males who consumed two drinks per day, 57% had detectable hEtG, while only 7.1% of 14 females who consumed one drink per day had detectable hEtG (Kronstrand et al., 2012).
Similar to our study, some prior reports have examined the effects of hair cosmetics and treatments on hEtG (Kerekes & Yegles, 2013; Morini, Politi, & Polettini, 2009; Morini, Zucchella, Polettini, Politi, & Groppi, 2010; Sporkert, Kharbouche, Augsburger, Klemm, & Baumgartner, 2012). However, two of these studies focused only on in vitro analysis of hair cosmetic treatments on the hair specimens (Kerekes & Yegles, 2013; Morini, Zucchella, et al., 2010) and another study analyzed the ability of a specific hair lotion to produce false positives (Sporkert et al., 2012). Analogous to our results, the study by Morini et al. (2009) demonstrated that hair treatments and the frequency of shampooing resulted in a lower area under the curve for hEtG, indicative of lower sensitivity and specificity.
Our study demonstrates a relatively low sensitivity of hEtG (19.1%). However, it was comparable to some biomarkers evaluated in the study (PEth, GGT) and was higher than others (%dCDT, uEtG, and uEtS). Such lower sensitivities for all biomarkers in our study might be a reflection of a low-to-moderate or intermittent binge-drinking alcohol consumption pattern in a majority of our study participants. Prior studies examining the validity of hEtG included study populations of heavy drinkers (Kronstrand et al., 2012; Morini, Politi, Acito, Groppi, & Polettini, 2009; Morini, Politi, & Polettini, 2009), patients with documented alcohol abuse or dependence (Morini et al., 2011; Pirro, Valente, et al., 2011), subjects with DUI records (Pirro, Di Corcia, et al., 2011), or patients undergoing alcohol detoxification treatment (Appenzeller, Agirman, Neuberg, Yegles, & Wennig, 2007; Politi et al., 2006; Yegles et al., 2004). Thus, it is not surprising that a much higher sensitivity of hEtG (typically > 60%) was reported in these studies. A recent meta-analysis of eight studies conducted in such high-risk non-pregnant populations demonstrated an overall hEtG sensitivity of 96% and a specificity of 99% for detecting alcohol consumption greater than 60 g per day (Boscolo-Berto et al., 2014). In our study, while many patients reported binge drinking early in gestation, average alcohol consumption after pregnancy recognition was 0.1 absolute ounces of alcohol per day (equivalent to 0.2 standard drinks per day).
We are aware of only three previous studies that examined hEtG in pregnant women. However, none established sensitivity and specificity of this biomarker relative to either self-report or other more established biomarkers. In a study of 103 pregnant Swedish women (Wurst et al., 2008), a positive hEtG test alone identified more women (13.6%) with some level of alcohol exposure than the AUDIT questionnaire (5.8%). In an Italian study by Morini et al. (2010), all 99 samples collected from mother-infant dyads from two hospitals in Italy and Spain tested negative for hEtG and hair fatty acid ethyl esters (hFAEE) in both maternal and neonatal samples, while 82.8% of meconium samples tested positive for EtG and 22.2% for FAEE (Morini, Marchei, et al., 2010). In another study of 151 mother-infant dyads from four Mediterranean public hospitals (Morini et al., 2013), 11.9% meconium samples tested positive (>2 nmol/g) for FAEEs and had detectable EtG levels between 0.5 and 1.5 nmol/g, whereas all maternal hair and nail samples tested negative for EtG. It is unclear whether these results reflect lower sensitivity of hEtG compared to meconium biomarkers or a high false positive rate of meconium FAEE, especially with samples that may have been collected later in postpartum (Zelner et al., 2012). It is also important to note that low concentrations of certain FAEEs have been detected in meconium of infants whose mothers had no history of drinking during pregnancy (Chan, Caprara, Blanchette, Klein, & Koren, 2004), which may also contribute to the higher rates of positive meconium FAEEs.
Several limitations should be taken into consideration when interpreting the results of our study. First, given hEtG’s broad window of detection, our specimen analysis could potentially capture alcohol use before pregnancy in some patients, thus decreasing specificity. For that reason, patients recruited before 10 gestational weeks were excluded from the analyses to minimize the effect of alcohol use before pregnancy on hEtG. Second, it should be acknowledged that the majority of the study population included polydrug users. Thus, the generalizability of the results might be somewhat limited. However, to our knowledge there are no known reasons to suggest that validity of hEtG, as a direct ethanol metabolite, is affected by illicit drug use.
Third, we acknowledge that in this field there is no true “gold standard” that can be used in the identification of prenatal alcohol exposure. However, one strength of our study is the use of a well-characterized cohort with repeated measures of alcohol consumption obtained by the TLFB in-depth interviews. While social desirability bias might affect the accuracy of self-report, validation of novel biomarkers against repeated TLFB interviews is a widely accepted approach (Crunelle, Cappelle, et al., 2014; Himes et al., 2015; Hjorthøj, Fohlmann, Larsen, Arendt, & Nordentoft, 2012). It should be noted that all ethanol biomarkers were compared to the same standard, allowing for direct comparison among them. Assuming that some subjects underreported alcohol use, especially after pregnancy recognition, the false positive rates (1 − specificity) could in fact indicate additional at-risk patients identified by biomarkers who were missed by self-report. Finally, we are aware that there is no unified cut-off concentration of hEtG for the identification of PAE. The Society of Hair Testing recommends 7 pg/mg as a general cutoff for repeated alcohol consumption, while we used the 8-pg/mg cut-off similar to other studies (Kerekes et al., 2009; Stewart et al., 2013). The same sensitivity and specificity estimates were obtained by re-analyzing the data with 7-pg/mg cut-off. Lowering the cut-off values might potentially improve sensitivity of identifying light drinkers. However, these cut-offs could not be examined in this sample given that there were no hEtG values between the LOD and 12 pg/mg.
The study also has several unique strengths. First, to our knowledge this is the first report that examined the validity of hEtG among more moderate drinkers as compared to prior studies. In addition, the study includes an ethnically diverse population with a large proportion of Hispanic/Latina and Native Americans patients who are frequently under-represented in other population-based studies. Nevertheless, this can also potentially limit generalizability of research findings to non-minority populations of pregnant women due to potential genetic and alcoholmetabolism differences.
Furthermore, our study examined the utility of hEtG in a battery alongside more established maternal ethanol biomarkers. An analysis of an array of biomarkers with varying detection windows may increase the likelihood of detecting drinking throughout pregnancy. While some previous studies have simultaneously assessed other biomarkers, such as CDT, MCV, GGT, FAEE, and EtS, along with hEtG, they did not specifically report the importance of using a combination of these biomarkers to ascertain alcohol exposure during pregnancy (Kharbouche et al., 2012; Pirro, Valente, et al., 2011). In our study, hEtG/PEth and hEtG/GGT combinations resulted in increased estimated sensitivity (27.5% and 32.5%, respectively) without a substantial drop in estimated specificity (86.0% and 83.3%, respectively) compared to an hEtG stand-alone test (sensitivity: 19.1%; specificity: 86.1%). A preliminary study conducted in 76 non-pregnant patients with chronic alcohol abuse found that estimated sensitivity increased to 73% for a hEtG/CDT combination (from 69% for hEtG and 27% for CDT stand-alone tests) without any change in the specificity (Morini et al., 2011). Finally, our study captures the effect of hair products as a potential effect modifier on hEtG.
Future follow-up studies should include a larger sample size and focus on examining other direct ethanol metabolites in hair samples, i.e., PEth and FAEE, as well as assessing the validity of hEtG in hair samples collected both during pregnancy and during the early postpartum period for each patient. It would also be important to examine the predictive ability of hEtG measured in pregnant women to identify future adverse neurodevelopmental outcomes in affected children. While collection of neonatal hair often presents logistical difficulties and might be associated with cultural sensitivities, we found that the sampling of maternal hair was well received by study participants. It would be important to further evaluate the effect of specific hair products on the validity of hEtG as a biomarker of PAE, examine validity of hEtG in non-head hair samples (Crunelle, Yegles, et al., 2014), and ascertain acceptability of collecting non-head hair in pregnant women. While hEtG appears to be a highly sensitive biomarker for identification of heavy chronic alcohol use in non-pregnant subjects, its utility for identification of a more moderate or episodic alcohol use pattern among pregnant women might be limited as a stand-alone test. However, hEtG’s utility in clinical practice can be improved when combined with other maternal ethanol biomarkers.
We examined validity of a hair EtG relative to 5 other biomarkers in pregnant women
Hair EtG alone reached 19.1% sensitivity and 86.1% specificity in this study
We found that the specificity of hair EtG was affected by the use of hair products
Hair EtG’s clinical utility can be improved when combined with other biomarkers
Acknowledgments
This work has been supported by research grants from NIAAA/NIH (1R03AA020170-01; 1P20AA017608), NCRR/NIH (8UL1TR000041), and the Alcohol Beverage Medical Research Foundation (ABMRF). Drs. Bakhireva’s, Hund’s, Rayburn’s, Leeman’s, and Savage’s efforts were partially supported by the R01 AA021771 grant from NIH/NIAAA. In addition, Dr. Bakhireva’s effort is partially supported by the R15 AA022242 from NIH/NIAAA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work has been previously presented in an abstract at the Research Society on Alcoholism 2014 Annual meeting.
References
- Anton RF, Youngblood M. Factors affecting %CDT status at entry into a multisite clinical treatment trial: experience from the COMBINE Study. Alcoholism: Clinical and Experimental Research. 2006;30:1878–1883. doi: 10.1111/j.1530-0277.2006.00225.x. [DOI] [PubMed] [Google Scholar]
- Appenzeller BM, Agirman R, Neuberg P, Yegles M, Wennig R. Segmental determination of ethyl glucuronide in hair: a pilot study. Forensic Science International. 2007;173:87–92. doi: 10.1016/j.forsciint.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Bakhireva LN, Cano S, Rayburn WF, Savich RD, Leeman L, Anton RF, et al. Advanced gestational age increases serum carbohydrate-deficient transferrin levels in abstinent pregnant women. Alcohol and Alcoholism. 2012;47:683–687. doi: 10.1093/alcalc/ags087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Leeman L, Savich RD, Cano S, Gutierrez H, Savage DD, et al. The validity of phosphatidylethanol in dried blood spots of newborns for the identification of prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2014;38:1078–1085. doi: 10.1111/acer.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhireva LN, Savage DD. Focus on: biomarkers of fetal alcohol exposure and fetal alcohol effects. Alcohol Research & Health. 2011;34:56–63. [PMC free article] [PubMed] [Google Scholar]
- Bianchi V, Ivaldi A, Raspagni A, Arfini C, Vidali M. Pregnancy and variations of carbohydrate-deficient transferrin levels measured by the candidate reference HPLC method. Alcohol and Alcoholism. 2011;46:123–127. doi: 10.1093/alcalc/agq092. [DOI] [PubMed] [Google Scholar]
- Boscolo-Berto R, Favretto D, Cecchetto G, Vincenti M, Kronstrand R, Ferrara SD, et al. Sensitivity and specificity of EtG in hair as a marker of chronic excessive drinking: pooled analysis of raw data and meta-analysis of diagnostic accuracy studies. Therapeutic Drug Monitoring. 2014;36:560–575. doi: 10.1097/FTD.0000000000000063. [DOI] [PubMed] [Google Scholar]
- Chan D, Caprara D, Blanchette P, Klein J, Koren G. Recent developments in meconium and hair testing methods for the confirmation of gestational exposures to alcohol and tobacco smoke. Clinical Biochemistry. 2004;37:429–438. doi: 10.1016/j.clinbiochem.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Crunelle CL, Cappelle D, Covaci A, van Nuijs AL, Maudens KE, Sabbe B, et al. Hair ethyl glucuronide as a biomarker of alcohol consumption in alcohol-dependent patients: role of gender differences. Drug and Alcohol Dependence. 2014;141:163–166. doi: 10.1016/j.drugalcdep.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Crunelle CL, Yegles M, van Nuijs AL, Covaci A, De Doncker M, Maudens KE, et al. Hair ethyl glucuronide levels as a marker for alcohol use and abuse: a review of the current state of the art. Drug and Alcohol Dependence. 2014;134:1–11. doi: 10.1016/j.drugalcdep.2013.10.008. [DOI] [PubMed] [Google Scholar]
- De Feo TM, Fargion S, Duca L, Mattioli M, Cappellini MD, Sampietro M, et al. Carbohydrate-deficient transferrin, a sensitive marker of chronic alcohol abuse, is highly influenced by body iron. Hepatology. 1999;29:658–663. doi: 10.1002/hep.510290326. [DOI] [PubMed] [Google Scholar]
- Harkey MR. Anatomy and physiology of hair. Forensic Science International. 1993;63:9–18. doi: 10.1016/0379-0738(93)90255-9. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Miyamoto I, Takeda K. Measurement of human hair growth by optical microscopy and image analysis. The British Journal of Dermatology. 1991;125:123–129. doi: 10.1111/j.1365-2133.1991.tb06058.x. [DOI] [PubMed] [Google Scholar]
- Hietala J, Puukka K, Koivisto H, Anttila P, Niemelä O. Serum gamma-glutamyl transferase in alcoholics, moderate drinkers and abstainers: effect on gt reference intervals at population level. Alcohol and Alcoholism. 2005;40:511–514. doi: 10.1093/alcalc/agh201. [DOI] [PubMed] [Google Scholar]
- Himes SK, Dukes KA, Tripp T, Petersen JM, Raffo C, Burd L, et al. Clinical sensitivity and specificity of meconium fatty acid ethyl ester, ethyl glucuronide, and ethyl sulfate for detecting maternal drinking during pregnancy. Clinical Chemistry. 2015;61:523–532. doi: 10.1373/clinchem.2014.233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorthøj CR, Fohlmann A, Larsen AM, Arendt M, Nordentoft M. Correlations and agreement between delta-9-tetrahydrocannabinol (THC) in blood plasma and timeline follow-back (TLFB)-assisted self-reported use of cannabis of patients with cannabis use disorder and psychotic illness attending the CapOpus randomized clinical trial. Addiction. 2012;107:1123–1131. doi: 10.1111/j.1360-0443.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jones J. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Analytical Methods. 2011;3:1101–1106. [Google Scholar]
- Jones J, Jones M, Plate C, Lewis D, Fendrich M, Berger L, et al. Liquid chromatography-tandem mass spectrometry assay to detect ethyl glucuronide in human fingernail: comparison to hair and gender differences. American Journal of Analytical Chemistry. 2012;3:83–91. doi: 10.4236/ajac.2012.31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Streissguth AP, Myrianthopoulos NC. Outcome in offspring of chronic alcoholic women. Lancet. 1974;1:1076–1078. doi: 10.1016/s0140-6736(74)90555-8. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bailey BA, Sokol RJ. Alcohol use in pregnancy: insights in screening and intervention for the clinician. Clinical Obstetrics and Gynecology. 2013;56:114–123. doi: 10.1097/GRF.0b013e31827957c0. [DOI] [PubMed] [Google Scholar]
- Kenan N, Larsson A, Axelsson O, Helander A. Changes in transferrin glycosylation during pregnancy may lead to false-positive carbohydrate-deficient transferrin (CDT) results in testing for riskful alcohol consumption. Clinica Chimica Acta. 2011;412:129–133. doi: 10.1016/j.cca.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Kerekes I, Yegles M. Coloring, bleaching, and perming: influence on EtG content in hair. Therapeutic Drug Monitoring. 2013;35:527–529. doi: 10.1097/FTD.0b013e31828ca246. [DOI] [PubMed] [Google Scholar]
- Kerekes I, Yegles M, Grimm U, Wennig R. Ethyl glucuronide determination: head hair versus non-head hair. Alcohol and Alcoholism. 2009;44:62–66. doi: 10.1093/alcalc/agn096. [DOI] [PubMed] [Google Scholar]
- Kharbouche H, Faouzi M, Sanchez N, Daeppen JB, Augsburger M, Mangin P, et al. Diagnostic performance of ethyl glucuronide in hair for the investigation of alcohol drinking behavior: a comparison with traditional biomarkers. International Journal of Legal Medicine. 2012;126:243–250. doi: 10.1007/s00414-011-0619-9. [DOI] [PubMed] [Google Scholar]
- Kronstrand R, Brinkhagen L, Nyström FH. Ethyl glucuronide in human hair after daily consumption of 16 or 32 g of ethanol for 3 months. Forensic Science International. 2012;215:51–55. doi: 10.1016/j.forsciint.2011.01.044. [DOI] [PubMed] [Google Scholar]
- Lange S, Shield K, Koren G, Rehm J, Popova S. A comparison of the prevalence of prenatal alcohol exposure obtained via maternal self-reports versus meconium testing: a systematic literature review and meta-analysis. BMC Pregnancy and Childbirth. 2014;14:127. doi: 10.1186/1471-2393-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine P, Harousseau H, Borteyru JP, Menuet JC. Children of alcoholic parents--observed anomalies: discussion of 127 cases. Therapeutic Drug Monitoring. 2003;25:132–136. doi: 10.1097/00007691-200304000-00002. [DOI] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134:855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morini L, Marchei E, Tarani L, Trivelli M, Rapisardi G, Elicio MR, et al. Testing ethylglucuronide in maternal hair and nails for the assessment of fetal exposure to alcohol: comparison with meconium testing. Therapeutic Drug Monitoring. 2013;35:402–407. doi: 10.1097/FTD.0b013e318283f719. [DOI] [PubMed] [Google Scholar]
- Morini L, Marchei E, Vagnarelli F, Garcia Algar O, Groppi A, Mastrobattista L, et al. Ethyl glucuronide and ethyl sulfate in meconium and hair-potential biomarkers of intrauterine exposure to ethanol. Forensic Science International. 2010;196:74–77. doi: 10.1016/j.forsciint.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Morini L, Politi L, Acito S, Groppi A, Polettini A. Comparison of ethyl glucuronide in hair with carbohydrate-deficient transferrin in serum as markers of chronic high levels of alcohol consumption. Forensic Science International. 2009;188:140–143. doi: 10.1016/j.forsciint.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Morini L, Politi L, Polettini A. Ethyl glucuronide in hair. A sensitive and specific marker of chronic heavy drinking. Addiction. 2009;104:915–920. doi: 10.1111/j.1360-0443.2009.02535.x. [DOI] [PubMed] [Google Scholar]
- Morini L, Varango C, Filippi C, Rusca C, Danesino P, Cheli F, et al. Chronic excessive alcohol consumption diagnosis: comparison between traditional biomarkers and ethyl glucuronide in hair, a study on a real population. Therapeutic Drug Monitoring. 2011;33:654–657. doi: 10.1097/FTD.0b013e318232950f. [DOI] [PubMed] [Google Scholar]
- Morini L, Zucchella A, Polettini A, Politi L, Groppi A. Effect of bleaching on ethyl glucuronide in hair: an in vitro experiment. Forensic Science International. 2010;198:23–27. doi: 10.1016/j.forsciint.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Pirro V, Di Corcia D, Pellegrino S, Vincenti M, Sciutteri B, Salomone A. A study of distribution of ethyl glucuronide in different keratin matrices. Forensic Science International. 2011;210:271–277. doi: 10.1016/j.forsciint.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Pirro V, Valente V, Oliveri P, De Bernardis A, Salomone A, Vincenti M. Chemometric evaluation of nine alcohol biomarkers in a large population of clinicallyclassified subjects: pre-eminence of ethyl glucuronide concentration in hair for confirmatory classification. Analytical and Bioanalytical Chemistry. 2011;401:2153–2164. doi: 10.1007/s00216-011-5314-7. [DOI] [PubMed] [Google Scholar]
- Politi L, Morini L, Leone F, Polettini A. Ethyl glucuronide in hair: Is it a reliable marker of chronic high levels of alcohol consumption? Addiction. 2006;101:1408–1412. doi: 10.1111/j.1360-0443.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline Follow-Back. In: Litten R, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. New York: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sporkert F, Kharbouche H, Augsburger MP, Klemm C, Baumgartner MR. Positive EtG findings in hair as a result of a cosmetic treatment. Forensic Science International. 2012;218:97–100. doi: 10.1016/j.forsciint.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Koch DG, Willner IR, Randall PK, Reuben A. Hair ethyl glucuronide is highly sensitive and specific for detecting moderate-to-heavy drinking in patients with liver disease. Alcohol and Alcoholism. 2013;48:83–87. doi: 10.1093/alcalc/ags109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. International Journal of Molecular Sciences. 2012;13:14788–14812. doi: 10.3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Sundström Poromaa I. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT--a pilot study in a population-based sample of Swedish women. American Journal of Obstetrics and Gynecology. 2008;198:407.e1–405.e1. doi: 10.1016/j.ajog.2007.10.801. [DOI] [PubMed] [Google Scholar]
- Yegles M, Labarthe A, Auwärter V, Hartwig S, Vater H, Wennig R, et al. Comparison of ethyl glucuronide and fatty acid ethyl ester concentrations in hair of alcoholics, social drinkers and teetotallers. Forensic Science International. 2004;145:167–173. doi: 10.1016/j.forsciint.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Zelner I, Hutson JR, Kapur BM, Feig DS, Koren G. False-positive meconium test results for fatty acid ethyl esters secondary to delayed sample collection. Alcoholism: Clinical and Experimental Research. 2012;36:1497–1506. doi: 10.1111/j.1530-0277.2012.01763.x. [DOI] [PubMed] [Google Scholar]