Abstract
Injectable nanomaterials have been designed for the treatment of myocardial infarction, particularly during the acute stages of inflammation and injury. Among these strategies, injectable nanofibrous hydrogel networks or nanoparticle complexes may be delivered alone or with a therapeutic to improve heart function. Intramyocardial delivery of these approaches localizes treatments to the site of injury. In an alternative approach, nanoparticles may be delivered intravenously, which provides the ultimate minimally invasive approach. These systems take advantage of the leaky vasculature after myocardial infarction, and may be designed to specifically target the injured region. The translational applicability of both intramyocardial and intravenous applications may provide safe and effective solutions upon optimizing the timing of the treatments and biodistribution.
Graphical abstract
Introduction
Myocardial infarction (MI) accounts for 1 in 6 of the total deaths in the U.S. [1]. Immediately after MI, the myocardium is unstable because of cell death and the physical and biological changes to the damaged extracellular matrix (ECM). As the ECM degrades, the left ventricle (LV) wall is weakened, thinning overtime [2]. Early healing processes involve the inflammatory response, causing the migration of neutrophils and macrophages to the injured site [3,4]. The ECM continues to degrade for over a week, and within three weeks, myofibroblasts invade the infarct area and a collagen scar begins to form. Late LV remodeling may continue for months to years, and may eventually lead to chronic heart failure [5]. Early intervention has the potential to minimize the adverse effects during the initial inflammatory stage and preserve borderzone cardiomyocytes, which are at risk of ongoing apoptosis, thereby slowing or inhibiting the progression of negative LV remodeling. In this review, we will focus on injectable nanomaterials recently under investigation for treating MI (Table 1).
Table 1.
Injectable nanomaterials for treating MI
| Material | Dimensions | Therapeutic | Animal | MI model | Control | Results compared to controls |
Reference |
|---|---|---|---|---|---|---|---|
| Surgical based injection of hydrogels | |||||||
| SAP | 5 nm fiber diameter | VEGF | SD rats and Lanyu minipigs | CAL | Saline, free VEGF, SAP alone | Increased neovascularization and improved cardiac function | [26] |
| SAP with heparin domain | 10 nm fiber diameter | VEGF | SD rats | CAL | SAP alone, SAP with heparin domain, saline | Improved cardiac function, decreased infarct size, and increased angiogenesis | [27] |
| MA gelatin and PEI with GO nanosheets | 30–40 nm nanosheets | VEGF-165 gene | Lewis rats | CAL | Hydrogel alone, hydrogel and VEGF-165, VEGF-165 alone, saline | Increased angiogenesis and cardiac function | [34] |
| Pericardial ECM | 200 nm fiber diameter | bFGF | SD rats | I/R | ECM alone, bFGF in collagen, collagen, saline | Increased vessel density | [30] |
| Catheter based injection of hydrogels | |||||||
| Cardiac ECM | 100 nm fiber diameter | - | Yucatan minipigs | Percutaneous coil embolism | Saline, untreated | Improved global and regional cardiac function and ventricular volumes; increased cardiac muscle and reduced infarct fibrosis. | [31] |
| Ureido-pyrimidinone and PEG | 75–100 nm fiber length | HGF and IGF | Dutch landrace pigs | Intracoronary balloon occlusion | Hydrogel alone, free HGF and IGF | Reduced infarct collagen content | [33] |
| Surgical based injection of nanoparticles | |||||||
| PLGA | 234 nm diameter | PGF | SD rats | CAL | Saline, free PGF | Improved cardiac function and reduced infarct expansion | [35] |
| PLGA | 60 nm diameter (PLGA) | IGF | FVB mice | CAL | Saline, free IGF, nanoparticles alone | Improved ejection fraction and reduced infarct size compared to the saline and free PGF groups | [36] |
| 74 nm diameter (with IGF) | |||||||
| IV delivery of nanoparticles | |||||||
| Gd micelles or liposomes | 15 nm diameter (micelle) | - | Swiss mice | CAL | Gadopentetic acid | Improved accumulation of micelles to infarct area monitored through noninvasive imaging Improved heart function and vascular density | [37] |
| 100 nm diameter (liposome) | |||||||
| Liposomes | 180 nm diameter | VEGF | SD rats | CAL | Free VEGF, liposomes only, no treatment | [38] | |
| DDFT nanoparticles | 250 nm diameter | Oxygen | C57BL/6J mice | CAL | Saline | Decreased infarct size | [39] |
| Myosin and TATp liposomes | 170 nm diameter (liposome) | - | Rats | CAL | Liposomes with nonspecific Ab | Increased accumulation in infarct zone, increased transfection of cardiomyocytes | [40] |
| 220 nm diameter (with TATp) | |||||||
| Ang-labeled liposomes | 142 nm diameter | - | C57BL/6J mice | CAL | Nanoparticles labeled with nonspecific peptide | Successful targeting to infarct zone | [41] |
| IMTP-CD-9R nanoparticle | 350 nm diameter | - | SD rats | I/R | Nanoparticles in healthy animals | Successful targeting to infarct zone, increased gene expression | [42] |
| PEG/Hoechst/Streptavidin nanocomplex | Dimensions not reported | IGF | Mice | I/R | Unlabeled complex, saline | Increased accumulation in infarct zone, improved heart function | [45] |
Ang: angiotensin; bFGF: basic fibroblast growth factor; CAL: coronary artery ligation; CD: cystamine bisacrylamide-diamino hexane polymer; D9: D-9-arginine; DDFP: dodecafluoropentane; ECM: extracellular matrix; Gd: gadolinium; GO: graphene oxide; HGF: hepatocyte growth factor; I/R; ischemia/reperfusion; IGF: insulin-like growth factor; IMTP: ischemic myocardium-targeted peptide; MA: methacrylate; PEG: polyethylene glycol; PEI: polyethylenimine; PGF: placental growth factor; PLGA: poly(lactic-co-glycolic acid); SAP: self-assembling peptide; SD: Sprague-Dawley; TATp: transcriptional activator peptide; VEGF: vascular endothelial growth factor.
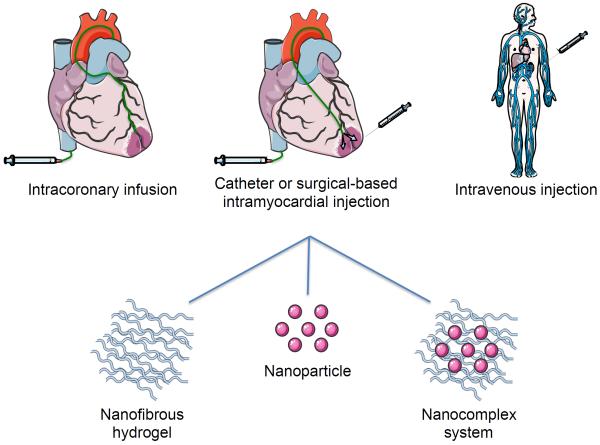
Injectable biomaterials designed to treat MI during the early stages of remodeling are frequently administered through intramyocardial, intracoronary, or intravenous (IV) routes (Figure 1). Intramyocardial injections have the advantage of localized therapy while minimizing potential systemic effects [5]. Intramyocardial injections may be accomplished by either catheter delivery or directly administered through a surgical approach with a syringe and needle. The former is a minimally invasive approach requiring only sedation, while the latter is an invasive surgery requiring general anesthesia [6]. When incorporated with a therapeutic, the bioactivity of the molecule should be maintained, released, and delivered in a sustained manner. Local intramyocardial delivery to the injured myocardium has been shown to reduce collagen scar formation and improve heart function. However, injecting a biomaterial during the acute time window is unlikely to be clinically acceptable, due to an increased risk of ventricular rupture [6–8]. The safety of intramyocardial injections in the very acute MI stages remains a clinical concern and should continue to be critically evaluated.
Figure 1.
Therapeutic delivery routes to the infarcted heart. Nanomaterials can be delivered to the heart through catheter-based intracoronary infusion, intramyocardial injection either via a transendocardial catheter or surgical-based direct injection, or through IV delivery and subsequent targeting to the site of infarction. Nanofibrous hydrogels or nanocomplexes have been delivered via intramyocardial injection, while nanoparticles have been delivered IV and have the potential for intracoronary infusion.
Intracoronary and IV delivery of biomaterials take advantage of the enhanced permeability and retention (EPR)-like effect that occurs after myocardial injury [9,10]. The leaky vasculature allows the transport of materials to enter the infarcted zone. In vivo studies investigating catheter-based intracoronary infusion of nanoscale biomaterials has not been demonstrated; however, interesting work has been published on alginate-derived hydrogels using this delivery method [11,12]. IV injection of biomaterials is the ultimate minimally invasive approach that delivers treatments directly into the blood stream with the goal of accumulating in the MI. Nanoparticles designed for IV delivery should not aggregate during injection or transport, leach functionalized therapies while in the bloodstream, nor cause systemic toxicity. IV treatments minimize potential procedure costs and time, but the biodistribution and clearance of the injected materials remains an important issue and is influenced by the size, shape, and charge of the molecule [13,14].
Intramyocardial delivery of nanoscale biomaterials: nanofibrous hydrogels and nanoparticle systems
Treatments developed to reduce LV remodeling include injectable hydrogels, which have shown promise in preventing heart failure [6,15,16]. Injectable hydrogels should form a gel in the injured area, and be biocompatible and biodegradable. While injectable hydrogels have been postulated to affect cardiac function via increasing wall thickness and reducing wall stress via La Place's Law [15], recent studies have shown that passive wall thickening does not increase function, but rather the bioactive or cell response to these materials influences positive remodeling outcomes and increases in cardiac function [17,18]. Hydrogels that are designed to have nanoscale fiber networks, mimic the native ECM, and thereby create a new scaffold to facilitate cell recruitment. Some nanofibrous hydrogel scaffolds have shown promise in vitro, but their delivery in an infarcted animal model remains uninvestigated [19,20]. Other hydrogel networks have been investigated as nanofibrous scaffolds to treat MI, but these invasive approaches have sutured the biomaterial directly to the heart [21,22]. Ideally an injectable hydrogel should be delivered via catheter to obviate the need for invasive surgery and general anesthesia. In this case, the hydrogel must also have the appropriate resistance and gelation kinetics to facilitate multiple injections required of a transendocardial delivery approach, and be hemocompatible and not result in embolism since some leakage into the blood stream is known to occur [6].
Injectable, self-assembling peptides are one class of nanofibrous hydrogels that have been examined for treating MI. These are composed of relatively short peptide sequences that assemble into a nanofibrous, hydrogel network under physiological conditions, as shown with RAD16-II, which forms fibers on the order of 5 nm in diameter [23–25]. The delivery of vascular endothelial growth factor (VEGF) has been explored using RAD16-II as a carrier in both rodent and porcine models [26]. VEGF was released from the nanofibrous hydrogel network at a steady rate over 14 days. Improved heart function and neovascularization was observed in the group treated with both VEGF and RAD16-II, compared to the saline and material only controls in both animal models. The self-assembling peptide RAD16-II has also been modified to contain a heparin-binding motif to sequester and deliver VEGF in a rodent MI model [27]. The newly designed material did not affect self-assembly properties and formed nanofibers roughly 10 nm in diameter. Improved heart function, decreased infarct size, and increased angiogenesis were observed in groups that received VEGF with either RAD16-II or modified with heparin, although all measures trended higher in the later combination.
Injectable hydrogels derived from decellularized ECM are another class of nanofibrous hydrogels that have been examined for treating MI [28–31]. To make an injectable hydrogel, the decellularized ECM is partially digested, creating a liquid material that self-assembles into a nanofibrous network upon injection with fiber diameters of approximately 40–100 nm [28]. A cardiac specific ECM hydrogel, derived from porcine ventricular myocardium, has been shown to be biocompatible, recruit cardiac progenitors, stimulate neovascularization, increase cardiac muscle, and reduce infarct fibrosis in small and large animal MI models [29,31]. The hydrogel, which is compatible with percutaneous transendocardial delivery, degrades upon cell infiltration within 2–3 weeks in vivo, yet significant increases in cardiac function were observed 3 months post-injection when delivered 2 weeks post-MI [31]. When processed appropriately, ECM derived hydrogels retain native sulfated glycosaminoglycans (sGAGs) [28,30], which can retain and enhance activity of delivered growth factors. This has been shown with a pericardial ECM hydrogel, where retention and increased activity of basic fibroblast growth factor (bFGF) and an engineered hepatocyte growth factor (HGF) were observed in rat MI models [30,32].
Several other nanoscale materials have also been explored as injectable delivery vehicles for treating for MI. In designing these systems, the molecule should be released at a controlled rate, from either nanofiber or nanoparticle carriers, while providing therapeutic effects in vivo. For example, a synthetic polymer composed of ureido-pyrimidinone and polyethylene glycol (PEG) was used to deliver hepatocyte growth factor (HGF) and insulin-like growth factor (IGF-1) via catheter injection [33]. Both IGF-1 and HGF were released from the hydrogel by 4 days in vitro. The initial burst release of IGF was lower than for HGF, most likely due to the size of the proteins. In another study, a hybrid nanosystem composed of a methacrylated gelatin hydrogel and polyethylenimine functionalized graphene oxide nanosheets was developed for gene delivery of VEGF-165 [34]. The researchers demonstrated slow release of VEGF-165 over 3 days in vitro. When injected 15 minutes post-ligation, treated groups did not show signs of toxicity or inflammation 7 days post-injection. Improved cardiac function, measured by ejection fraction, and angiogenesis was also observed in treated groups compared to the control groups 14 days post-injection.
Systemic treatment with nanoparticles through passive delivery
Nanoparticles are attractive for minimally invasive delivery because they may be administered with IV injection and target the heart through the EPR effect that is present in the acute stages of MI. Similar to trends observed for intramyocardial injections, the size of the nanoparticle may affect accumulation and distribution with IV injections. For example, gadolinium-containing micelles and liposomes, 15 and 100 nm in diameter, respectively, were injected IV into infarcted mice 24 hours post-injury [37]. Both the micelles and liposomes demonstrated longer circulation times than gadolinium alone. The micelles accumulated more quickly than the liposomes, and both materials remained in the infarct 24 hours post-injection. Two days after injection, the micelles were only observed in the kidneys, while the liposomes were present in the spleen and liver. When injected 7 days post-MI, both sets of particles accumulated in the infarct and were cleared within an hour. These results suggest that both particle size and timing of injection after injury play important roles in accumulation and circulation.
An advantage of IV delivery is the ability to deliver at very early time points post-MI, which has the potential to mitigate detrimental negative LV remodeling. For example, VEGF-encapsulated liposomes were designed as an approach to safely deliver VEGF [38]. When the liposomes were injected IV immediately after ligation, significant improvements in heart function and vascular density were observed 4 weeks after injection compared to groups that received liposomes alone or free VEGF. Another example of immediate delivery has been shown with dodecafluoropentane nanoparticles to deliver oxygen [39]. The therapy was injected IV immediately after ligation, and a 60% decrease in infarct size was observed compared to those treated with saline.
Systemic treatment with nanoparticles through targeted delivery
In addition to taking advantage of passive targeting with the EPR effect, nanoparticles can also be designed to bind to specific targets that are upregulated in the infarct. A dual gene delivery system was designed to target both extra- and intracellular areas of the ischemic heart by combining transactivating transcriptional activator peptide and monoclonal anti-myosin antibody 2G4 within a liposome carrier [40]. The complex was labeled with green fluorescent protein (GFP) and injected IV 30 minutes after occlusion of the coronary artery and infused for over 10 minutes. Accumulation and higher GFP expression were observed in the infarct up to 48 hours after injection compared to groups treated with liposomes complexed with a nonspecific antibody. In a separate study, liposomes labeled with angiotensin were injected IV into infarcted mice 1, 4, and 7 days post-MI, and accumulation was observed mainly in the left ventricle for all time points 24 hours post-injection [41]. Liposomes conjugated with a nonspecific peptide sequence were designed as a negative control, however accumulation was also observed when injected up to 1 week post-injury. These results suggest that while the system was designed to specifically target the angiotensin receptor post-MI, the therapeutic delivery is mainly due to the EPR effect.
Polymeric nanoparticles have also been explored for targeting the acute MI. For example, a peptide-polymer system targeting ischemic myocardium was developed using a peptide sequence that was identified via phage display to have a high affinity for infarcted myocardium [42,43]. The particles were composed of a cystamine bisacrylamide-diamino hexane polymer carrier conjugated with the ischemic myocardium targeted peptide (IMTP) for targeting and D-9-arginine (D9) for increased transfection. Following IV injection into a rat MI model 20 minutes after reperfusion, successful targeting to the damaged region and increased gene expression was shown. In another study, tetravalent streptavidin was used as the nanoparticle core, which was conjugated with biotinylated IGF and biotinylated PEG-Hoechst [44]. Hoechst binds to DNA, which is released as cells undergo necrosis in acute MI. After IV injection in a mouse MI model, the delivery of IGF to the infarcted region was significantly higher for the Hoechst-containing complex than the unlabeled control [45]. Improved heart function was also demonstrated 28 days after injection.
Conclusion
Using nanomaterials with minimally invasive strategies to treat MI may reduce debilitating, late stage effects of cardiovascular disease. Injectable hydrogel networks, such as ECM hydrogels or self-assembling peptides, form nanofibrous scaffolds that can facilitate endogenous cell infiltration or therapeutic delivery. A number of additional scaffolds have been designed to treat MI, but many have not been fully characterized to examine their nanoscale properties nor have they been investigated in an in vivo model. Intramyocardial delivery of nanofibrous hydrogels, nanoparticles, or in combination with therapeutic molecules localizes treatments to the site of injury using catheter or surgical based injections. By delivering the materials through a catheter, invasive surgical procedures may be avoided, reducing patient recovery times and chance of infection. The infarct region is, however, unstable early after MI, and intramyocardial injections may increase the risk of ventricular rupture, resulting in safety concerns in acute MI patients. The concern using this approach may be addressed by delivery through IV injection or coronary infusion, as the leaky vasculature of the acute MI allows nanoparticles to enter the injured site. Hydrogel therapies are beginning to reach the clinic; however, translating nanoparticle therapies is likely to be a longer and more expensive process given the greater potential for off target effects [46]. The Food and Drug Administration recently issued guidelines for nanotechnology [8], which will be necessary to design safe and effective nanotherapeutics. Continued work and investment in this area, examining both safety and efficacy of the different materials and delivery approaches, will be critical to realize new nanomaterial based therapeutics for treating MI.
Highlights.
Delivery of nanomaterials to treat MI has the potential for clinical application.
Approaches for delivery include intracoronary, intramyocardial, and IV injection.
Biomaterials explored to treat MI include nanofibrous hydrogels and nanoparticles.
Acknowledgments
This work was supported by a NIH Director's Transformative Research Award (5R01HL117326) and the NHLBI (5R01HL113468). We would also like to acknowledge Dr. Roberto Gaetani, Dr. Andrea Luthi, Jean Wang, and Jessica Ungerleider for their valuable input during the preparation of this review. Dr. Christman is co-founder, board member, and holds equity interest in Ventrix, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, et al. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. Journal of Molecular and Cellular Cardiology. 2010;48(3):504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutton MGS, Sharpe N. Left ventricular remodeling after myocardial infarction - pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoutte D, Schellings M, Pinto Y, Heymans S. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: A temporal and spatial window. Cardiovascular Research. 2006;69(3):604–613. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomaterialia. 2011;7(1):1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Johnson TD, Christman KL. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opinion on Drug Delivery. 2013;10(1):59–72. doi: 10.1517/17425247.2013.739156. [DOI] [PubMed] [Google Scholar]; This review provides a comprehesive discussion on different strategies to deliver injectable biomaterials to an infarct.
- 7.van der Laan AM, Nahrendorf M, Piek JJ. Healing and adverse remodelling after acute myocardial infarction: Role of the cellular immune response. Heart. 2012;98(18):1384–1390. doi: 10.1136/heartjnl-2012-301623. [DOI] [PubMed] [Google Scholar]
- 8.Ungerleider JL, Christman KL. Concise review: Injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: Translational challenges and progress. Stem Cells Translational Medicine. 2014;3(9):1090–1099. doi: 10.5966/sctm.2014-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovascular research. 2002;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 10.Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C, Teillon J, Le Jan S, Bouleti C, Briois G, Philippe J, et al. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation. 2012;125(1):140–149. doi: 10.1161/CIRCULATIONAHA.111.049072. [DOI] [PubMed] [Google Scholar]
- 11.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, Petneházy Ö, Landa N, Feinberg MS, Konen E, Goitein O, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in swine. J Am Coll Cardiol. 2009;54(11):1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Frey N, Linke A, Süselbeck T, Müller-Ehmsen J, Vermeersch P, Schoors D, Rosenberg M, Bea F, Tuvia S, Leor J. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after st-elevation myocardial infarction: A first-in-man study. Circulation: Cardiovascular Interventions. 2014 doi: 10.1161/CIRCINTERVENTIONS.114.001478. DOI: 10.1161/circinterventions.114.001478. [DOI] [PubMed] [Google Scholar]
- 13.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Clares B, Ruiz MA, Gallardo V, Arias JL. Drug delivery to inflammation based on nanoparticles surface decorated with biomolecules. Current Medicinal Chemistry. 2012;19(19):3203–3211. doi: 10.2174/092986712800784676. [DOI] [PubMed] [Google Scholar]; The review discusses key design criteria that must be addressed with nanoparticle therapeutics, including biodistribution and effect on particle size. Lipid and polymeric-based systems are emphasized.
- 15.Tous E, Purcell B, Ifkovits JL, Burdick JA. Injectable acellular hydrogels for cardiac repair. Journal of cardiovascular translational research. 2011;4(5):528–542. doi: 10.1007/s12265-011-9291-1. [DOI] [PubMed] [Google Scholar]
- 16.Rane AA, Christman KL. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2011;58(25):2615–2629. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Rane AA, Chuang JS, Shah A, Hu DP, Dalton ND, Gu Y, Peterson KL, Omens JH, Christman KL. Increased infarct wall thickness by a bio-inert material is insufficient to prevent negative left ventricular remodeling after myocardial infarction. PLoS ONE. 2011;6(6):e21571. doi: 10.1371/journal.pone.0021571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGarvey JR, Pettaway S, Shuman JA, Novack CP, Zellars KN, Freels PD, Echols RL, Jr., Burdick JA, Gorman JH, 3rd, Gorman RC, Spinale FG. Targeted injection of a biocomposite material alters macrophage and fibroblast phenotype and function following myocardial infarction: Relation to left ventricular remodeling. The Journal of pharmacology and experimental therapeutics. 2014;350(3):701–709. doi: 10.1124/jpet.114.215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kharaziha M, Nikkhah M, Shin SR, Annabi N, Masoumi N, Gaharwar AK, Camci-Unal G, Khademhosseini A. PGS:Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials. 2013;34(27):6355–6366. doi: 10.1016/j.biomaterials.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venugopal J, Rajeswari R, Shayanti M, Sridhar R, Sundarrajan S, Balamurugan R, Ramakrishna S. Xylan polysaccharides fabricated into nanofibrous substrate for myocardial infarction. Materials Science & Engineering C-Materials for Biological Applications. 2013;33(3):1325–1331. doi: 10.1016/j.msec.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Bhaarathy V, Venugopal J, Gandhimathi C, Ponpandian N, Mangalaraj D, Ramakrishna S. Biologically improved nanofibrous scaffolds for cardiac tissue engineering. Materials science & engineering C, Materials for biological applications. 2014;44:268–277. doi: 10.1016/j.msec.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Lin YD, Ko MC, Wu ST, Li SF, Hu JF, Lai YJ, Harn HIC, Laio IC, Yeh ML, Yeh HI, Tang MJ, et al. A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomaterials Science. 2014;2(4):567–580. doi: 10.1039/c3bm60289c. [DOI] [PubMed] [Google Scholar]
- 23.Davis ME, Motion JPM, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang SG, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111(4):442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y-D, Yeh M-L, Yang Y-J, Tsai D-C, Chu T-Y, Shih Y-Y, Chang M-Y, Liu Y-W, Tang ACL, Chen T-Y, Luo C-Y, et al. Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation. 2010;122(11 suppl 1):S132–S141. doi: 10.1161/CIRCULATIONAHA.110.939512. [DOI] [PubMed] [Google Scholar]
- 25.Davis ME, Hsieh PCH, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proceedings of the National Academy of Sciences. 2006;103(21):8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Lin Y-D, Luo C-Y, Hu Y-N, Yeh M-L, Hsueh Y-C, Chang M-Y, Tsai D-C, Wang JN, Tang M-J, Wei EIH, Springer ML, et al. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Science Translational Medicine. 2012;4(146):146ra109. doi: 10.1126/scitranslmed.3003841. [DOI] [PubMed] [Google Scholar]; In this study, the nanofiber delivery system showed improvements in heart function in both small and large animal models.
- 27.Guo H, Cui G, Yang J, Wang C, Zhu J, Zhang L, Jiang J, Shao S. Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochemical and Biophysical Research Communications. 2012;424(1):105–111. doi: 10.1016/j.bbrc.2012.06.080. [DOI] [PubMed] [Google Scholar]
- 28.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30(29):5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, Kinsey AM, et al. Catheterdeliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. Journal of the American College of Cardiology. 2012;59(8):751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]; Catheter-based injections are less invasive than surgical based intramyocardial delivery, and therefore have greater translational applicablity. This work is the first demonstration of percutaneous transendocardial delivery of a nanofibrous hydrogel.
- 30.Seif-Naraghi SB, Horn D, Schup-Magoffin PJ, Christman KL. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomaterialia. 2012;8(10):3695–3703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Science Translational Medicine. 2013;5(173):173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates clinical applicability of a cardiac specific decellularized ECM hydrogel in a large animal model.
- 32.Sonya Sonnenberg AAR, Liu Cassie J., Rao Nikhil, Bajaj Vaibhav, Zhang Shirley, Braden Rebecca, Schup-Magoffin Pamela J., Kwan Oi Ling, DeMaria Anthony N., Cochran Jennifer R., Christman Karen L. Delivery of an engineered HGF fragment in an extracellular matrix-derived hydrogel prevents negative lv remodeling post-myocardial infarction. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.12.021. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Bastings MMC, Koudstaal S, Kieltyka RE, Nakano Y, Pape ACH, Feyen DAM, van Slochteren FJ, Doevendans PA, Sluijter JPG, Meijer EW, Chamuleau SAJ, et al. A fast pH-switchable and self-healing supramolecular hydrogel carrier for guided, local catheter injection in the infarcted myocardium. Advanced Healthcare Materials. 2014;3(1):70–78. doi: 10.1002/adhm.201300076. [DOI] [PubMed] [Google Scholar]; Designing materials that allow for catheter deliver will faciltate translation. In this study, a synthetic polymer was designed to be injectable via a transendocardial catheter.
- 34.Paul A, Hasan A, Al Kindi H, Gaharwar AK, Rao VTS, Nikkhah M, Shin SR, Krafft D, Dokmeci MR, Shum-Tim D, Khademhosseini A. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. Acs Nano. 2014;8(8):8050–8062. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Z-x, Mao L-l, Lian F, He J, Zhang W-t, Dai C-y, Xue S, Lu W-g, Zhu H-s. Cardioprotective activity of placental growth factor in a rat model of acute myocardial infarction: Nanoparticle-based delivery versus direct myocardial injection. Bmc Cardiovascular Disorders. 2014;14(53) doi: 10.1186/1471-2261-14-53. DOI:10.1186/1471-2261-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Chang MY, Yang YJ, Chang CH, Tang ACL, Liao WY, Cheng FY, Yeh CS, Lai JJ, Stayton PS, Hsieh PCH. Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. Journal of Controlled Release. 2013;170(2):287–294. doi: 10.1016/j.jconrel.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 37*.Paulis LE, Geelen T, Kuhlmann MT, Coolen BF, Schaefers M, Nicolay K, Strijkers GJ. Distribution of lipid-based nanoparticles to infarcted myocardium with potential application for mri-monitored drug delivery. Journal of Controlled Release. 2012;162(2):276–285. doi: 10.1016/j.jconrel.2012.06.035. [DOI] [PubMed] [Google Scholar]; Tuning nanoparticle size is a key parameter for enhancing retention in the heart. This work demonstrates differences in nanoparticle accumulation and distribution between two differently sized liposomes.
- 38.Scott RC, Rosano JM, Ivanov Z, Wang B, Chong PL-G, Issekutz AC, Crabbe DL, Kiani MF. Targeting VEGF-encapsulated immunoliposomes to MI heart improves vascularity and cardiac function. Faseb Journal. 2009;23(10):3361–3367. doi: 10.1096/fj.08-127373. [DOI] [PubMed] [Google Scholar]
- 39.Swyer TW, Strom J, Larson DF. Nanoparticle oxygen delivery to the ischemic heart. Perfusion. 2014;29(6):539–43. doi: 10.1177/0267659114534290. [DOI] [PubMed] [Google Scholar]
- 40.Ko YT, Hartner WC, Kale A, Torchilin VP. Gene delivery into ischemic myocardium by double-targeted lipoplexes with anti-myosin antibody and TAT peptide. Gene Therapy. 2009;16(1):52–59. doi: 10.1038/gt.2008.135. [DOI] [PubMed] [Google Scholar]
- 41.Dvir T, Bauer M, Schroeder A, Tsui JH, Anderson DG, Langer R, Liao R, Kohane DS. Nanoparticles targeting the infarcted heart. Nano Letters. 2011;11(10):4411–4414. doi: 10.1021/nl2025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Won YW, McGinn AN, Lee M, Bull DA, Kim SW. Targeted gene delivery to ischemic myocardium by homing peptide-guided polymeric carrier. Molecular Pharmaceutics. 2013;10(1):378–385. doi: 10.1021/mp300500y. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrates the applicability of an ischemic myocardium-targeted peptide-based nanoparticle system to treat MI. Authors report successful targeting to the injured area and enhanced gene expression using an IV delivery route.
- 43.Kanki S, Jaalouk DE, Lee S, Yu AY, Gannon J, Lee RT. Identification of targeting peptides for ischemic myocardium by in vivo phage display. Journal of molecular and cellular cardiology. 2011;50(5):841–848. doi: 10.1016/j.yjmcc.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dasari M, Lee S, Sy J, Kim D, Lee S, Brown M, Davis M, Murthy N. Hoechst-IR: An imaging agent that detects necrotic tissue in vivo by binding extracellular DNA. Organic Letters. 2010;12(15):3300–3303. doi: 10.1021/ol100923d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Khan RS, Martinez MD, Sy JC, Pendergrass KD, Che P-l, Brown ME, Cabigas EB, Dasari M, Murthy N, Davis ME. Targeting extracellular DNA to deliver IGF-1 to the injured heart. Scientific Reports. 2014;4(4257):1–7. doi: 10.1038/srep04257. [DOI] [PMC free article] [PubMed] [Google Scholar]; Methods to target and retain nanoparticles in the MI are likely to enhance therapeutic efficacy over passive targeting. In this study a DNA-binding, Hoechst-labeled delivery system was used to target necrotic cardiomyocytes in the acute MI and deliver relatively high concentrations of IGF.
- 46.Eifler A, Thaxton CS. Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. In: Hurst SJ, editor. Biomedical nanotechnology. Vol. 726. Humana Press; 2011. pp. 325–338. [DOI] [PubMed] [Google Scholar]