Abstract
The steady state compositions of omega-6 and omega-3 polyunsaturated fatty acids (PUFA) throughout the various viscera and tissues within the whole body of rats have not previously been described in a comprehensive manner. Dams consumed diets containing 10 wt% fat (15% linoleate and 3% α-linolenate). Male offspring (n=9) at 7-wks of age were euthanized and dissected into 25 compartments. Total lipid fatty acids for each compartment were quantified by GC/FID and summed for the rat whole body; total n-6 PUFA was 12 wt% and total n-3 PUFA was 2.1% of total fatty acids. 18:2n-6 accounted for 84% of the total n-6 PUFA, 20:4n-6 was 12%, 18:3n-3 was 59% of the total n-3 PUFA, 20:5n-3 was 2.1%, and 22:6n-3 was 32%. The white adipose tissue contained the greatest amounts of 18:2n-6 (1.5 g) and 18:3n-3 (0.2 g). 20:4n-6 was highest in muscle (60 mg) and liver (57 mg), while 22:6n-3 was greatest in muscle (46 mg), followed by liver (27 mg) and carcass (20 mg). In terms of fatty acid composition expressed as a percentage, 18:2n-6 was the highest in the heart (13 wt%), while 18:3n-3 was about 1.3 wt% for skin, white adipose tissue and fur. 20:4n-6 was highest (21–25 wt%) in the circulation, kidney, and spleen, while 22:6n-3 was highest in the brain (12 wt%), followed by the heart (7.9 wt%), liver (5.9 wt%), and spinal cord (5.1 wt%). Selectivity was greatest when comparing 22:6n-3 in brain (12%) to white adipose (0.08%) (68-fold) and 22:5n-6 in testes (15.6%) compared to white adipose (0.02%), 780-fold.
Keywords: alpha-linolenic acid, linoleic acid, docosahaexenoic acid, arachidonic acid, n-3, n-6
1. INTRODUCTION
Omega-6 (n-6) and omega-3 (n-3) polyunsaturated fatty acids (PUFA) are essential fatty acids (EFA) and play a vital role in cellular and physiological functions [1, 2]. They serve key functions in various organ systems and contribute to growth and development, cardiovascular health, immune responses, psychological health as well as the prevention for many diseases [3]. The precursors, both linoleic acid and α-linolenic acid, cannot be synthesized de novo in animals, but must be supplied from the diet. Their longer chain and more unsaturated metabolites are then synthesized from their respective precursors though the synthesis rates are quite low [4, 5].
Despite decades of interest in essential fatty acids, little is known about the complete profiles of PUFA distribution within viscera and tissues throughout the whole body. It has long been appreciated that particular PUFAs are selectively concentrated in particular organs and tissues, some examples being linoleic acid enrichment in liver [6, 7], α-linolenic acid enrichment in skin and fur [8], docosahaexenoic acid (DHA) in brain [9], and n-6 docosapentaenoic acid (DPAn-6) in testes [10]. Many studies have described PUFA profiles in major organs in young rats [11] such as, rat blood, muscle and some viscera [12], as well as autopsy studies in human subjects [13]. In addition, some studies have investigated the PUFA as a whole in animals with a variety of methods, applying whole body balance methods to determine the oxidation of two precursors [14–16].
Our previous stable isotope tracer study [10] described the uptake of precursors, deuterated-18:2n-6 and -18:3n-3, as well as their deuterated metabolites de novo into viscera and other tissues in rats. However, to our knowledge, a thorough and quantitative description of the endogenous fatty acid composition in various compartments throughout the whole body of any mammal has not been conducted. In this steady state study, fatty acids ranging from C10 to C24, including the saturated, monounsaturated, n-6 and n-3 PUFA, were quantified in 25 compartments of the rat body, detailing both essential PUFA families. We investigated rats that were fed to equilibrium on a defined diet and quantified the tissue selectivity for each tissue when the same fatty acid substrates were available to the tissues via the diet.
2. MATERIALS & METHODS
2.1 Animals, diet, and tissue collection
All animal procedures were carried out in accordance with the NIH animal care and welfare guidelines; the protocol was approved by the NIAAA Animal Care and Use Committee. The animals in this study were the same as those used in a previous study [10] on PUFA metabolism using a stable isotope tracer technique coupled with a GC/MS negative chemical ionization assay. The details of the animals, diets and tissue collection were thus reported previously in Lin et al [10]. Briefly, male Long-Evans hooded rats were weaned onto the same defined, pelleted custom diet as their parents, which was modified from the AIN-93G formulation [17] as previously described as an n-3 adequate diet [18]. Lipid extracted casein was used; carbohydrate sources were modified and fat sources (10 wt %) were controlled. Fat sources were 77 g of hydrogenated coconut oil, 18 g of safflower oil and 5 g flaxseed oil per kg of diet. The fatty acid distribution of the diet was as follows: 77% saturates, 4% monounsaturates, 15% linoleate, 3% α-linolenate and only traces of longer chain C20 and C22 EFAs. The animals were euthanized and the tissues were dissected out into 25 compartments when the animals were seven to eleven weeks of age, with a mean body weight of 246 ± 25 g (mean ± SEM).
2.2 Chemicals
Methanol and chloroform were purchased from Burdick & Jackson (Muskegon, MI); hexane from EMD chemicals Inc. (Gibbstown, NJ); boron trifluoride in methanol (14 g/L) was from Sigma Chemical (St. Louis, MO); docosatrienoic ethyl ester (22:3n-3) and the GC reference standard GLC–462 were purchased from Nu–Chek Prep (Elysian, MN). All chemicals were of analytical grade and used without further purification.
2.3 Homogenization and lipid extraction of various tissues and derivatization reactions
The processing of tissues was as previously described [10]. In brief, large organs/tissues were dissected and thoroughly diced into fine pieces at 4°C prior to extraction. Organs were homogenized in methanol containing 0.2 mM BHT (10 mL for 1 g of tissue). One aliquot of homogenate (about 100 mg of tissue wet weight) was used for total lipid extraction, following a Folch total fatty acid lipid extraction [19]. The total lipid extract was dried under a stream of nitrogen and transmethylated using boron trifluoride in methanol as described by Morrison and Smith [20] and modified by Salem et al [21]. The internal standard, 22:3n-3 ethyl ester (20 µg) was added to each sample prior to lipid extraction.
2.4. Gas liquid chromatographic analysis
HP-5890 (series II) gas chromatograph (Hewlett-Packard, Palo Alto, CA) coupled with a flame ionization detector was employed to quantify fatty acids. An aliquot of fatty acid methyl ester (FAME) from each sample was injected onto a DB-FFAP fused silica capillary column (30 m × 0.25 mm i.d. × 0.25 µm, J&W Scientific, Folsom, CA) through a split/splitless inlet (50:1). The oven temperature was programmed as previously reported [22]. A reference standard GLC-462 containing 28 fatty acid methyl esters was used to identify the retention time of FAME peaks on GC chromatograms. PUFA in a mixture of adrenal gland, thyroid gland and mandibular lymph nodes (ATL), and bladder were quantified employing gas chromatography-mass spectrometry, negative chemical ionization as reported previously [10].
2.5. Calculation
Unless indicated in the text, data were expressed as mean ± SEM (n=9) in concentrations, µg of fatty acid per mg of wet tissue weight (µg/mg) or per mL of plasma or red blood cell (µg/mL), or the proportion of each fatty acid in the weight of the total fatty acids in each sample (wt%). The concentrations were calculated by comparing the integrated areas of each fatty acid peak in the gas chromatograms with that of the known amount of internal standard (22:3n-3) added in the sample prior to total lipid extraction. The total amount of each fatty acid in rat whole body was summed from the values from all compartments and the carcass.
3. RESULTS
3.1. Fatty acid profiles of rat whole body
The fatty acid compositions in the rat whole body are shown in Table 1 and Figure 1. The average, total amount of identified fatty acids in each rat were 20.6 ± 4.4 g (n = 9). The rat whole body was composed primarily of saturated fatty acids (48.4 wt% of total fatty acids), whilst monounsaturated fatty acids were present in the second greatest amount (34.8%). Within these fatty acid categories, 16:0 (25.8%) and 18:1n-9 (24.0%) were present in the highest amounts, respectively. For the polyunsaturates, the total n-6 PUFA (12.0%) was more than 5-fold greater than the total n-3 PUFA (2.1%) in the whole rat body tissue. The n-6 PUFA with the highest content was 18:2n-6 (10.1%), followed by 20:4n-6 (1.4%). The n-3 PUFA with the highest fatty acid composition was 18:3n-3 (1.2%), followed by the end product 22:6n-3 (0.66%). Despite five-fold more 18:2n-6 in the diet compared to 18:3n-3, the end product of n-6 PUFA metabolism, 22:5n-6 was over 7-fold more abundant (0.09 wt%) compared to the end product of n-3 PUFA metabolism, 22:6n-3 (0.66 wt%). In contrast, little EPA (20:5n-3) was found (0.04%) in comparison to 20:4n-6 (1.45%). The selectivity of individual n-6 and n-3 PUFA in the 25 tissue compartments are presented in the following tables and figures with varying measures.
Table 1.
Fatty acid composition in rat whole body (wt%)
| Fatty acids | Mean | SEM | |
|---|---|---|---|
| 10:0 | 0.53 | ± | 0.06 |
| 12:0 | 9.08 | ± | 0.56 |
| 14:0 | 7.93 | ± | 0.49 |
| 16:0 | 25.8 | ± | 0.3 |
| 18:0 | 4.87 | ± | 0.09 |
| 20:0 | 0.08 | ± | 0.00 |
| 22:0 | 0.04 | ± | 0.00 |
| 24:0 | 0.09 | ± | 0.01 |
| 14:1 | 0.54 | ± | 0.02 |
| 16:1n-7 | 6.92 | ± | 0.22 |
| 18:1n-9 | 24.0 | ± | 1.0 |
| 18:1n-7 | 3.05 | ± | 0.16 |
| 20:1n-9 | 0.17 | ± | 0.01 |
| 22:1n-9 | 0.02 | ± | 0.00 |
| 24:1n-9 | 0.15 | ± | 0.02 |
| 18:2n-6 | 10.1 | ± | 0.30 |
| 18:3n-6 | 0.06 | ± | 0.01 |
| 20:2n-6 | 0.09 | ± | 0.01 |
| 20:3n-6 | 0.09 | ± | 0.01 |
| 20:4n-6 | 1.45 | ± | 0.07 |
| 22:4n-6 | 0.14 | ± | 0.01 |
| 22:5n-6 | 0.09 | ± | 0.01 |
| 18:3n-3 | 1.23 | ± | 0.03 |
| 20:5n-3 | 0.04 | ± | 0.01 |
| 22:5n-3 | 0.14 | ± | 0.01 |
| 22:6n-3 | 0.66 | ± | 0.03 |
| 20:3n-9 | 0.09 | ± | 0.00 |
| Summary | |||
| Saturated | 48.4 | ± | 0.9 |
| Monounsaturated | 34.8 | ± | 1.3 |
| n-6 PUFA | 12.0 | ± | 0.3 |
| n-3 PUFA | 2.07 | ± | 0.05 |
| n-6% HUFA | 67.6 | ± | 0.5 |
| n-6/n-3 PUFA | 5.8 | ± | 0.1 |
| Total identified fatty acids | 97.4 | ± | 0.2 |
| Total fatty acids (g) | 20.6 | ± | 4.4 |
Footnote: data were presented as mean ± SEM (n=8–9). “0.00” indicates values were less than 0.005. Data were not included for saturated and monounsaturated fatty acids in adrenal gland, thyroid gland, mandibular lymph nodes, and bladder. The total fatty acid accounted for all fatty acid detected by gas chromatography. HUFA was defined as fatty acids with 20 or more carbons and at least 3 carbon-carbon double bonds, including 20:3n-6, 20:4n-6, 22:4n-6, 22:5n-6, 20:5n-3, 22:5n-3, and 22:6n-3.
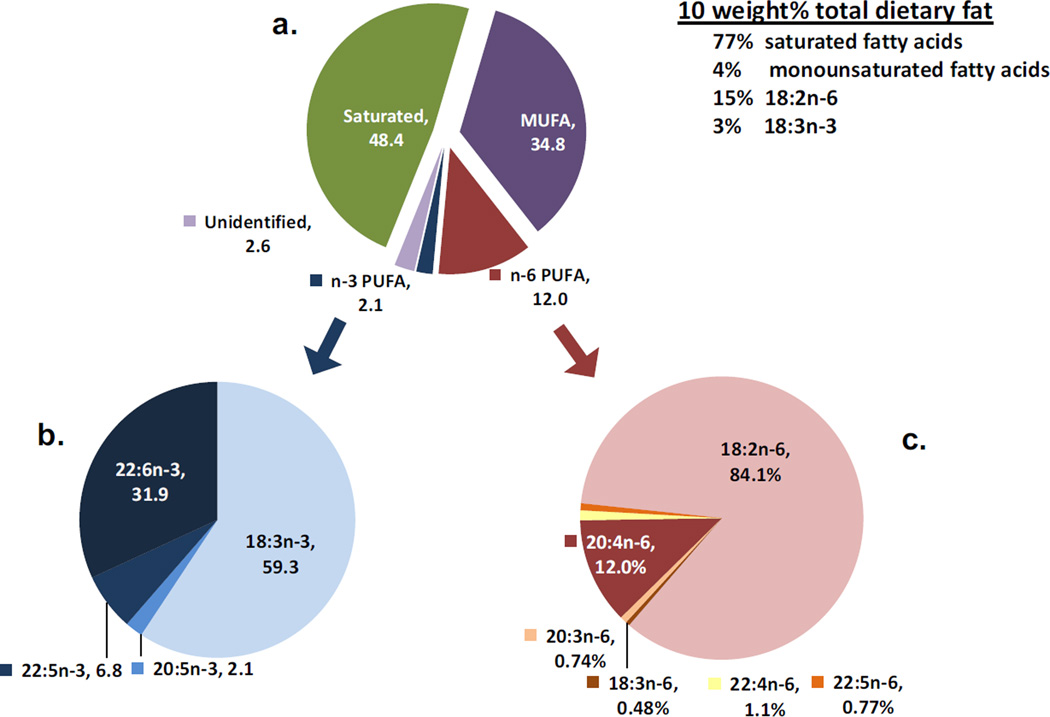
Figure 1.
Proportions of various species of fatty acids in rat whole body. 1a) categorized fatty acids; 1b) major n-3 PUFA as % of total n-3 PUFA; 1c) major n-6 PUFA as % of total n-6 PUFA. Data were presented as an average of nine animals except eight for data from brown adipose tissue, bladder, and heart.
3.2. The weight percentage of individual PUFA in each compartment
The major n-6 and n-3 PUFAs, for each compartment, expressed as weight percent of total fatty acids are shown in Table 2. For the n-6 PUFA, the tissue with the highest wt% in 18:2n-6 was heart (12.9%), followed by skin (11.8%). Carcass, salivary gland, muscle, and bone were slightly lower at 11 wt%. Plasma, pancreas, adipose white, and fur were all about 10 wt%. Eye, spinal cord, and brain had substantially lower 18:2n-6 (1%) than the other tissues. The tissues with the highest 20:4n-6 were plasma (25.3%), followed by kidney, red blood cells, and spleen, which ranged from 23.4–18.7% while brown adipose, white adipose and eye contained only very low amounts (< 1.0%). The n-6 end product of metabolism, 22:5n-6, was markedly greater in the testes (15.6%) in comparison with the remaining tissues (Figure 2) especially white adipose (0.02%), a 780-fold difference. The second highest weight percentage for 22:5n-6 was heart tissue (1.0%), which was about 15-fold lower than that observed in the testes.
Table 2.
PUFA profiles in each compartment (weight % of total fatty acids)
| 18:2n-6 | 18:3n-6 | 20:2n-6 | 20:3n-6 | 20:4n-6 | 22:4n-6 | 22:5n-6 | n-6PUFA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADB | 6.60 | ± | 0.72 | 0.02 | ± | 0.00 | 0.11 | ± | 0.01 | 0.13 | ± | 0.03 | 0.96 | ± | 0.17 | 0.20 | ± | 0.04 | 0.09 | ± | 0.02 | 8.12 | ± | 1.04 |
| ADW | 10.0 | ± | 0.30 | 0.05 | ± | 0.01 | 0.08 | ± | 0.01 | 0.04 | ± | 0.00 | 0.25 | ± | 0.01 | 0.04 | ± | 0.00 | 0.02 | ± | 0.00 | 10.52 | ± | 0.31 |
| Bone | 10.6 | ± | 0.38 | 0.06 | ± | 0.00 | 0.19 | ± | 0.01 | 0.43 | ± | 0.02 | 7.35 | ± | 0.18 | 1.01 | ± | 0.03 | 0.44 | ± | 0.02 | 20.12 | ± | 0.56 |
| Brain | 0.58 | ± | 0.03 | 0.00 | ± | 0.00 | 0.16 | ± | 0.00 | 0.42 | ± | 0.01 | 8.39 | ± | 0.11 | 2.82 | ± | 0.02 | 0.60 | ± | 0.02 | 12.98 | ± | 0.15 |
| Carcass | 11.3 | ± | 0.38 | 0.06 | ± | 0.01 | 0.16 | ± | 0.01 | 0.44 | ± | 0.03 | 6.96 | ± | 0.41 | 0.78 | ± | 0.05 | 0.41 | ± | 0.03 | 20.13 | ± | 0.80 |
| Eye | 1.43 | ± | 0.13 | 0.17 | ± | 0.03 | 0.00 | ± | 0.00 | 0.10 | ± | 0.01 | 0.90 | ± | 0.09 | 0.17 | ± | 0.02 | 0.05 | ± | 0.01 | 2.82 | ± | 0.21 |
| Fur | 9.57 | ± | 0.47 | 0.05 | ± | 0.01 | 0.10 | ± | 0.01 | 0.13 | ± | 0.01 | 1.32 | ± | 0.09 | 0.33 | ± | 0.02 | 0.10 | ± | 0.01 | 11.60 | ± | 0.49 |
| Heart | 12.9 | ± | 0.79 | 0.03 | ± | 0.00 | 0.12 | ± | 0.01 | 0.43 | ± | 0.03 | 18.7 | ± | 0.67 | 0.92 | ± | 0.03 | 0.99 | ± | 0.05 | 34.07 | ± | 1.11 |
| Intestine | 6.44 | ± | 0.24 | 0.05 | ± | 0.00 | 0.21 | ± | 0.02 | 0.84 | ± | 0.08 | 12.3 | ± | 0.86 | 1.99 | ± | 0.13 | 0.37 | ± | 0.02 | 22.17 | ± | 1.12 |
| Kidney | 6.20 | ± | 0.32 | 0.06 | ± | 0.01 | 0.16 | ± | 0.01 | 0.79 | ± | 0.03 | 23.4 | ± | 1.01 | 0.52 | ± | 0.01 | 0.19 | ± | 0.01 | 31.37 | ± | 0.85 |
| Liver | 7.86 | ± | 0.98 | 0.20 | ± | 0.05 | 0.11 | ± | 0.02 | 0.32 | ± | 0.05 | 12.2 | ± | 1.43 | 0.28 | ± | 0.05 | 0.32 | ± | 0.03 | 21.28 | ± | 1.85 |
| Lung | 5.60 | ± | 0.09 | 0.07 | ± | 0.00 | 0.16 | ± | 0.01 | 0.43 | ± | 0.02 | 11.1 | ± | 0.36 | 2.12 | ± | 0.08 | 0.39 | ± | 0.02 | 19.89 | ± | 0.40 |
| Muscle | 10.7 | ± | 0.37 | 0.05 | ± | 0.00 | 0.10 | ± | 0.01 | 0.26 | ± | 0.04 | 3.82 | ± | 0.49 | 0.29 | ± | 0.04 | 0.29 | ± | 0.04 | 15.50 | ± | 0.88 |
| Pancreas | 10.1 | ± | 0.35 | 0.28 | ± | 0.02 | 0.13 | ± | 0.01 | 0.61 | ± | 0.06 | 17.6 | ± | 1.26 | 0.61 | ± | 0.06 | 0.41 | ± | 0.03 | 29.75 | ± | 1.51 |
| Plasma | 10.2 | ± | 0.42 | 0.22 | ± | 0.04 | 0.09 | ± | 0.01 | 0.44 | ± | 0.05 | 25.3 | ± | 1.64 | 0.34 | ± | 0.05 | 0.45 | ± | 0.04 | 37.05 | ± | 1.55 |
| RBC | 6.15 | ± | 0.27 | 0.06 | ± | 0.01 | 0.20 | ± | 0.01 | 0.56 | ± | 0.03 | 23.4 | ± | 0.42 | 1.43 | ± | 0.09 | 0.71 | ± | 0.02 | 32.54 | ± | 0.65 |
| SG | 10.8 | ± | 0.30 | 0.22 | ± | 0.01 | 0.27 | ± | 0.01 | 2.98 | ± | 0.15 | 16.5 | ± | 0.27 | 0.57 | ± | 0.01 | 0.37 | ± | 0.02 | 31.63 | ± | 0.65 |
| Skin | 11.8 | ± | 0.29 | 0.06 | ± | 0.01 | 0.09 | ± | 0.01 | 0.08 | ± | 0.01 | 1.29 | ± | 0.14 | 0.15 | ± | 0.02 | 0.05 | ± | 0.00 | 13.49 | ± | 0.37 |
| SPC | 0.78 | ± | 0.03 | 0.01 | ± | 0.00 | 0.39 | ± | 0.01 | 0.70 | ± | 0.02 | 4.55 | ± | 0.07 | 1.95 | ± | 0.04 | 0.21 | ± | 0.01 | 8.59 | ± | 0.09 |
| Spleen | 4.59 | ± | 0.17 | 0.03 | ± | 0.01 | 0.41 | ± | 0.02 | 0.82 | ± | 0.04 | 22.1 | ± | 0.30 | 2.69 | ± | 0.07 | 0.60 | ± | 0.02 | 31.23 | ± | 0.31 |
| Stomach | 7.97 | ± | 0.43 | 0.07 | ± | 0.01 | 0.10 | ± | 0.01 | 0.38 | ± | 0.16 | 5.34 | ± | 1.00 | 0.46 | ± | 0.08 | 0.13 | ± | 0.02 | 14.45 | ± | 1.42 |
| Testes | 4.31 | ± | 0.14 | 0.11 | ± | 0.01 | 0.18 | ± | 0.01 | 1.04 | ± | 0.03 | 16.4 | ± | 0.31 | 2.52 | ± | 0.09 | 15.6 | ± | 0.78 | 40.15 | ± | 0.75 |
| Thymus | 5.12 | ± | 0.19 | 0.03 | ± | 0.00 | 0.62 | ± | 0.04 | 0.74 | ± | 0.06 | 14.6 | ± | 0.70 | 1.60 | ± | 0.09 | 0.28 | ± | 0.01 | 23.04 | ± | 0.96 |
| 18:3n-3 | 20:5n-3 | 22:5n-3 | 22:6n-3 | n-3PUFA | n-6%HUFA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADB | 0.54 | ± | 0.04 | 0.04 | ± | 0.01 | 0.14 | ± | 0.04 | 0.38 | ± | 0.08 | 1.10 | ± | 0.17 | 72.3 | ± | 1.1 |
| ADW | 1.33 | ± | 0.03 | 0.01 | ± | 0.00 | 0.03 | ± | 0.00 | 0.08 | ± | 0.01 | 1.46 | ± | 0.03 | 74.5 | ± | 0.7 |
| Bone | 0.68 | ± | 0.03 | 0.15 | ± | 0.02 | 0.93 | ± | 0.08 | 3.54 | ± | 0.13 | 5.30 | ± | 0.20 | 66.7 | ± | 0.6 |
| Brain | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.17 | ± | 0.01 | 12.2 | ± | 0.10 | 12.43 | ± | 0.11 | 49.6 | ± | 0.2 |
| Carcass | 0.82 | ± | 0.03 | 0.15 | ± | 0.02 | 0.83 | ± | 0.07 | 3.50 | ± | 0.24 | 5.30 | ± | 0.28 | 65.8 | ± | 0.7 |
| Eye | 0.14 | ± | 0.02 | 0.10 | ± | 0.01 | 0.08 | ± | 0.01 | 0.77 | ± | 0.09 | 1.09 | ± | 0.09 | 56.5 | ± | 1.0 |
| Fur | 1.26 | ± | 0.06 | 0.03 | ± | 0.00 | 0.11 | ± | 0.01 | 0.36 | ± | 0.03 | 1.76 | ± | 0.08 | 79.3 | ± | 0.9 |
| Heart | 0.25 | ± | 0.02 | 0.15 | ± | 0.01 | 1.18 | ± | 0.09 | 7.85 | ± | 0.49 | 9.42 | ± | 0.49 | 69.7 | ± | 0.6 |
| Intestine | 0.43 | ± | 0.04 | 0.23 | ± | 0.03 | 0.69 | ± | 0.06 | 2.08 | ± | 0.13 | 3.43 | ± | 0.17 | 83.7 | ± | 0.5 |
| Kidney | 0.26 | ± | 0.03 | 0.42 | ± | 0.04 | 0.48 | ± | 0.04 | 2.78 | ± | 0.07 | 3.94 | ± | 0.14 | 87.0 | ± | 0.7 |
| Liver | 0.53 | ± | 0.12 | 0.30 | ± | 0.05 | 0.55 | ± | 0.08 | 5.85 | ± | 0.65 | 7.24 | ± | 0.72 | 65.9 | ± | 1.0 |
| Lung | 0.41 | ± | 0.02 | 0.29 | ± | 0.02 | 1.03 | ± | 0.07 | 1.93 | ± | 0.06 | 3.66 | ± | 0.10 | 81.2 | ± | 0.5 |
| Muscle | 0.82 | ± | 0.05 | 0.11 | ± | 0.02 | 0.65 | ± | 0.11 | 2.81 | ± | 0.38 | 4.40 | ± | 0.45 | 56.9 | ± | 0.8 |
| Pancreas | 0.36 | ± | 0.04 | 1.52 | ± | 0.12 | 0.59 | ± | 0.06 | 2.40 | ± | 0.17 | 4.88 | ± | 0.29 | 80.9 | ± | 0.7 |
| Plasma | 0.51 | ± | 0.05 | 0.60 | ± | 0.08 | 0.59 | ± | 0.09 | 4.60 | ± | 0.32 | 6.30 | ± | 0.41 | 82.0 | ± | 1.0 |
| RBC | 0.13 | ± | 0.02 | 0.39 | ± | 0.04 | 1.68 | ± | 0.15 | 4.32 | ± | 0.13 | 6.52 | ± | 0.27 | 80.4 | ± | 0.7 |
| SG | 0.29 | ± | 0.02 | 0.89 | ± | 0.05 | 0.71 | ± | 0.04 | 2.17 | ± | 0.07 | 4.06 | ± | 0.12 | 84.4 | ± | 0.4 |
| Skin | 1.34 | ± | 0.02 | 0.03 | ± | 0.00 | 0.10 | ± | 0.01 | 0.26 | ± | 0.03 | 1.72 | ± | 0.04 | 80.6 | ± | 0.4 |
| SPC | 0.03 | ± | 0.00 | 0.04 | ± | 0.00 | 0.28 | ± | 0.01 | 5.09 | ± | 0.14 | 5.44 | ± | 0.15 | 57.9 | ± | 0.6 |
| Spleen | 0.11 | ± | 0.01 | 0.28 | ± | 0.02 | 1.52 | ± | 0.09 | 2.59 | ± | 0.06 | 4.50 | ± | 0.11 | 85.6 | ± | 0.4 |
| Stomach | 0.78 | ± | 0.06 | 0.13 | ± | 0.02 | 0.19 | ± | 0.03 | 0.56 | ± | 0.09 | 1.65 | ± | 0.12 | 87.6 | ± | 0.7 |
| Testes | 0.06 | ± | 0.02 | 0.09 | ± | 0.01 | 0.11 | ± | 0.01 | 2.61 | ± | 0.17 | 2.86 | ± | 0.20 | 92.7 | ± | 0.6 |
| Thymus | 0.28 | ± | 0.02 | 0.13 | ± | 0.02 | 0.38 | ± | 0.04 | 0.77 | ± | 0.04 | 1.57 | ± | 0.07 | 93.1 | ± | 0.3 |
Footnote: data were presented as mean ± SEM (n=8–9). Data for adrenal gland (ATL) and bladder were not available. “0.0” indicates values were less than 0.05, “0.00” indicates values were less than 0.005.
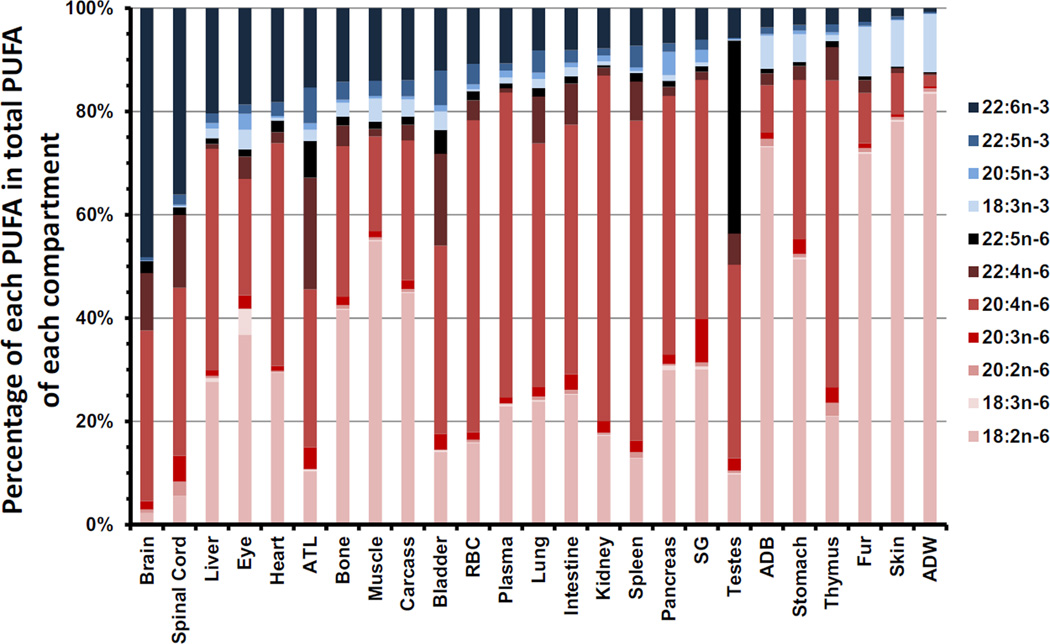
Figure 2.
The weight percentage of individual n-6 and n-3 PUFA (wt%) in each compartment. The compartment percentages of DHA are decreasing from left to right. N-3 PUFA are depicted in shades of blue, whereas n-6 are in red/black. Data were presented as the mean of nine animals. ATL, adrenal gland, thyroid gland and mandibular lymph nodes; RBC, red blood cell; SG, salivary gland; ADB, brown adipose tissue; ADW, white adipose tissue
Regarding n-3 PUFAs, 18:3n-3 had a considerably lower tissue content compared to 18:2n-6. The highest contents of 18:3n-3 were observed in skin, white adipose, and fur (all were 1.3%). The remaining tissues all contained less than 1.0 wt% of 18:3n-3. The only tissue that had 20:5n-3 in an amount greater than 1.0% was pancreas (1.5%). 22:5n-3 was also not abundant: red blood cell, spleen, heart, and lung had the highest values which were between 1.0–1.7%. However, 22:6n-3 was highest in the brain (12.2%), followed by heart (7.8%), then liver, spinal cord, plasma, and red blood cell, which were in the range of 5.8–4.3%. Brain tissue contained more than 7-fold as much 22:6n-3 than did tissues with the maximal levels of the other long chain n-3 PUFAs, e.g., 20:5n-3 (pancreas: 1.5%) and 22:5n-3 (red blood cell: 1.7%). N-6 highly unsaturated fatty acids (HUFA%) were greater than the n-3 HUFA% in every tissue except the brain (n-3 HUFA, 50.4 %). Eye, muscle, and spinal cord also had high levels of n-3 HUFA% with values between 43–42%. The ratio of 20:4n-6 to 20:5n-3 was greatest in brain (839), but was also very high in testes (11.5), heart, thymus, and spinal cord. In contrast the only tissue that had more 22:5n-6 than 22:6n-3 was the testes (6). The lowest ratios of 22:5n-6 compared to 22:6n-3 (greater relative abundance of 22:6n-3) were observed in the spinal cord, brain, liver, kidney, and eye (all below 0.1).
3.3. Percentage of individual PUFA in total n-6 and n-3 PUFA
Another perspective regarding the wt% of individual n-6 and n-3 PUFAs in each compartment is presented in Figure 2. The 25 tissues were listed from the highest DHA (expressed as the wt% of total PUFAs) content to the lowest. The highest values for 18:2n-6 were found in adipose white tissue (83.5%), skin (78.0%), adipose brown tissue (73.1%), fur (71.8%), muscle (54.9%), and stomach (51.4%). Only testes, spinal cord, and brain were found to have less than 10% of their PUFA content as 18:2n-6. 18:3n-6 was found in the highest proportion of PUFAs in the eye (5.0%) which was about 6-fold greater than the next highest tissue, which was the pancreas (0.8%). There was no 18:3n-6 detected in the brain. 20:2n-6 was highest in the spinal cord and thymus, at levels above 2.4% of PUFAs. However, there was no 20:2n-6 detected in the eye, ATL or bladder. 20:3n-6 was greatest in the salivary gland (8.4%), followed by the spinal cord, ATL tissues, bladder, intestine, and thymus, which were between 3.0–5.0%. 20:4n-6 occurred in amounts above 50% in the kidney, spleen, red blood cell, thymus, plasma, and pancreas, but was less than 10% in the fur, adipose brown tissue, skin and adipose white tissue. 22:4n-6 was highest in the ATL tissues (21.6%), followed by the bladder, spinal cord, and brain, which ranged from 11.1–17.8%. The testes showed a very high accretion of 22:5n-6 (37.4%) compared to the other tissues. The testes were unique in that 22:5n-6 was observed as the most abundant PUFA. An interesting observation was that the testes had the lowest amount (0.2%) of 22:5n-3 yet its n-6 PUFA isomer, 22:5n-6, accreted in the greatest amount. The second highest value for accretion of 22:5n-6 was found in the ATL tissues (7.1%), which were more than 5-fold lower than in the testes, followed by the bladder (4.6%), which was about 8-fold lower than that accreted in the testes. 18:3n-3 was highest in the adipose white tissue (11.3%) followed by the fur (9.5%) and skin (8.9%). The lowest amount of 18:3n-3 was found in the brain (<0.1%). 20:5n-3 was highest in the pancreas (4.5%), eye (3.1%) and salivary gland (2.4%), and was once again lowest in the brain (<0.1%). 22:5n-3 was detected in the greatest amount in the ATL tissues, bladder, red blood cell, lung, and spleen, which ranged between 4.2–6.9%. Finally, 22:6n-3 was found in the greatest amounts in the brain (48.2%), spinal cord (36.1%), liver (20.4%), eye (18.6%) and heart (18.2%). The thymus, fur, skin, and white adipose tissue had the lowest proportion of 22:6n-3, which ranged between 0.7 and 3.1%. When comparing the 22:6n-3 as the % of total PUFA from tissue to the lowest, there was a 68 fold higher amount in brain compared to adipose white tissues, demonstrating very marked tissue selectivity.
3.4. Total amount of individual PUFA in each compartment
The total amounts (mg) of individual PUFA in each compartment within the rat body are shown in Table 3. 18:2n-6 was found in the highest amounts (in mg/compartment) in the white adipose tissue (1506), followed by skin (234), and muscle (180). 18:3n-6, 20:2n-6, and 20:3n-6 were all found in the highest amounts in the adipose white tissue (6.9, 12.2, and 5.7, respectively). 20:4n-6 was found highest in muscle (60), then liver (57), adipose white (39), and carcass (38). 22:4n-6 was found at the highest level in adipose white (7.1), muscle (4.7), and carcass (4.2); this was similar to the tissues that 22:5n-6 was found in highest amounts: muscle, adipose white, and carcass, but also the testes, which were between 2.3 and 4.7. 18:3n-3 was highest in: adipose white (204), skin (26.8) and muscle (14.7). 20:5n-3 was also found in the highest amount in adipose white, followed by muscle and liver, which were between 1.4 and 2.1. Once elongated to 22:5n-3, this fatty acid was then highest in muscle (9.7), adipose white (4.9), carcass (4.4), and liver (2.5). The end product of n-3 PUFA metabolism, 22:6n-3, was greatest in muscle (45.9), followed by liver (27.0), carcass (19.7), white adipose (13.0), brain (6.3) and bone (4.2). The total amount of all fatty acids was greatest in the white adipose (15 g), followed by tissues of about 2 g or less, such as skin (2.0), muscle (1.7), carcass (0.57), and liver (0.49).
Table 3.
Total amount of PUFA in each compartment (mg)
| 18:2n-6 | 18:3n-6 | 20:2n-6 | 20:3n-6 | 20:4n-6 | 22:4n-6 | 22:5n-6 | n-6PUFA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADB | 6.05 | ± | 1.77 | 0.02 | ± | 0.00 | 0.11 | ± | 0.05 | 0.10 | ± | 0.03 | 0.76 | ± | 0.16 | 0.19 | ± | 0.08 | 0.07 | ± | 0.03 | 7.30 | ± | 2.1 |
| ADW | 1506 | ± | 371 | 6.90 | ± | 0.85 | 12.2 | ± | 2.80 | 5.72 | ± | 1.10 | 39.2 | ± | 10.1 | 7.12 | ± | 2.15 | 3.46 | ± | 1.01 | 1581 | ± | 389 |
| ATL | 0.03 | ± | 0.01 | 0.00 | ± | 0.00 | 0.00 | ± | 0.00 | 0.01 | ± | 0.00 | 0.10 | ± | 0.01 | 0.07 | ± | 0.01 | 0.02 | ± | 0.00 | 0.25 | ± | 0.0 |
| Bladder | 0.04 | ± | 0.01 | 0.00 | ± | 0.00 | 0.00 | ± | 0.00 | 0.01 | ± | 0.00 | 0.11 | ± | 0.02 | 0.05 | ± | 0.01 | 0.01 | ± | 0.00 | 0.24 | ± | 0.0 |
| Bone | 12.3 | ± | 1.27 | 0.06 | ± | 0.00 | 0.21 | ± | 0.02 | 0.49 | ± | 0.04 | 8.61 | ± | 1.04 | 1.19 | ± | 0.16 | 0.52 | ± | 0.07 | 23.4 | ± | 2.6 |
| Brain | 0.30 | ± | 0.01 | 0.00 | ± | 0.00 | 0.08 | ± | 0.00 | 0.22 | ± | 0.01 | 4.34 | ± | 0.10 | 1.46 | ± | 0.05 | 0.31 | ± | 0.01 | 6.72 | ± | 0.2 |
| Carcass | 63.4 | ± | 6.39 | 0.33 | ± | 0.02 | 0.86 | ± | 0.07 | 2.38 | ± | 0.13 | 38.2 | ± | 3.38 | 4.23 | ± | 0.35 | 2.31 | ± | 0.26 | 112 | ± | 10 |
| Eye | 0.41 | ± | 0.04 | 0.06 | ± | 0.01 | 0.00 | ± | 0.00 | 0.03 | ± | 0.00 | 0.25 | ± | 0.02 | 0.05 | ± | 0.00 | 0.02 | ± | 0.00 | 0.80 | ± | 0.1 |
| Fur | 2.72 | ± | 0.29 | 0.02 | ± | 0.00 | 0.03 | ± | 0.00 | 0.04 | ± | 0.00 | 0.37 | ± | 0.04 | 0.09 | ± | 0.01 | 0.03 | ± | 0.00 | 3.30 | ± | 0.3 |
| Heart | 2.13 | ± | 0.15 | 0.00 | ± | 0.00 | 0.02 | ± | 0.00 | 0.07 | ± | 0.00 | 3.11 | ± | 0.23 | 0.15 | ± | 0.01 | 0.16 | ± | 0.01 | 5.65 | ± | 0.4 |
| Intestine | 2.78 | ± | 0.59 | 0.02 | ± | 0.00 | 0.09 | ± | 0.02 | 0.33 | ± | 0.05 | 5.33 | ± | 1.19 | 0.88 | ± | 0.20 | 0.15 | ± | 0.03 | 9.59 | ± | 2.1 |
| Kidney | 2.13 | ± | 0.15 | 0.02 | ± | 0.00 | 0.06 | ± | 0.00 | 0.27 | ± | 0.02 | 8.28 | ± | 0.90 | 0.19 | ± | 0.02 | 0.07 | ± | 0.00 | 11.0 | ± | 1.0 |
| Liver | 36.8 | ± | 4.05 | 0.95 | ± | 0.24 | 0.49 | ± | 0.07 | 1.48 | ± | 0.22 | 56.9 | ± | 6.06 | 1.25 | ± | 0.16 | 1.49 | ± | 0.08 | 99.4 | ± | 6.1 |
| Lung | 1.12 | ± | 0.06 | 0.01 | ± | 0.00 | 0.03 | ± | 0.00 | 0.09 | ± | 0.00 | 2.23 | ± | 0.12 | 0.43 | ± | 0.03 | 0.08 | ± | 0.00 | 3.99 | ± | 0.2 |
| Muscle | 180 | ± | 27 | 0.78 | ± | 0.10 | 1.61 | ± | 0.23 | 3.99 | ± | 0.49 | 60.1 | ± | 9.73 | 4.66 | ± | 0.84 | 4.71 | ± | 0.97 | 256 | ± | 36 |
| Pancreas | 0.91 | ± | 0.12 | 0.03 | ± | 0.00 | 0.01 | ± | 0.00 | 0.05 | ± | 0.01 | 1.52 | ± | 0.19 | 0.05 | ± | 0.01 | 0.04 | ± | 0.00 | 2.61 | ± | 0.3 |
| Plasma | 4.36 | ± | 0.52 | 0.09 | ± | 0.01 | 0.04 | ± | 0.01 | 0.20 | ± | 0.04 | 11.2 | ± | 1.76 | 0.15 | ± | 0.03 | 0.19 | ± | 0.03 | 16.3 | ± | 2.3 |
| RBC | 1.48 | ± | 0.27 | 0.01 | ± | 0.00 | 0.05 | ± | 0.01 | 0.14 | ± | 0.03 | 5.66 | ± | 1.06 | 0.36 | ± | 0.09 | 0.17 | ± | 0.03 | 7.88 | ± | 1.5 |
| SG | 0.37 | ± | 0.04 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.10 | ± | 0.01 | 0.57 | ± | 0.06 | 0.02 | ± | 0.00 | 0.01 | ± | 0.00 | 1.10 | ± | 0.1 |
| Skin | 234 | ± | 31.4 | 1.11 | ± | 0.11 | 1.71 | ± | 0.18 | 1.48 | ± | 0.07 | 23.9 | ± | 2.54 | 2.85 | ± | 0.35 | 0.95 | ± | 0.10 | 266 | ± | 34 |
| SPC | 0.16 | ± | 0.01 | 0.00 | ± | 0.00 | 0.08 | ± | 0.01 | 0.14 | ± | 0.01 | 0.94 | ± | 0.07 | 0.41 | ± | 0.04 | 0.04 | ± | 0.00 | 1.78 | ± | 0.1 |
| Spleen | 0.26 | ± | 0.02 | 0.00 | ± | 0.00 | 0.02 | ± | 0.00 | 0.05 | ± | 0.00 | 1.26 | ± | 0.10 | 0.15 | ± | 0.01 | 0.03 | ± | 0.00 | 1.78 | ± | 0.1 |
| Stomach | 2.73 | ± | 0.38 | 0.02 | ± | 0.00 | 0.03 | ± | 0.01 | 0.15 | ± | 0.09 | 1.64 | ± | 0.16 | 0.14 | ± | 0.01 | 0.04 | ± | 0.00 | 4.76 | ± | 0.6 |
| Testes | 0.89 | ± | 0.09 | 0.02 | ± | 0.00 | 0.04 | ± | 0.00 | 0.22 | ± | 0.03 | 3.40 | ± | 0.37 | 0.54 | ± | 0.09 | 3.39 | ± | 0.58 | 8.49 | ± | 1.1 |
| Thymus | 0.29 | ± | 0.02 | 0.00 | ± | 0.00 | 0.03 | ± | 0.00 | 0.04 | ± | 0.00 | 0.81 | ± | 0.03 | 0.09 | ± | 0.00 | 0.02 | ± | 0.00 | 1.28 | ± | 0.0 |
| 18:3n-3 | 20:5n-3 | 22:5n-3 | 22:6n-3 | n-3PUFA | n-6/n-3PUFA | TFA | Tissue Wt (g) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADB | 0.53 | ± | 0.16 | 0.03 | ± | 0.01 | 0.10 | ± | 0.03 | 0.31 | ± | 0.09 | 0.97 | ± | 0.3 | 7.56 | ± | 0.74 | 102 | ± | 29 | 0.3 | ± | 0.1 |
| ADW | 204 | ± | 53.1 | 2.06 | ± | 0.37 | 4.90 | ± | 1.25 | 13.0 | ± | 4.24 | 224 | ± | 59 | 7.21 | ± | 0.31 | 15261 | ± | 3888 | 28.3 | ± | 6.3 |
| ATL | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.02 | ± | 0.00 | 0.05 | ± | 0.01 | 0.09 | ± | 0.0 | 2.92 | ± | 0.27 | 0.34 | ± | 0.0 | 0.1 | ± | 0.01 |
| Bladder | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.02 | ± | 0.00 | 0.04 | ± | 0.01 | 0.07 | ± | 0.0 | 3.26 | ± | 0.27 | 0.31 | ± | 0.1 | 0.1 | ± | 0.02 |
| Bone | 0.79 | ± | 0.09 | 0.17 | ± | 0.01 | 1.03 | ± | 0.07 | 4.21 | ± | 0.59 | 6.20 | ± | 0.7 | 3.81 | ± | 0.23 | 118 | ± | 14.5 | 9.8 | ± | 1.3 |
| Brain | 0.004 | ± | 0.00 | 0.01 | ± | 0.00 | 0.09 | ± | 0.00 | 6.34 | ± | 0.18 | 6.44 | ± | 0.2 | 1.04 | ± | 0.03 | 51.9 | ± | 1.6 | 1.8 | ± | 0.04 |
| Carcass | 4.70 | ± | 0.62 | 0.80 | ± | 0.04 | 4.42 | ± | 0.23 | 19.7 | ± | 2.67 | 29.6 | ± | 3.4 | 3.83 | ± | 0.32 | 572 | ± | 70 | 40.4 | ± | 4.3 |
| Eye | 0.04 | ± | 0.01 | 0.03 | ± | 0.01 | 0.02 | ± | 0.00 | 0.21 | ± | 0.01 | 0.30 | ± | 0.0 | 2.64 | ± | 0.42 | 29.8 | ± | 3.6 | 0.5 | ± | 0.03 |
| Fur | 0.36 | ± | 0.04 | 0.01 | ± | 0.00 | 0.03 | ± | 0.00 | 0.10 | ± | 0.01 | 0.50 | ± | 0.1 | 6.62 | ± | 0.30 | 28.9 | ± | 3.5 | 2.9 | ± | 0.4 |
| Heart | 0.04 | ± | 0.00 | 0.02 | ± | 0.00 | 0.19 | ± | 0.00 | 1.32 | ± | 0.14 | 1.57 | ± | 0.1 | 3.67 | ± | 0.52 | 16.6 | ± | 0.9 | 1.0 | ± | 0.05 |
| Intestine | 0.20 | ± | 0.06 | 0.09 | ± | 0.01 | 0.27 | ± | 0.04 | 0.90 | ± | 0.20 | 1.46 | ± | 0.3 | 6.47 | ± | 0.41 | 44.1 | ± | 9.9 | 2.7 | ± | 0.5 |
| Kidney | 0.09 | ± | 0.01 | 0.14 | ± | 0.01 | 0.16 | ± | 0.01 | 0.97 | ± | 0.07 | 1.36 | ± | 0.1 | 8.06 | ± | 1.20 | 35.2 | ± | 3.1 | 1.9 | ± | 0.2 |
| Liver | 2.48 | ± | 0.52 | 1.42 | ± | 0.23 | 2.48 | ± | 0.19 | 27.0 | ± | 2.22 | 33.4 | ± | 2.0 | 2.98 | ± | 0.27 | 492 | ± | 49.5 | 9.3 | ± | 1.0 |
| Lung | 0.08 | ± | 0.01 | 0.06 | ± | 0.00 | 0.20 | ± | 0.01 | 0.39 | ± | 0.02 | 0.73 | ± | 0.0 | 5.46 | ± | 0.35 | 20.1 | ± | 1.2 | 1.1 | ± | 0.03 |
| Muscle | 14.7 | ± | 2.78 | 1.63 | ± | 0.13 | 9.66 | ± | 1.18 | 45.9 | ± | 9.93 | 71.9 | ± | 13 | 3.68 | ± | 0.74 | 1716 | ± | 268 | 58.3 | ± | 7.4 |
| Pancreas | 0.03 | ± | 0.01 | 0.14 | ± | 0.02 | 0.05 | ± | 0.01 | 0.21 | ± | 0.02 | 0.43 | ± | 0.0 | 6.14 | ± | 0.61 | 8.91 | ± | 1.1 | 0.4 | ± | 0.04 |
| Plasma | 0.21 | ± | 0.03 | 0.26 | ± | 0.05 | 0.26 | ± | 0.05 | 2.03 | ± | 0.31 | 2.76 | ± | 0.4 | 6.00 | ± | 1.00 | 43.1 | ± | 5.2 | 9.8 | ± | 1.0 |
| RBC | 0.03 | ± | 0.01 | 0.09 | ± | 0.01 | 0.37 | ± | 0.04 | 1.01 | ± | 0.16 | 1.50 | ± | 0.2 | 5.04 | ± | 0.61 | 24.1 | ± | 4.4 | 4.4 | ± | 0.4 |
| SG | 0.01 | ± | 0.00 | 0.03 | ± | 0.00 | 0.02 | ± | 0.00 | 0.08 | ± | 0.01 | 0.14 | ± | 0.0 | 7.82 | ± | 0.55 | 3.48 | ± | 0.4 | 0.2 | ± | 0.0 |
| Skin | 26.8 | ± | 3.77 | 0.55 | ± | 0.03 | 1.71 | ± | 0.11 | 4.76 | ± | 0.59 | 33.9 | ± | 4.2 | 7.83 | ± | 0.27 | 2005 | ± | 286 | 30.0 | ± | 3.7 |
| SPC | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.06 | ± | 0.00 | 1.04 | ± | 0.06 | 1.12 | ± | 0.1 | 1.59 | ± | 0.10 | 20.7 | ± | 1.6 | 0.4 | ± | 0.03 |
| Spleen | 0.01 | ± | 0.00 | 0.02 | ± | 0.00 | 0.08 | ± | 0.01 | 0.15 | ± | 0.01 | 0.25 | ± | 0.0 | 6.98 | ± | 0.58 | 5.71 | ± | 0.5 | 0.6 | ± | 0.1 |
| Stomach | 0.29 | ± | 0.06 | 0.04 | ± | 0.00 | 0.06 | ± | 0.00 | 0.17 | ± | 0.01 | 0.55 | ± | 0.1 | 8.69 | ± | 0.93 | 36.1 | ± | 6.1 | 1.0 | ± | 0.1 |
| Testes | 0.01 | ± | 0.00 | 0.02 | ± | 0.00 | 0.02 | ± | 0.00 | 0.52 | ± | 0.03 | 0.57 | ± | 0.0 | 14.6 | ± | 3.5 | 21.0 | ± | 2.6 | 2.2 | ± | 0.3 |
| Thymus | 0.02 | ± | 0.00 | 0.01 | ± | 0.00 | 0.02 | ± | 0.00 | 0.04 | ± | 0.00 | 0.09 | ± | 0.0 | 14.8 | ± | 1.5 | 5.63 | ± | 0.4 | 0.5 | ± | 0.03 |
Footnote: data were presented as mean ± SEM (n=8–9). “0.0” indicates values were less than 0.05, “0.00” indicates values were less than 0.005. TFA, total fatty acids, which included saturated, monounsaturated, n-3 and n-6 PUFA. Tissue Wt (g) was the total weight of each compartment, except total volume was used for plasma and RBC (mL).
3.5. Concentrations of major PUFA in each compartment
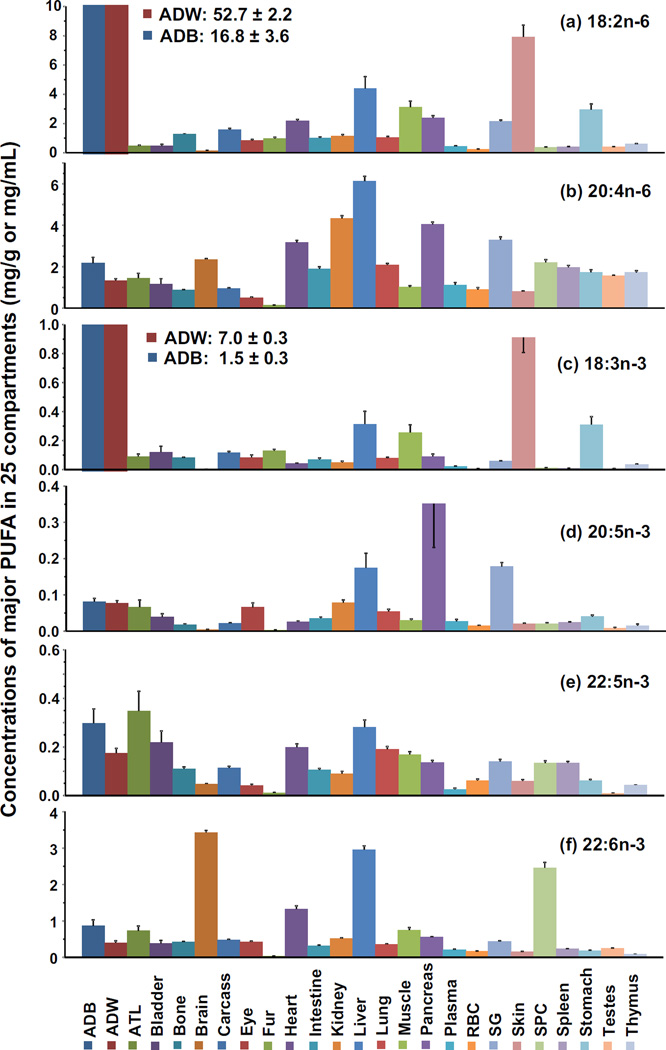
The concentrations (mg/g wet tissue or mg/mL for blood/plasma) of 18:2n-6, 20:4n-6, 18:3n-3, 20:5n-3, and 22:6n-3 in the 25 tissue compartments are presented in a bar graph in Figure 3. The highest fatty acid concentration was found in 18:2n-6 in the white adipose tissue (52.7), followed by brown adipose tissue (16.8), skin (7.9), and liver (4.4), whereas the lowest concentrations were below 0.2 and found in brain and RBC. 20:4n-6 was found at the highest concentration in liver (6.1), followed by kidney (4.3), pancreas (4.0), salivary gland (3.3), and heart (3.2), but was found in the lowest concentrations in the eye (0.5) and fur (0.1). For the n-3 fatty acids, 18:3n-3 showed a similar tissue concentration pattern as 18:2n-6, but with smaller values. The white adipose tissue had the highest concentration (7.0), followed by a drop in values with only 0.3–1.5 present in adipose brown, skin, and liver. Interestingly, the lowest concentration of 18:3n-3 occurred in the brain (0.002) which was almost one order of magnitude lower than the next lowest tissue concentration. 20:5n-3 was found in the highest concentration in pancreas, salivary gland and liver, which ranged between 0.2–0.3, but was once again markedly lower in brain (0.004). Conversely, 22:6n-3 was found in the highest concentration in the brain (3.4), liver (3.0), and spinal cord (2.5) but lowest in the thymus (0.1) and fur (<0.1). This result was particularly interesting because the concentration of 22:6n-3 was 858- and 1715-fold higher than 20:5n-3 and 18:3n-3 in brain tissue, again demonstrating remarkable selectivity. There also appears to be selectivity with respect to 18:2n-6 and 18:3n-3 in the adipose white and brown tissues as shown by the high accumulation of these fatty acids in adipose tissues and low levels in the brain.
Figure 3.
The concentrations of 18:2n-6, 20:4n-6, 18:3n-3, 20:5n-3, 22:5n-3, and 22:6n-3 in 25 compartments expressed as mg/g of wet tissue or mg/mL of blood. 18:2n-6 and 18:3n-3 in white and brown adipose tissues were off scale and thus numbers indicating their magnitude are given. Their values were labeled for clarification. Data were presented as mean ± SEM (n=8–9). For abbreviations see the legend to Figure 2.
3.6. The proportions of n-6 and n-3 PUFA in the rat whole body
In summary, the proportions of total and major n-6 and n-3 PUFA in the rat whole body are presented in the pie charts in Figure 1. Figure 1a showed that, in rat whole body, the saturated fatty acids accounted for 48.4% of the total fatty acids, the monounsaturated fatty acids 35%, while n-6 PUFA was 12% and n-3 PUFA was 2.1%. For the total n-6 PUFA, shown in pie chart 1c, 18:2n-6 accounted for 84.1% of the total n-6 PUFA, which was over 6-fold larger than the amount of 20:4n-6 (12.0%) in the rat body. The remaining n-6 PUFAs, 18:3n-6, 20:3n-6, 22:4n-6 and 22:5n-6 were all below 1.1%. These data indicate that 22:6n-3 comprises a greater part of the n-3 family of fatty acids than does 20:4n-6 in the n-6 family where the C18 precursor, 18:2n-6 makes up the bulk of the fatty acid. In pie chart 1b, out of the 2.1% of total fatty acids represented by n-3 PUFAs, the main n-3 species occurring in the rat was the diet-supplied precursor, 18:3n-3 (59.3%), followed by the end product of 18:3n-3 metabolism, 22:6n-3, 31.9%. The intermediates 20:5n-3 and 22:5n-3 were present at 2.1% and 6.8% of total n-3 PUFAs, respectively.
DISCUSSION
The design of this study wherein the rat carcass was divided into 25 compartments and each analyzed quantitatively allows a complete and thorough assessment of the selectivity with which each tissue is able to construct its particular fatty acid composition. Our data conclusively show that many tissues have an apparent selectivity in which particular fatty acids predominate. It is critical in such experiments to define carefully not only the basal diet but also the fatty acid and PUFA content of the diet, as this is known to be a key variable defining fatty acid composition of tissues [23]. The diet here was a semi-synthetic one with 18:2n-6 and 18:3n-3 at 15 and 3 wt% of total fatty acids, respectively [10, 18].
It is instructive to consider the disposition of the main dietary PUFAs in terms of their main body depots as well as that of their key longer chain and more unsaturated metabolites. Not surprisingly, with the diet enriched in 18:2n-6, this was the predominant fatty acid in the rat whole body making up 10 wt% of the calculated total fatty acid content (Table 1) and about 72% of the total PUFAs. The Institute of Medicine report on mean fat intake in Americans [24] indicates that about 15g/d of 18:2n-6 is consumed and in Canadians it is similarly high at 5 en% [25]. Thus from the present observation in rats and the similarity of the diets with respect to the high 18:2n-6 intake, it may be surmised that many Westerners also have this omega-6 fatty acid as their predominant one in obtainable tissue samples. Analyses of needle biopsies of human adipose tissue corroborate this suggestion [26].
Our data showed clearly that both 18:2n-6 and 18:3n-3 were mainly deposited in white adipose tissue with a total content of these two fatty acids being 1506 and 204 mg, respectively. A distant second was the skin compartment with 234 and 26.8 mg, respectively, followed by the muscle. Fu and Sinclair have previously suggested that a large fraction of the total 18:3n3 was associated with a skin and fur fraction in guinea pigs using a radiotracer approach [8]. Our data are consistent with that of Cunnane et al who have previously reported that 19% of dietary 18:2n-6 and 11% of dietary 18:3n-3 were stored in the tissues of growing rats and that 60% of that was in visceral fat [7].
These C18 essential fatty acids were metabolized in the rats to longer chain and more unsaturated fatty acids with 20:4n-6 being the predominant n-6 metabolite and 22:6n-3 the predominant n-3 metabolite (Figure 1). The 20:4n-6 is a major PUFA in nearly every tissue and is the major PUFA in most internal organs. The testes was an exception with the predominant n-6 fatty acid being 22:5n-6 as has been previously noted [27]. In the nervous system, however, the major PUFA is 22:6n-3 with a brain and spinal cord content of 48 and 36% of total PUFAs, respectively.
This study highlights the difference in selectivity of the brain for 22:6n-3 and that of most other tissues in the mammalian organism. The very high content of the brain has been appreciated for a long time [28] but more recently some of the underlying mechanisms have been elucidated. A particularly interesting aspect of brain fatty acid composition is its low content of 20:5n-3 as well as the C18 precursor. A recent review of such studies indicates that EPA undergoes more rapid β-oxidation and metabolism relative to 22:6n-3 but that its uptake into brain is similar, and that this may help to explain the great disparity in final concentrations [29].
This study has some limitations and indications for future studies. It is likely that the proportion of tissues may have changed somewhat over the 25 days of this study, because the rats were sacrificed at different time points, e.g., by gaining visceral fat content. This was in part mitigated by feeding both the dam and the pups the same diet throughout their lifetimes and by a period of diet equilibration for 7 weeks prior to the first sampling time point. Of course, the study could be extended by analyzing how all of the tissues respond to changing the PUFA intake in the diet such as in the very extensive study by Gibson et al [30] or by adding longer chain n-3 and/or n-6 PUFAs to the diet.
In conclusion, after having reached equilibrium on a defined diet composed of 10% total fat, of which 15% was 18:2n-6 and 3% was 18:3n-3, the rats retained 12 wt% as total n-6 PUFA and 2.1% as total n-3 PUFA in the body. Among these, 18:2n-6 accounted for 84% while 20:4n-6 was 12% of the total n-6 PUFA. For the total n-3 PUFA, 18:3n-3 was 59%, 20:5n-3 was 2.1%, and 22:6n-3 was 32%. The white adipose tissue contained the most C18 precursors and the muscle had the most of 20:4n-6 and 22:6n-3. Through these data, we have shown that in the rat body, fatty acid profiles differ greatly from tissue to tissue not only in fatty acid species, but also in carbon length and degree of unsaturation. Here we have demonstrated that there is remarkable specificity of fatty acid tissue accretion in the rat tissues.
ACKNOWLEDGEMENTS
Thanks also to Dr. Lee Chedester and Mr. Marshall Jones for their valuable advice and expert assistance with the animal work. This project was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Abbreviations
- ATL
adrenal gland, thyroid and mandibular lymph nodes
- ADB
brown adipose
- ADW
white adipose
- BHT
butylated hydroxytoluene
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EFA
essential fatty acids
- EPA
eicosapentaenoic acid
- FAME
fatty acid methyl esters
- FID
flame ionization detector
- GC
gas liquid chromatography
- HUFA
highly unsaturated fatty acids
- MS
mass spectrometry
- PUFA
polyunsaturated fatty acids
- RBC
red blood cell
- SG
salivary glands
- SPC
spinal cord
- TFA
total fatty acid
- wt%
weight percent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 2.Tinoco J, Babcock R, Hincenbergs I, Medwadowski B, Miljanich P, Williams MA. Linolenic acid deficiency. Lipids. 1979;14:166–173. doi: 10.1007/BF02533868. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Government. Dietary Guidelines for Americans. 7th Ed. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]
- 4.Salem N, Jr, Pawlosky R, Wegher B, Hibbeln J. In vivo conversion of linoleic acid to arachidonic acid in human adults. Prostaglandins, leukotrienes, and essential fatty acids. 1999;60:407–410. doi: 10.1016/s0952-3278(99)80021-0. [DOI] [PubMed] [Google Scholar]
- 5.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257–1265. [PubMed] [Google Scholar]
- 6.Fu Z, Attar-Bashi NM, Sinclair AJ. 1-14C-linoleic acid distribution in various tissue lipids of guinea pigs following an oral dose. Lipids. 2001;36:255–260. doi: 10.1007/s11745-001-0715-7. [DOI] [PubMed] [Google Scholar]
- 7.Cunnane SC, Anderson MJ. The majority of dietary linoleate in growing rats is beta-oxidized or stored in visceral fat. Journal of Nutrition. 1997;127:146–152. doi: 10.1093/jn/127.1.146. [DOI] [PubMed] [Google Scholar]
- 8.Fu Z, Sinclair AJ. Novel pathway of metabolism of alpha-linolenic acid in the guinea pig. Pediatric research. 2000;47:414–417. doi: 10.1203/00006450-200003000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Innis SM. Essential fatty acids in growth and development. Progress in lipid research. 1991;30:39–103. doi: 10.1016/0163-7827(91)90006-q. [DOI] [PubMed] [Google Scholar]
- 10.Lin YH, Salem N., Jr Whole body distribution of deuterated linoleic and alpha-linolenic acids and their metabolites in the rat. J Lipid Res. 2007;48:2709–2724. doi: 10.1194/jlr.M700369-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.DeMar JC, Jr, DiMartino C, Baca AW, Lefkowitz W, Salem N., Jr Effect of dietary docosahexaenoic acid on biosynthesis of docosahexaenoic acid from alpha-linolenic acid in young rats. J Lipid Res. 2008;49:1963–1980. doi: 10.1194/jlr.M800117-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark KD, Lim SY, Salem N., Jr Artificial rearing with docosahexaenoic acid and n-6 docosapentaenoic acid alters rat tissue fatty acid composition. J Lipid Res. 2007;48:2471–2477. doi: 10.1194/jlr.M700317-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain research bulletin. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Chen ZY, Cunnane SC. Application of the balance method to determining accumulation, metabolism, and apparent oxidation of linoleic and alpha-linolenic acids in the pregnant rat. Metabolism: clinical and experimental. 1994;43:940–944. doi: 10.1016/0026-0495(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 15.Cunnane SC. Application of new methods and analytical approaches to research on polyunsaturated fatty acid homeostasis. Lipids. 2001;36:975–979. doi: 10.1007/s11745-001-0808-3. [DOI] [PubMed] [Google Scholar]
- 16.Domenichiello AF, Chen CT, Trepanier MO, Stavro PM, Bazinet RP. Whole body synthesis rates of DHA from alpha-linolenic acid are greater than brain DHA accretion and uptake rates in adult rats. J Lipid Res. 2014;55:62–74. doi: 10.1194/jlr.M042275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. The Journal of nutrition. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 18.Moriguchi T, Lim SY, Greiner R, Lefkowitz W, Loewke J, Hoshiba J, Salem N. Effects of an n-3-deficient diet on brain, retina, and liver fatty acyl composition in artificially reared rats. J Lipid Res. 2004;45:1437–1445. doi: 10.1194/jlr.M400087-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethyl acetals from lipids with boron tri-fluoride-methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 21.Salem N, Jr, Reyzer M, Karanian J. Losses of arachidonic acid in rat liver after alcohol inhalation. Lipids. 1996;31(Suppl):S153–S156. doi: 10.1007/BF02637068. [DOI] [PubMed] [Google Scholar]
- 22.Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 23.Salem N., Jr . Omega-3 fatty acids: molecular and biochemical aspects. In: Spiller GA, Scala J, editors. Current Topics in Nutrition and Disease: New Protective Roles for Selected Nutrients. New York: Alan R Liss; 1989. pp. 109–228. [Google Scholar]
- 24.Trumbo P, Schlicker S, Yates AA, Poos M Food, T.N.A. Nutrition Board of the Institute of Medicine, Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Journal of the American Dietetic Association. 2002;102:1621–1630. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 25.Health. Canada. [Accessed 4/24/15]; http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/art-nutr-child-enf-eng.php. [Google Scholar]
- 26.Handelman GJ, Epstein WL, Machlin LJ, van Kuijk FJ, Dratz EA. Biopsy method for human adipose with vitamin E and lipid measurements. Lipids. 1988;23:598–604. doi: 10.1007/BF02535604. [DOI] [PubMed] [Google Scholar]
- 27.Bieri JG, Prival EL. Lipid composition of testes from various species. Comparative biochemistry and physiology. 1965;15:275–282. doi: 10.1016/0010-406x(65)90131-3. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- 29.Chen CT, Bazinet RP. beta-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins, leukotrienes, and essential fatty acids. 2015;92:33–40. doi: 10.1016/j.plefa.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Gibson RA, Neumann MA, Lien EL, Boyd KA, Tu WC. Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids. Prostaglandins, leukotrienes, and essential fatty acids. 2013;88:139–146. doi: 10.1016/j.plefa.2012.04.003. [DOI] [PubMed] [Google Scholar]