Abstract
Mesenchymal stem cells isolated from rats are frequently used for tissue engineering research. However, considerable differences have been identified between rat mesenchymal stem cells and those derived from humans, and no defined panel of markers currently exists for the isolation of these cells. The aim of this study was to examine the effects of cell sorting for CD29+/CD90+ cells from rat adipose and bone marrow tissues on their differentiation and expression of stem cell–associated genes. Flow cytometry showed 66% and 78% CD29+/CD90+ positivity within passage 1 of adipose and bone marrow cultures, respectively. CD29+/CD90+ cells showed a reduction in both osteogenic and adipogenic differentiation when compared with unsorted cells, as determined by alizarin red and Oil Red-O staining, respectively. These findings could not entirely be explained by fluorescence-activated cell sorting–induced cell injury as sort recovery was only modestly affected in adipose-derived cells. Maintaining cells in fluorescence-activated cell sorting buffer did not affect adipose-derived cell viability, but a significant (p < 0.05) reduction was found in bone marrow–derived cell viability. Additionally, CD29+/CD90+ selection was associated with a significant decrease in the expression of Lin28, Sox2, Nanog and CD73 in adipose-derived cell cultures, whereas differences in stem cell–associated gene expression were not observed in sorted bone marrow–derived cell cultures. In summary, this study demonstrated that fluorescence-activated cell sorting had differential effects on adipose-derived cells and bone marrow–derived cells, and both CD29+/CD90+ cells displayed a significantly reduced capacity for osteogenic/adipogenic differentiation. In conclusion, we identify that maintaining heterogeneity within the mesenchymal stem cell population may be important for optimal differentiation.
Keywords: Stem cell, adipose, bone marrow, flow cytometry, osteogenic, adipogenic, CD29, CD90, rat
Introduction
Mesenchymal stem cells (MSCs) have been defined as multipotent cells that adhere to tissue culture polystyrene (TCP) and present a characteristic panel of surface markers, including CD73, CD90 and CD105.1,2 However, the majority of early phenotyping studies have been most frequently conducted using human or murine MSCs and, as such, are not necessarily translatable for the identification of MSCs derived from other species. Indeed, variations between cell surface markers have been consistently observed between MSCs isolated from humans and non-humans, such as rats.1,3,4 Due to the frequent use of rat MSCs for tissue engineering research,5–7 publications that identify effective selection and culture conditions for rat MSCs may be of considerable benefit for tissue engineering applications.
Notwithstanding the specificity of MSC markers, it has been argued that MSCs should be purified/sorted if they are to be used for tissue engineering applications, since the presence of a heterogeneous mixture of cell types may compromise the proliferation and/or differentiation potential of stem/progenitor cells and limit their potential for regenerative applications.8–10 Additionally, since the MSC population is inherently heterogeneous, containing cells with different proliferation and differentiation capacities,11–13 studies examining the effects of cell selection criteria on multi-differentiation potential will be of significant value for the development of targeted regenerative therapies. Unfortunately, the commercial availability of antibodies raised against rats is limited when compared with those raised against humans and mice, limiting the breadth of marker profiling studies in this species. However, throughout the literature, the combined expression of CD29 and CD90 is one of the most consistent identifying phenotypic features among MSCs, irrespective of species variations.3,14,15 Furthermore, a recent study has indicated that CD29+/CD90+ expression is likely conserved among stem cells isolated from adipose tissue irrespective of harvesting site and age, further making this an appealing population for tissue engineering research.16 Therefore, characterisation of CD29+/CD90+ cells derived from major MSC stores, such as adipose and bone marrow tissues, is of importance to identify their potential suitability as a source of stem cells for pre-clinical use.
It must be highlighted that no single unique marker exists for the identification of MSCs, and that when used independently, the presence of CD29 or CD90 cannot effectively distinguish MSCs from other resident cells present within the stem cell niche, such as endothelial cells, fibroblasts, immunocompetent cells and adipocytes.17,18 CD90, also known as thymocyte antigen 1 (Thy-1), represents a 25- to 37-kDa glycosylphosphatidylinositol (GPI)-linked membrane protein commonly associated with osteoprogenitor cells.19 CD29 (integrin beta-1) is frequently used, in combination with other cluster of differentiation (CD) markers such as CD90, for the identification of MSCs and has been shown to be expressed by adipocyte progenitors.20 When used in combination, previous studies have shown that CD29+/CD90+ cells isolated from rats have demonstrated the capacity for osteogenic and adipogenic differentiation.21,22 However, to our knowledge, none of these studies have examined whether enriching for a CD29+/CD90+ population was able to enhance the differentiation capacity of these cells when compared with TCP-adherent controls. Therefore, despite some promising initial studies, the benefits of selecting CD29+/CD90+ cells for tissue engineering applications remain largely unknown.
In this study, we examined the potential of CD29+/CD90+ cell selection for the isolation of MSC-like cells that can be used for tissue engineering applications. These cells were compared with unsorted TCP-adherent cells to identify whether CD29+/CD90+ enrichment enhanced MSC-associated characteristics, such as osteogenic and adipogenic differentiation. In these experiments, all cells were first expanded on TCP to maximise cell number and provide an initial means of MSC selection, as was first identified by Friedenstein et al.23 during his initial pioneering work with bone marrow stem cells.24,25
Materials and methods
Cell isolation and culture
Six-week-old male Wistar Han rats (weight: ~120 g) were sacrificed by cervical dislocation (Aston University, Pharmaceutical Sciences Animal House, Birmingham, UK; ethical approval reference: BCHDent286.1471.TB). Adipose tissue was collected from inguinal fat pads and bone marrow from femora. Adipose-derived cells (ADCs) were isolated by mincing inguinal adipose tissue into ~1 mm3 pieces, which were incubated with 0.01% type-I collagenase (Sigma, UK) for 30 min at 37°C in a rotary incubator (SI20H; Stuart Scientific, UK) and centrifuged at 1000g for 5 min to pellet the stromal vascular fraction (SVF).26,27 Bone marrow–derived cells (BMDCs) were flushed from rodent femora by inserting a 22-gauge needle attached to a 20-mL syringe containing growth medium (alpha-modified minimum essential medium (α-MEM), with 10% foetal bovine serum (FBS) and 1% penicillin/streptomycin; Sigma) into the femoral cavity. Bone marrow was flushed and collected in a 50-mL Falcon® tube and centrifuged at 900g for 5 min to pellet the BMDCs.28 ADCs and BMDCs were re-suspended and cultured in growth medium until approximately 80% confluent.
Cell viability analysis
To study the effects of maintaining cells in fluorescence-activated cell sorting (FACS) buffer during blocking, antibody labelling and sorting stages, cell viability was analysed at 30-min intervals over a total period of 270 min. Passage 1 ADCs and BMDCs were cultured until ~80% confluent. Cells were then detached using 0.25% trypsin (2.5 g/L of trypsin in 0.38 g/L of ethylenediaminetetraacetic acid (EDTA)) for 5 min at 37°C (Gibco, UK). Following detachment, the cells were centrifuged (Eppendorf 5804R; Eppendorf, UK) at 900g for 5 min, neutralised with growth medium and the resulting suspensions transferred to 15 mL Falcon tubes. Cell suspensions were incubated in FACS buffer (sterile phosphate-buffered saline (PBS) + 1% FBS) and maintained at 4°C under constant agitation using an orbital shaker to prevent sedimentation. The number of viable cells was determined every 30 min by adding 0.4% (w/v) Trypan blue solution (Sigma–Aldrich, UK) to an equal volume of cell suspension and manually counting the cells using a modified Neubauer haemocytometer (Hawksley, UK).
FACS
Passage 1 ADCs and BMDCs were detached with 0.2% (w/v) EDTA (Gibco) at 37°C for approximately 5 min and centrifuged at 400g (Eppendorf 5804R; Eppendorf) for 5 min. The supernatant was discarded and 1 µg/mL of anti-rat Fc block (BD Pharmingen, UK) in sterile PBS was added to block non-specific binding sites. Cells were washed using sterile PBS, centrifuged at 400g for 5 min and the pellet re-suspended in sterile FACS buffer (PBS, 1% FBS) containing allophycocyanin (APC)-conjugated CD29 antibody (eBiosciences, 17-0291, UK) and fluorescein isothiocyanate (FITC)-conjugated CD90 antibody (eBiosciences, 11-0900, UK) for 30 min. FACS buffer was maintained at room temperature. Unsorted cells (i.e. not processed through the FACS instrument) and unlabelled cells (i.e. not exposed to primary antibody, but processed through the FACS instrument) were used as controls. For cell selection, a FACSAria II instrument (BD Biosciences, Illinois, USA) was equipped with an 85-µm nozzle, and cells were sorted at a low sort rate using a pressure of 45 lbf/in2 (3.1 bar). Cells were acquired and gated using forward scatter (FSC) and side scatter (SSC) parameters to exclude cell debris and aggregates. Propidium iodide (PI) staining (eBiosciences, 00-6990-50, UK) was used to label non-viable cells. These cells were excluded prior to analysis. CD29+/CD90+ cells were recovered in growth medium containing 10% FBS to maximise viability. Cells were rested for a period of approximately 15 min after being sorted. All data were analysed using FlowJo software (TreeStar, USA).
Sort recovery was measured immediately following FACS using the Trypan blue exclusion assay, as previously described (section ‘Cell viability analysis’).
Differentiation assays
To assess osteogenic and adipogenic differentiation, FACS-recovered cells were seeded at 1 × 105 cells per well in 35 mm2 culture dishes (Sarstedt, UK) containing growth medium for 24 h. Following expansion, growth medium was aspirated and replaced with differentiation medium as follows.
Passage 1 CD29+/CD90+, unlabelled (i.e. cells that had not been exposed to primary antibody but processed through the FACS instrument) and unsorted ADCs and BMDCs were cultured in osteogenic medium (50 µg/mL ascorbic acid (Sigma), 10 mM β-glycerophosphate (Sigma) and 10−9 M dexamethasone (Sigma)) for a period of 21 days.29,30 Cultures were fixed with 10% (w/v) formaldehyde for 20 min (VWR, UK) and stained using 40 mM alizarin red (AR), adjusted to pH 4.2 using ammonium hydroxide, for 20 min at room temperature under constant agitation (Sigma).31 Unbound stain was removed with successive washes in PBS. Images of stained cultures were captured using a Nikon TE-DH100W (Nikon, UK) camera attached to a Nikon Eclipse TE300 microscope (Nikon). To quantify the amount of calcium present in each culture, the bound AR stain was eluted using 10% (v/v) acetic acid and quantified using a spectrophotometer at a wavelength of 405 nm (ultraviolet (UV)/visible spectrometer; Philips, UK).
To examine adipogenic differentiation, ADCs and BMDCs were cultured in adipogenic medium (0.5 mM 1-methyl-3-isobutylxanthine (Sigma), 60 µM indomethacin (Sigma) and 0.5 µM hydrocortisone (Sigma)) for a period of 21 days.32 Following differentiation, adipogenic medium was aspirated and the cultures fixed with 10% (w/v) formaldehyde for a period of 20 min (VWR). After fixation, each culture was stained with 0.25% (w/v) Oil Red-O (ORO; VWR) dissolved in dH2O (VWR) for 40 min at room temperature under constant agitation. Cultures were then washed twice with PBS to remove unbound ORO. Bound ORO dye was eluted through the addition of 200 µL of 60% (v/v) propan-2-ol (VWR). Cultures were incubated at room temperature for 20 min with constant agitation. A total of 60 µL of the eluate was transferred to an opaque-walled 96-well assay plate (Corning, UK) and the absorbance measured at 500 nm using an ELx800 plate-reader (BioTek, UK) and normalised according to cell number, which was calculated using Trypan blue cell counts (section ‘Cell viability analysis’).
Semi-quantitative reverse-transcription polymerase chain reaction
RNA was isolated from passage 1 CD29+/CD90+ cells directly after FACS, as well as from unsorted controls using the Qiagen RNeasy Mini Kit, following the manufacturer’s instructions (Qiagen, UK). RNA was reverse transcribed into complementary DNA (cDNA) using the Qiagen Omniscript RT Kit according to the manufacturer’s instructions (Qiagen). A REDTaq mastermix was produced, comprising 12.5 µL of REDTaq ready mix (Sigma–Aldrich), 12.5 µL of molecular grade water (BDH Laboratory Supplies, UK), 1 µL of 1 µM forward primer (Invitrogen, UK) and 1 µL of 1 µM reverse primer (Invitrogen). A 6-µL PCR MasterMix was transferred to individual 0.2 mL PCR tubes (Appleton Woods, UK) to which 50–100 ng of cDNA was added. All components were mixed thoroughly using a vortex mixer and transferred to a GeneAmp 2700 PCR Thermocycler (Applied Biosystems, UK). Amplification parameters were optimised for each gene (see Table 1). Expression of genes associated with a stem cell phenotype (Table 1) was quantified following amplification by analysing the PCR products on a 1.5% (w/v) agarose gel containing 0.01% ethidium bromide (Invitrogen). Gels were then transferred to a G:BOX gel documentation and analysis unit (Syngene, UK) where the presence of amplified products was detected under UV illumination. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to normalise gene expression. All primers were manufactured commercially (Invitrogen), and details of DNA sequences and gene product sizes are provided in Table 1.
Table 1.
Details of primer sequences, product sizes, annealing temperatures, cycle numbers used, and NCBI gene accession numbers.
| Gene | Sequence | Product size (bp) | Annealing temperature (°C) | Cycle number | Accession number |
|---|---|---|---|---|---|
| Normalisation | |||||
| GAPDH | F-CCCATCACCATCTTCCAGGAGC; | 473 | 60.5 | 21 | NM_017008 |
| R-CCAGTGAGCTTCCCGTTCAGC | |||||
| Pluripotent markers | |||||
| Nanog | F-TATCGTTTTGAGGGGTGAGG; | 356 | 60.5 | 31 | NM_001100781 |
| R-CAGCTGGCACTGGTTTATCA | |||||
| Lin28 | F-TTTCTTGTTTCCCCCAAATG; | 630 | 60.5 | 30 | NM_001109269 |
| R-AGAGGGGCTGGTTGTAAGGT | |||||
| Sox2 | F-ATACAAGGGAATTGGGAGGG; | 414 | 60.5 | 29 | NM_001109181 |
| R-AAACCCAGCAAGAACCCTTT | |||||
| Multipotent markers | |||||
| CD29 | F-AATGGAGTGAATGGGACAGG; | 2397 | 60.5 | 25 | NM_017022.2 |
| R-TCTGTGAAGCCCAGAGGTTT | |||||
| CD73 | F-GGACTGATTGATCCCCTCCT; | 401 | 60.5 | 25 | NM_002526 |
| R-TTGTCCCTGGATTTGAGAGG | |||||
| CD90 | F-AGCTCTTTGATCTGCCGTGT; | 486 | 60.5 | 26 | NM_012673 |
| R-CTGCAGGCAATCCAATTTTT | |||||
| CD105 | F-TTCAGCTTTCTCCTCCGTGT; | 325 | 60.5 | 28 | NM_001010968 |
| R-TGTGGTTGGTACTGCTGCTC | |||||
GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
All data were analysed using SPSS 10.0 for Windows (SPSS Inc., USA). Statistical analyses were performed using Student’s t-test or a one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test. p < 0.05 was considered to indicate statistical significance.
Results
Flow cytometry for a CD29+/CD90+ cell population
The total percentage of CD29+, CD90+ and CD29+/CD90+ cells within the whole population of adipose and bone marrow tissues was analysed using FACS (Figure 1). Freshly isolated (P0) ADC cultures contained significantly (p < 0.05) higher numbers of CD29+, CD90+ and CD29+/CD90+ cells than BMDC cultures (ADCs: 91% CD29+, 57% CD90+ and 51% CD29+/CD90+; BMDCs: 64% CD29+, 36% CD90+ and 28% CD29+/CD90+). No significant (p < 0.05) differences were identified between ADCs and BMDCs at P1 (ADCs: 98% CD29+, 73% CD90+ and 66% CD29+/CD90+; BMDCs: 99% CD29+, 84% CD90+ and 78% CD29+/CD90+).
Figure 1.
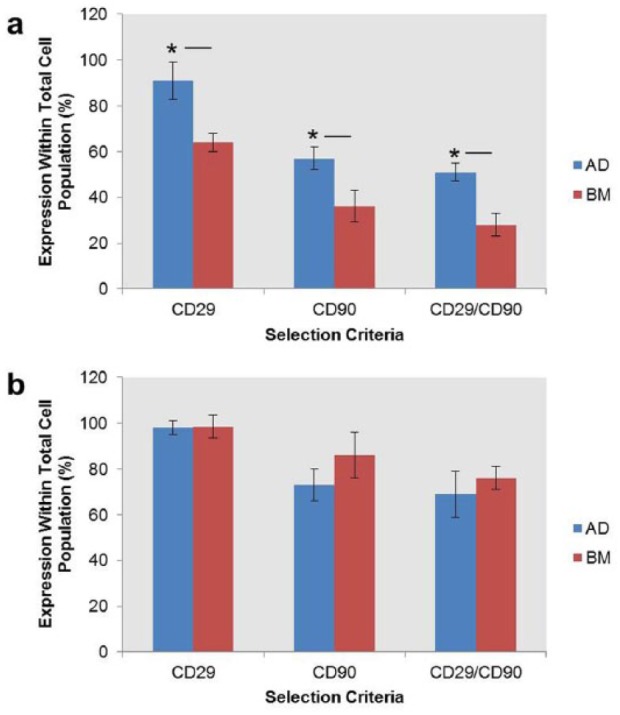
Proportion of CD29+, CD90+ and CD29+/CD90+ cells within (a) passage 0 and (b) passage 1 ADC and BMDC cultures. Percentage positivity was analysed within the total cell population. Non-viable cells were excluded using propidium iodide staining. Data were analysed using Student’s t-test.
*p < 0.05, n = 5.
Sort recovery and cell viability in FACS buffer
The influence of time spent by isolated cells in FACS buffer on cell viability was determined. Data demonstrated that ADCs and BMDCs stored in FACS buffer for up to 60 min (Figure 2(a)) displayed >90% viability. ADCs showed >90% viability over the total 270-min incubation, while BMDCs only showed >90% viability for up to 150 min. Following 270 min in FACS buffer, cell viability decreased by 8% for ADCs and 23% for bone marrow stromal cells (BMSCs) when compared with time point 0. Significant (p < 0.05) differences in cell viability were observed between ADCs and BMDCs at time points of 120, 180 and 210 min.
Figure 2.
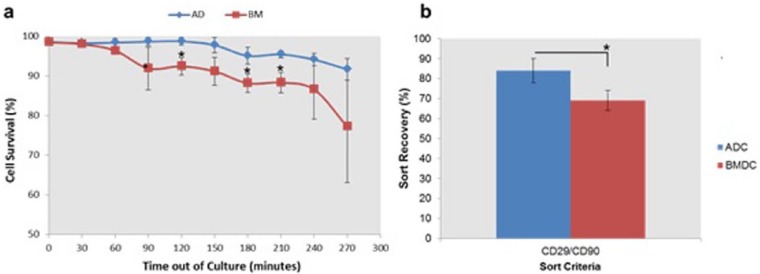
(a) Viability of ADCs and BMDCs maintained in FACS buffer (sterile PBS + 1% FBS) over a period of 270 min. Cell viability counts were performed using Trypan blue staining at 30-min intervals and (b) sort recovery of ADCs and BMDCs following FACS, as determined by Trypan blue staining.
*p < 0.05, n = 5.
The number of viable cells retrieved after FACS (sort recovery) was determined using Trypan blue cell counts, which were performed immediately after cell selection. ADCs (84%) demonstrated a significantly (p < 0.05) higher sort recovery when compared with BMDCs (69%) (Figure 2(b)).
Differentiation
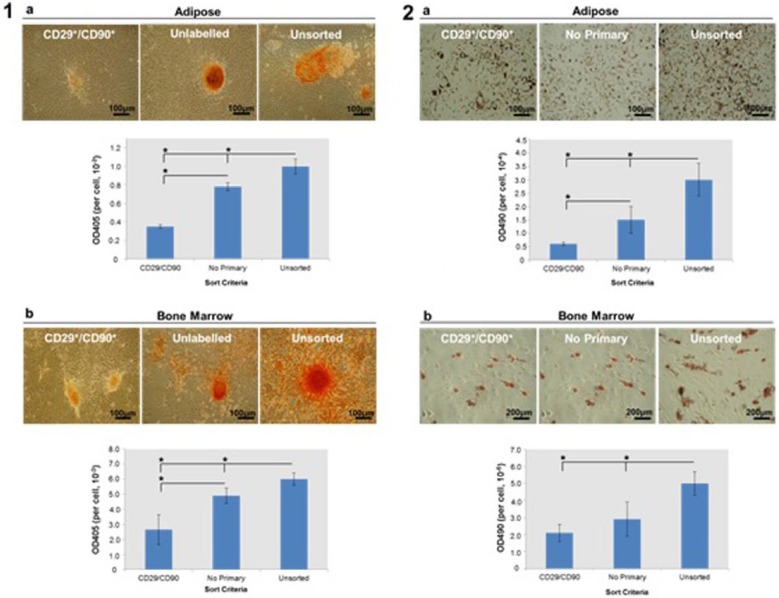
Osteogenic differentiation was analysed using AR staining and quantification. AR staining indicated that calcium deposition was significantly (p < 0.05) reduced in CD29+/CD90+ cultures when compared with unsorted and unlabelled controls (Figure 3(1)). Significant (p < 0.05) differences in AR staining were observed between the CD29+/CD90+ and the unlabelled controls (i.e. cells processed through the FACS instrument but lacking primary antibody) in both ADC and BMDC cultures. A significant (p < 0.05) reduction in AR staining was also observed when unlabelled cells were compared with unsorted cells.
Figure 3.
Osteogenic and adipogenic differentiation analysed by (1) alizarin red (AR) and (2) Oil Red-O (ORO) staining, respectively. AR staining and quantification were used to identify calcium deposition. ORO staining and quantification were used to identify lipid accumulation. For both assays, CD29+/CD90+, unlabelled and unsorted (a) ADC and (b) BMDC cultures were compared. Both analyses were performed following a 21-day culture in osteogenic or adipogenic medium.
*p < 0.05, n = 3.
Adipogenic differentiation was analysed using ORO staining and quantification. CD29+/CD90+ ADCs and BMDCs showed significantly (p < 0.05) reduced intracellular lipid formation, as assessed by ORO staining and quantification, when compared with unsorted controls (Figure 3(2)). No statistically significant (p < 0.05) differences in ORO staining were identified between the CD29+/CD90+ and the unlabelled controls in ADC and BMDC cultures. A significant (p < 0.05) reduction in ORO staining was observed when unlabelled cells were compared with unsorted cells.
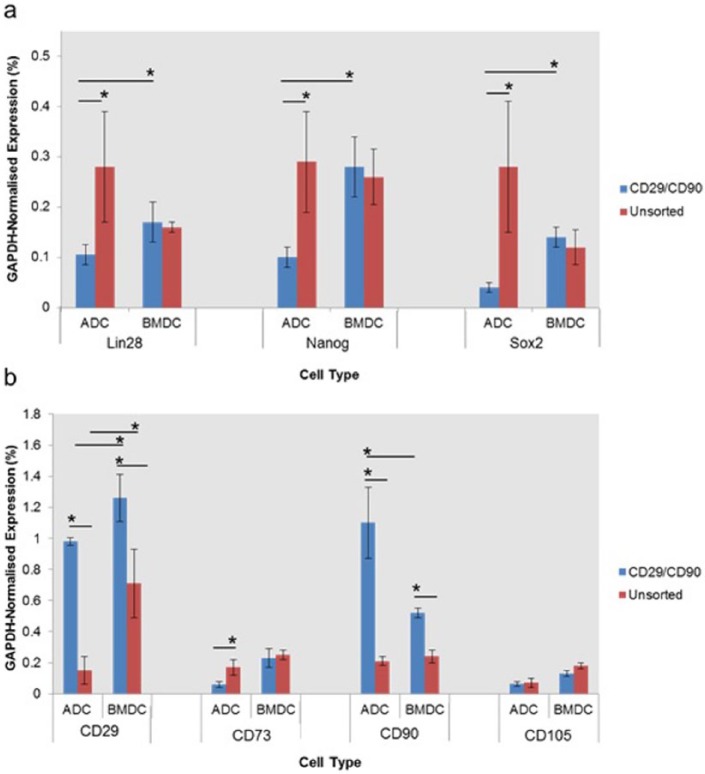
Gene expression analysis for stem cell markers
To further analyse the effects of enrichment for CD29+/CD90+ on stem cell phenotype, cells sorted using these surface markers were compared with unsorted controls. Semi-quantitative reverse-transcription polymerase chain reaction (sqRT-PCR) analysis demonstrated that CD29+/CD90+ selection significantly reduced (p < 0.05) expression levels of pluripotent marker genes (Nanog, Lin28 and Sox2) within primary ADC cultures, but had no significant (p < 0.05) effect on the expression of these genes in BMDC cultures (Figure 4(a)). CD29+/CD90+ BMDCs showed a significantly (p < 0.05) higher expression of Lin28, Nanog and Sox2 compared with CD29+/CD90+ ADCs. Expression of CD29 and CD90 was significantly (p < 0.05) increased in CD29+/CD90+ ADCs and BMDCs, supporting the validity of the FACS process. The expression of CD73 within the adipose fraction was significantly (p < 0.05) decreased following CD29+/CD90+ selection. CD29+/CD90+ selection had no significant (p > 0.05) effect on the expression of CD73 within BMDCs (Figure 4(b)). No significant (p > 0.05) changes were observed in CD105 expression for ADC and BMDC cultures following CD29+/CD90+ selection. CD29+/CD90+ BMDCs showed a significantly (p < 0.05) higher expression of CD29 and CD73 than CD29+/CD73+ ADCs. CD29+/CD90+ ADCs displayed significantly higher expression of CD90 than BMDCs.
Figure 4.
Gene expression analysis comparing levels of transcripts associated with stem cell: (a) pluripotency and (b) multipotency for CD29+/CD90+ ADCs and BMDCs isolated using FACS, with unsorted controls. All analyses were performed on passage 1 cells that had reached ~80% confluence.
*p < 0.05, n = 3.
Discussion
Previous studies have demonstrated that CD29+/CD90+ expression is preserved among MSCs isolated from many different species,3,33 with these markers being used to isolate cells that have some level of osteogenic/adipogenic potential.10,19,21,22 However, to our knowledge, no previous studies have compared CD29+/CD90+ cells with TCP-adherent controls to confirm that this selection criterion enhances culture responses important for tissue engineering applications. This is an important distinction, since adherence to TCP was the initial method of stem cell selection first identified by Friedenstein et al.,23 and regimes utilising ex vivo expansion with only minimal surface marker characterisation currently represent popular and easily employable methods for generating autologous cells for clinical transplantation.34,35 However, the immunological consequences of such procedures are still subject to intensive research.35
In this study, we identified that selection of CD29+/CD90+ cells within BMDC cultures caused no significant (p < 0.05) changes in any of the stem cell markers analysed. Moreover, CD29+/CD90+ ADC populations showed significantly (p < 0.05) lower levels of expression of several pluripotent marker genes (Lin28, Sox2 and Nanog) when compared with unsorted controls. These genes represent members of a panel of pluripotent factors required for stem cell self-renewal and maintenance that can be transfected, along with OCT4, to induce reversion to an induced pluripotent state.36,37 In corroboration with these gene expression data, this study also demonstrated that CD29+/CD90+ enrichment significantly (p < 0.05) decreased the capacity of both ADCs and BMDCs to differentiate towards an osteogenic/adipogenic phenotype when compared with unsorted controls. We identify that these data may have implications when considering the role of accessory cells located within the adipose and bone marrow niche for stem cell function and differentiation, which shall be discussed below.
Previous studies have shown that MSC selection using TCP-adherence gives an initially heterogeneous population of cells that becomes increasingly homogeneous as they are propagated on TCP.38,39 In fact, it has been shown that MSCs isolated using TCP-adherence reach a similar level of homogeneity to those isolated using flow cytometry after three passages.37 This study identified that in the case of CD29+/CD90+ cells, achieving homogeneity using TCP-selection is at faster rate for BMDCs than for ADCs. This may be due to the persistent presence of more committed colony-forming CD29+/CD90− adipocyte progenitors within ADC cultures.20,40 At passage 1, CD29+/CD90+ ADCs and BMDCs make up 66% and 78% of the total cell population, respectively. Therefore, unsorted passage 1 cells used in this study should be identified as a largely heterogeneous population, rather than a pure MSC population. However, previous studies have shown that such heterogeneity may be of benefit, with many reports highlighting the importance of maintaining the stem cell niche for optimal MSC function.41,42 Furthermore, the use of explant cultures within tissue engineering studies highlights the importance of maintaining features of the stem cell niche in vitro.43,44 This is due to multifaceted effect that the stem cell niche has been shown to have in coordinating and controlling the participation of MSCs in tissue maintenance, repair and regeneration through the action of paracrine factors.45,46 Therefore, exploiting or mimicking elements of the MSC niche in vitro is becoming increasingly important for improving the efficacy of current tissue engineering research.47,48 Findings presented in this study, together with our previously published data, support the hypothesis that maintaining cellular heterogeneity within adipose and bone marrow cultures facilitates MSC differentiation.25 Additionally, employing TCP-adherence rather than targeted cell selection for the isolation of MSCs increases the initial degree of heterogeneity, potentially reducing phenotypic changes known to occur when MSCs are removed from their native environment, and thereby facilitating a more stable ex vivo transition.49
The differentiation capacity of unlabelled cells was significantly increased when compared with CD29+/CD90+ cells. However, this study identified that unlabelled cells had a decreased capacity for differentiation when compared with unsorted cells. This suggests that cell selection at a low sort rate using a cuvette-based cell sorter (FACSAria II) had a small yet identifiable effect on MSC differentiation. Flow cytometry both profiles and sorts cells via the application of hydrodynamic pressure, which is used to align the cells into single droplets that can be accurately analysed. However, previous studies have indicated that changes in gene expression can result from nuclear distortions occurring from the application of pressure to cells, with MSCs being particularly susceptible due to the presence of large nuclei and relatively minimal cytoplasm.50–52 Additionally, mechanical stimulation has been found to influence the expression of pluripotent genes such as Nanog and Sox2 through rearrangement of the actin cytoskeleton.53,54 In light of this information, it could be hypothesised that hydrodynamic forces encountered during FACS may cause distortions in cell and/or nuclear shape, and that these changes may be associated with alterations in gene expression and differentiation, which were observed for ADCs and BMDCs in this study. In light of this information, it becomes necessary to further define the benefits or drawbacks of using a cuvette-based flow cytometer when isolating cells for tissue engineering applications. We identify that further studies will need to be conducted in order to compare the effects of cuvette-based flow cytometry, highlighted in this study, with the alternative ‘jet-in-air’ approaches. Finally, we suggest that immunomagnetic cell sorting may represent a comparatively sensitive approach when compared with the cuvette-based FACS system. As such, this approach may represent more appropriate methods of cell selection if isolating MSCs for downstream applications.55,56
In addition to hydrodynamic stresses encountered during FACS, antibody labelling, blocking, washing and transportation, which precede the FACS process, require the storage of cells in a low-serum FACS buffer, which we hypothesised may have a negative effect on cell viability. In this study, cell viability was assessed over a 270-min period to investigate differences in protocol, as well as differences in possible transport times, which would be useful for those that do not have a FACS instrument located on site. Our data indicated site-specific differences in the ability of CD29+/CD90+ cells isolated from two different sources to survive during maintenance in FACS buffer and following FACS, with ADCs seemingly representing a more robust cell type than BMDCs. Data supporting the comparative robustness of stem/progenitor cells derived from adipose tissue are corroborated by a previous study showing that ADCs maintain their phenotype with age and increasing time spent in culture.57 The ability of ADCs to survive out of culture for longer periods than BMDCs and survive hydrodynamic stresses encountered during FACS may also be related to the discovery of a population of multi-lineage differentiating stress-enduring (Muse) cells within adipose tissue.58 These cells are able to endure extreme stresses such as hypoxia, serum deprivation, long-term exposure to proteolytic enzymes such as collagenase and low temperatures.59,60 Similar cell populations with the ability to survive extreme stress have not yet been identified within bone marrow.
To conclude, this study showed that CD29+/CD90+ cells isolated from rat adipose and bone marrow tissues demonstrated a reduced differentiation capacity when compared with MSCs isolated using TCP-adherence. As a result of this study, we identify that CD29+/CD90+ pre-sorting may not be the most appropriate selection criterion for the isolation of multipotent cells from rats. We also identify that maintaining heterogeneity within MSC cultures may be of benefit for improved differentiation. We acknowledge that further characterisation of rat MSCs will be required if they are to provide accurate and translatable tissue engineering models that can be used in pre-clinical studies. Finally, our findings also have implications for the use of cuvette-based flow cytometry for MSC isolation for translational tissue regeneration applications. Given the prevalence of this method of flow cytometry for the isolation of MSCs preceding tissue engineering, further studies into the effects of hydrodynamic forces experienced during cell sorting on cellular function are of importance.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This study was supported by the College of Medical and Dental Sciences, University of Birmingham, PhD award (to Dr O. Davies).
References
- 1. Dominici M, Le Blanc Mueller I, Slaper-Cortenbach I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2005; 8(4): 315–317. [DOI] [PubMed] [Google Scholar]
- 2. Hermida-Gomez T, Fuentes-Boquete I, Gimeno-Longas EJ, et al. Quantification of cells expressing mesenchymal stem cell markers in healthy and osteoarthritic synovial membranes. J Rheumatol 2011; 38(2): 339–349. [DOI] [PubMed] [Google Scholar]
- 3. Harting M, Jimenez F, Pati S, et al. Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy 2008; 10: 243–253. [DOI] [PubMed] [Google Scholar]
- 4. Scuteri A, Donzelli E, Foudah D, et al. Mesengenic differentiation: comparison of human and rat bone marrow mesenchymal stem cells. Int J Stem Cells 2014; 7(2): 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shin M, Yoshimoto H, Vacanti JP. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng 2004; 10(1–2): 33–41. [DOI] [PubMed] [Google Scholar]
- 6. Osugi M, Katagiri W, Yoshimi R, et al. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A 2012; 18(13–14): 1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yao S, He H, Gutierrez DL, et al. Expression of bone morphogenetic protein-6 in dental follicle stem cells and its effect on osteogenic differentiation. Cells Tissues Organs 2013; 198(6): 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lennon DP, Haynesworth SE, Arm DM, et al. Dilution of human mesenchymal stem cells with dermal fibroblasts and the effects on in vitro and in vivo osteochondrogenesis. Dev Dyn 2000; 219: 50–62. [DOI] [PubMed] [Google Scholar]
- 9. Schrepfer S, Deuse T, Lange C, et al. Simplified protocol to isolate, purify, and culture expand mesenchymal stem cells. Stem Cells Dev 2007; 16: 105–107. [DOI] [PubMed] [Google Scholar]
- 10. Rada T, Reis RL, Gomes ME. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev 2011; 7: 64–76. [DOI] [PubMed] [Google Scholar]
- 11. Guilak F, Lott KE, Awad HA, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol 2006; 206(1): 229–237. [DOI] [PubMed] [Google Scholar]
- 12. Zhang L, Chan C. Isolation and enrichment of rat mesenchymal stem cells (MSCs) and separation of single-colony derived MSCs. J Vis Exp 2010; 37 DOI: 10.3791/1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manini I, Gulino L, Gava B, et al. Multi-potent progenitors in freshly isolated and cultured human mesenchymal stem cells: a comparison between adipose and dermal tissue. Cell Tissue Res 2011; 344(1): 85–95. [DOI] [PubMed] [Google Scholar]
- 14. Wieczorek G, Steinhoff C, Schulz R, et al. Gene expression profile of mouse bone marrow stromal cells determined by cDNA microarray analysis. Cell Tissue Res 2003; 311(2): 227–237. [DOI] [PubMed] [Google Scholar]
- 15. Vieira NM, Brandalise V, Zucconi E, et al. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant 2010; 19(3): 279–289. [DOI] [PubMed] [Google Scholar]
- 16. Jung HG, Ahn EK, Lee JH, et al. Effects of harvesting sites and ages of adipose tissue-derived stem cells in rat. Tissue Eng Regen Med 2014; 11(2): 137–142. [Google Scholar]
- 17. Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466(7308): 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim WS, Han J, Hwang SJ, et al. An update on niche, composition, signalling and functional regulation of the adipose-derived stem cells. Expert Opin Biol Ther 2014; 14(8): 1091–1102. [DOI] [PubMed] [Google Scholar]
- 19. Chung MT, Liu C, Hyun JS, et al. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 2013; 19: 989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 2008; 135(2): 240–249. [DOI] [PubMed] [Google Scholar]
- 21. Zavan B, Giorgi C, Bagnara GP, et al. Osteogenic and chondrogenic differentiation: comparison of human and rat bone marrow mesenchymal stem cells cultured into polymeric scaffolds. Eur J Histochem 2007; 51(Suppl. 1): 1–8. [PubMed] [Google Scholar]
- 22. Hayashi O, Katsube Y, Hirose M, et al. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int 2008; 82: 238–247. [DOI] [PubMed] [Google Scholar]
- 23. Friedenstein AJ, Piatetzky-Shapiro, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 1966; 16(3): 381–390. [PubMed] [Google Scholar]
- 24. Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 1988; 136: 42–60. [DOI] [PubMed] [Google Scholar]
- 25. Davies OD, Cooper PR, Shelton RM, et al. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab. Epub ahead of print 6 July 2014. DOI: 10.1007/s00774-014-0601-y. [DOI] [PubMed] [Google Scholar]
- 26. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13: 4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Francis MP, Sachs PC, Elmore LW, et al. Isolating adipose-derived mesenchymal stem cells from lipoaspirate blood and saline fraction. Organogenesis 2010; 6(1): 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 2009; 4: 102–106. [DOI] [PubMed] [Google Scholar]
- 29. Castano-Izquierdo H, Alvarez-Barreto J, van den Dolder J, et al. Pre-culture period of mesenchymal stem cells in osteogenic media influences their in vivo bone forming potential. J Biomed Mater Res A 2007; 82(1): 129–138. [DOI] [PubMed] [Google Scholar]
- 30. Kyllonen L, Haimi S, Mannerstrom B, et al. Effects of different serum conditions on osteogenic differentiation of human adipose stem cells in vitro. Stem Cell Res Ther 2013; 4(1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gregory CA, Gunn WG, Piester A, et al. An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 2004; 1: 77–84. [DOI] [PubMed] [Google Scholar]
- 32. Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res 2002; 81: 531–535. [DOI] [PubMed] [Google Scholar]
- 33. Penny J, Harris P, Shakesheff KM, et al. The biology of equine mesenchymal stem cells: phenotypic characterisation, cell surface markers and multilineage differentiation. Front Biosci 2012; 17: 892–908. [DOI] [PubMed] [Google Scholar]
- 34. Lorenzo S, Gitto S, Grandini E, et al. Stem cells for end stage liver disease: how far have we got? World J Gastroenterol 2008; 14: 4593–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kebriaei P, Madden T, Wang X, et al. Intravenous BU plus Mel: an effective, chemotherapy-only transplant conditioning regimen in patients with ALL. Bone Marrow Transplant 2013; 48(1): 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thomson JA, ItskovitzEldor J, Shapira SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–1147. [DOI] [PubMed] [Google Scholar]
- 37. Tomioka I, Maeda T, Shimada H, et al. Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes Cells 2010; 15(9): 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tondreau T, Lagneaux L, Dejeneffe M, et al. Isolation of BM mesenchymal stem cells by plastic adhesion or negative selection: phenotype, proliferation kinetics and differentiation potential. Cytotherapy 2004; 6(4): 372–379. [DOI] [PubMed] [Google Scholar]
- 39. Cavallo C, Cuomo C, Fantini S, et al. Comparison of alternative mesenchymal stem cell sources for cell banking and musculoskeletal advanced therapies. J Cell Biochem 2011; 112(5): 1418–1430. [DOI] [PubMed] [Google Scholar]
- 40. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007; 100(9): 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Birmingham E, Niebur GL, McHugh PE, et al. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater 2012; 23: 13–27. [DOI] [PubMed] [Google Scholar]
- 42. Sharma MB, Limaye LS, Kale VP. Mimicking the functional hematopoietic stem cell niche in vitro: recapitulation of marrow physiology by hydrogel-based three-dimensional cultures of mesenchymal stromal cells. Haematologica 2012; 97(5): 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spath L, Rotilio V, Alessandrini M, et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J Cell Mol Med 2010; 14(6B): 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Priya N, Sarcar S, Majumdar AS, et al. Explant culture: a simple, reproducible, efficient and economic technique for isolation of mesenchymal stromal cells from human adipose tissue and lipoaspirate. J Tissue Eng Regen Med 2014; 8(9): 706–716. [DOI] [PubMed] [Google Scholar]
- 45. Mitsiadis TA, Barrandon O, Rochat A, et al. Stem cell niches in mammals. Exp Cell Res 2007; 313(16): 3377–3385. [DOI] [PubMed] [Google Scholar]
- 46. Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 2013; 502(7472): 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peerani R, Zandstra PW. Enabling stem cell therapies through synthetic stem cell-niche engineering. J Clin Invest 2010; 120(1): 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu M, Liu N, Zang R, et al. Engineering stem cell niches in bioreactors. World J Stem Cells 2013; 5(4): 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum 2002; 46: 3349–3360. [DOI] [PubMed] [Google Scholar]
- 50. Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res 2008; 102: 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karaoz E, Aksoy A, Ayhan S, et al. Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem Cell Biol 2009; 132: 533–546. [DOI] [PubMed] [Google Scholar]
- 52. Bray MA, Adams WJ, Geisse NA, et al. Nuclear morphology and deformation in engineered cardiac myocytes and tissues. Biomaterials 2010; 31: 5143–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Teramura T, Takehara T, Onodera Y, et al. Mechanical stimulation of cyclic tensile strain induces reduction of pluripotent related gene expressions via activation of Rho/ROCK and subsequent decreasing of AKT phosphorylation in human induced pluripotent stem cells. Biochem Biophys Res Commun 2012; 417: 836–841. [DOI] [PubMed] [Google Scholar]
- 54. Horiuchi R, Akimoto T, Hong Z, et al. Cyclic mechanical strain maintains Nanog expression through PI3K/Akt signaling in mouse embryonic stem cells. Exp Cell Res 2012; 318: 1726–1732. [DOI] [PubMed] [Google Scholar]
- 55. Miltenyi S, Muller W, Weichel W, et al. High gradient magnetic cell separation with MACS. Cytometry 1990; 11(2): 231–238. [DOI] [PubMed] [Google Scholar]
- 56. Seidl J, Knuechel R, Kunz-Schughart LA. Evaluation of membrane physiology following fluorescence activated or magnetic cell separation. Cytometry 1999; 36: 102–111. [DOI] [PubMed] [Google Scholar]
- 57. Wu W, Niklason L, Steinbacher DM. The effect of age on human adipose-derived stem cells. Plast Reconstr Surg 2013; 131: 27–37. [DOI] [PubMed] [Google Scholar]
- 58. Ogura F, Wakao S, Kuroda Y, et al. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev 2014; 23(7): 717–728. [DOI] [PubMed] [Google Scholar]
- 59. Heneidi S, Simerman AA, Keller E, et al. Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PLoS ONE 2013; 8: e64752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kuroda Y, Wakao S, Kitada M, et al. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc 2013; 8: 1391–1415. [DOI] [PubMed] [Google Scholar]