Abstract
Platelet-rich plasma has been used to treat articular cartilage defects, with the expectations of anabolic and anti-inflammatory effects. However, its role on cellular chondrogenic or fibrogenic commitment is still a controversy. Herein, the role of platelet-rich plasma releasate, the product obtained following platelet-rich plasma activation, on cellular commitment toward the chondrogenic lineage was evaluated in vitro. Human nasoseptal chondrogenic cells and human bone marrow mesenchymal stromal cells were used as cell types already committed to the chondrogenic lineage and undifferentiated cells, respectively, as different concentrations of platelet-rich plasma releasate were tested in comparison to commonly used fetal bovine serum. Low concentration of platelet-rich plasma releasate (2.5%) presented similar effects on cellular growth compared to 10% fetal bovine serum, for both cell types. In a three-dimensional culture system, platelet-rich plasma releasate alone did not induce full nasoseptal chondrogenic cells cartilage-like pellet formation. Nonetheless, platelet-rich plasma releasate played a significant role on cell commitment as high-passage nasoseptal chondrogenic cells only originated cartilage-like pellets when expanded in the presence of platelet-rich plasma releasate rather than fetal bovine serum. Histological analyses and measurements of pellet area demonstrated that even low concentrations of platelet-rich plasma releasate were enough to prevent nasoseptal chondrogenic cells from losing their chondrogenic potential due to in vitro expansion thereby promoting their recommitment. Low concentration of platelet-rich plasma releasate supplemented in chondrogenic medium also increased the chondrogenic potential of mesenchymal stromal cells seeded on collagen-hyaluronic acid scaffolds, as observed by an increase in chondrogenic-related gene expression, sulfated glycosaminoglycan production, and compressive modulus following in vitro culture. On the contrary, higher concentration of platelet-rich plasma releasate (10%) hampered some of these features. In conclusion, platelet-rich plasma releasate was able to prevent cellular chondrogenic capacity loss, inducing regain of their phenotype, and modulate cell commitment. Our data support the hypothesis of platelet-rich plasma chondrogenic potential, allowing fetal bovine serum substitution for platelet-rich plasma releasate at specific concentrations in culture medium when chondrogenic commitment is desired on specific cell types and moments of culture.
Keywords: Platelet-rich plasma, chondrogenesis, cartilage repair, mesenchymal stromal cells, nasoseptal chondrogenic cells
Introduction
Articular cartilage is an avascular, alymphatic, aneural, and hypocellular tissue. Such characteristics contribute to its low self-regenerative capacity.1 Chondrocytes produce cartilage extracellular matrix (ECM) and, under physiological conditions, these cells efficiently contribute to tissue turnover. Nonetheless, under pathological conditions, such as large chondral defects, chondrocytes are unable to proliferate, migrate, and synthesize de novo cartilage ECM in order to promote tissue regeneration.2 Current treatments are based on palliative strategies such as analgesics and nonsteroidal anti-inflammatory drugs administration; nonreparative such as debridement, chondral shaving, and knee joint lavage; and reparative such as microfracture, and subchondral drilling, which are based on stimulation of proliferation and differentiation of subchondral bone marrow progenitor cells resulting in fibrous tissue formation.3
Cell-based therapies emerged as a promising clinical intervention in cartilage defect repair. Autologous chondrocyte implantation (ACI) is one such approach whereby autologous chondrocytes are isolated from a healthy nonweight bearing region of the joint, expanded in vitro, and subsequently implanted into the lesion.4,5 Second- and third-generation ACI approaches, which rely on the use of collagen membranes and porous scaffolds, respectively, have demonstrated more advanced regenerative potential.6 However, the drawbacks associated with the use of autologous chondrocytes still prevail, including donor site morbidity and limited tissue source. Moreover, fibrous tissue formation after cell implantation is associated with a major drawback, chondrocyte dedifferentiation during in vitro expansion.7,8 Although the use of recombinant factors such as TGF-β1, FGF-2, PDGF-BB, and IGF-1 can effectively promote chondrocyte redifferentiation in vitro,9–11 the production of such therapeutics represents a high cost procedure. These and other factors such as TGF-β2,12 HGF,13 and BMP-2,14 which have also been shown to stimulate chondrogenesis in vitro, are present in platelet alpha granules and are released upon platelet activation.15 These observations indicate that platelet-rich plasma releasate (PRPr) might be an interesting source of chondrogenic factors.
Platelets can be easily and cost-effectively separated from other blood components, concentrated in plasma to form platelet-rich plasma (PRP), and when activated with exogenous thrombin or calcium can trigger endogenous thrombin formation and importantly the release of high concentrations of different cytokines and growth factors.16 It has been recently reviewed that although preclinical and clinical reports generally show promising histological and clinical results when PRP is used in vivo, there is still inconclusive scientific evidence to support its use for the repair of cartilage defects.17–19
Besides articular chondrocytes, many cell types have been identified as promising for future cell therapy protocols to treat cartilage defects.20,21 Some progenitors are found in an undifferentiated state, as mesenchymal stromal cells (MSCs), and others are already committed to the chondrogenic lineage, as human nasoseptal chondrogenic cells (NCCs). MSCs are characterized mostly by their multipotency and ability to originate colonies of fibroblast-like cells in vitro.22,23 These cells can be isolated from a range of different tissue types such as bone marrow, adipose tissue, and synovia.24 Although MSCs could be used therapeutically for cartilage repair,3 the addition of growth factors is traditionally required to promote their differentiation toward the chondrogenic lineage in vitro, in particular, factors from the TGF-β superfamily.25 Human NCCs, on the other hand, residing on the surface of the nasoseptal hyaline cartilage, can be easily harvested and cultured, and have been demonstrated to have an intrinsic chondrogenic commitment. In the absence of growth factors, NCCs up to the third passage are able to synthesize a hyaline-like cartilage in a pellet culture system.26 The chondrogenic capacity of progenitor cells from the nasal septum is also being investigated by other groups.27–29 Using two different cell culture models, NCCs and MSCs, the aim of this study was to investigate the effect of PRPr on cell proliferation and commitment (or loss of it) toward the chondrogenic lineage in vitro.
Materials and methods
PRP preparation
Two methods for PRPr preparation were used. The first protocol was based on using platelet concentrate bags, which would not be used in clinics, following approval from the Research Ethics Committee of the Instituto Estadual de Hematologia Arthur de Siqueira Cavalcanti (201042).
Platelet concentrate (n = 7) was transferred to 50 mL Falcon tubes (Corning Incorporated, Corning, NY, USA). Platelet counting was performed by the Brilliant Blue Cresyl (Sigma Chemical Co., St Louis, MO, USA) staining and observation under hemocytometer. For inducing platelet activation, 1 M CaCl2 solution was added to tubes, in a proportion of 1:50, reaching a final concentration of 20 mM CaCl2. Tubes were maintained at 37°C for 1 h, after that a clot was formed. Tubes were then incubated overnight at 4°C for clot retraction. Finally, tubes were centrifuged at 3000 r/min for 5 min (Centrifuge 5810 R; Eppendorf). The final supernatant (PRPr) was frozen at −20°C for future in vitro analysis.
The second protocol was based on immediate use of blood collection, following consent and under the Royal College of Surgeons in Ireland (RCSI) Ethics Committee approval (REC676), as previously described, with minor modifications.30
Briefly, 54 mL peripheral blood from six donors (n = 6) was collected into 60-mL syringe filled with 6 mL of 3.2% sodium citrate solution (Sigma). Blood was transferred to 50-mL tube (Corning) and centrifuged at 300g for 5 min. The buffy coat and the red blood cell layer remained intact, while the upper fraction was transferred to new tubes, which was further centrifuged at 700g for 17 min. After second centrifugation step, platelets pellet was resuspended with the supernatant (platelet-poor plasma), forming the inactivated PRP. Platelets were counted using a hematology analyzer (Sysmex, Kobe, Japan). The platelet activation was achieved as previously described for protocol 1.
Scaffold fabrication
Collagen-hyaluronic acid (CHyA) scaffolds were fabricated as previously described.31 Briefly, collagen type I derived from bovine Achilles tendon (Collagen Matrix, Oakland, NJ, USA) and hyaluronic acid (HyA) sodium salt derived from streptococcus equi (Sigma-Aldrich, Arklow, Ireland) were blended with a 0.5 M acetic acid (Sigma) solution to give suspensions with final concentrations of 0.5% (w/v) collagen and 0.05% (w/v) HyA. Subsequently, the suspensions were freeze-dried (Virtis Genesis 25EL; Biopharma, Winchester, UK) to a final freezing temperature of −10°C with an additional annealing step prior to drying. The resultant scaffolds were then crosslinked using dehydrothermal treatment (DHT) under vacuum of 50 mTorr and a temperature of 105°C in a vacuum oven (VacuCell; MMM, Germany) for a duration of 24 h.
NCC isolation and culture
Human NCCs were isolated and cultured as previously described.26 Briefly, cartilage fragments from nasoseptal were obtained from three donors (n = 3) that underwent aesthetic surgery procedures, with informed consent and with the approval of the Research Ethics Committee of the Clementino Fraga Filho University Hospital, Federal University of Rio de Janeiro, Brazil (766/09). Fragments were rapidly incubated with collagenase IA (Sigma). Cells were harvested by centrifugation and cultured in tissue culture flasks with Iscove’s Modified Dulbecco’s Medium (IMDM; Sigma Chemical Co.) containing 10% fetal bovine serum (FBS; LGC), 100 U/mL penicillin, and 100 µg/mL streptomycin. Cultures were maintained at 37°C in a humid atmosphere with 5% CO2, and the medium was changed twice a week until cell monolayer reached 80%–90% confluence. By this level, cells were harvested with 0.78 mM ethylenediaminetetraacetic acid (EDTA; Gibco BRL, Rockville, MD, USA) and 0.125% trypsin (Gibco) and re-seeded at a density of 104 cells/cm2. Dissociation with trypsin followed by re-seeding for cell expansion was denominated “passage.” Time elapsed on each passage was about 1 week. Freshly isolated cells, that is, isolated from fragments but not underwent trypsin harvesting yet, were considered to be on passage 0. Until passage 1, all cells were cultured with 10% FBS. For three-dimensional (3D) culture, NCCs in third and fifth passages were transferred to pellet culture system (n = 3, for each condition).32 For third passage pellets, cells were cultured in passage 2 in 10% FBS (control condition), 2.5% PRPr or 10% PRPr. For fifth passage pellets, cells were cultured in passages 2, 3, and 4 in 10% FBS (control condition), 2.5% PRPr, or 10% PRPr. In another experiment, cells were cultured in 10% FBS until passage 3 and on passage 4 the medium was changed to 2.5% PRPr or 10% PRPr. The PRPr used in these experiments was pooled from four different donors. For pellet assembly, cells were harvested and centrifuged at 300g for 10 min into 15-mL tubes (Corning) at a concentration of 2 × 105 cells/tube. Media was replaced twice a week, while the pellets remained intact. Chondrogenic control media was composed of IMDM (Gibco) supplemented with 1 µM insulin (Sigma), 8 × 10−8 M transferrin (Sigma), 2 × 10−8 M bovine serum albumin (Sigma), and 2.5 × 10−4 M ascorbic acid (Sigma). For the chondrogenic inductor medium, 10 ng/mL TGF-β2 (R&D Systems, MN) was added to the chondrogenic control medium. In some experiments, IMDM supplemented with 10% FBS (control condition), 1% PRPr, 2.5% PRPr, or 10% PRPr were directly used to culture pellets. After 21 and 35 days, cultures were fixed in 4% paraformaldehyde and embedded in paraffin for histological analysis. The culture conditions for NCCs are summarized in a table of culture conditions (Table 1).
Table 1.
Table of culture conditions.
| Passage 2 | Passage 3 | Passage 4 | Passage 5 | Passage 6 | Passage 7 (in scaffold) | Condition | |
|---|---|---|---|---|---|---|---|
| NCCs | 10% FBS | Pellet | X | X | X | X | Passage 2 in 10% FBS |
| 2.5% PRPr | X | X | X | X | Passage 2 in 2.5% PRPr | ||
| 10% PRPr | X | X | X | X | Passage 2 in 10% PRPr | ||
| 10% FBS | Pellet | X | X | Passages 2 to 4 in 10% FBS | |||
| 2.5% PRPr | X | X | Passages 2 to 4 in 2.5% PRPr | ||||
| 10% PRPr | X | X | Passages 2 to 4 in 10% PRPr | ||||
| 10% FBS | 2.5% PRPr | X | X | Passage 4 in 2.5% PRPr | |||
| 10% FBS | 10% PRPr | X | X | Passage 4 in 10% PRPr | |||
| MSCs | 10% FBS | 2.5% PRPr | 2.5% PRPr | ||||
| 10% FBS | 10% PRPr | 10% PRPr | |||||
| 10% FBS | CM + 2.5% PRPr | CM + 2.5% PRPr | |||||
| 10% FBS | CM + 10% PRPr | CM + 10% PRPr | |||||
| 10% FBS | 2.5% PRPr | CM + 10% FBS | CM + 10% FBS_PRPr exp | ||||
| 10% FBS | CM + 10% FBS | CM + 10% FBS_FBS exp |
NCCs: nasoseptal chondrogenic cells; MSCs: mesenchymal stromal cells; FBS: fetal bovine serum; PRPr: platelet-rich plasma releasate; CM: chondrogenic medium.
All culture conditions summarized for NCCs and MSCs. Passages where no culture or experiment was performed on the determined condition are marked with an X symbol. Light green shade corresponds to 10% FBS treatment, yellow to 2.5% PRPr, light blue to 10% PRPr, orange to CM + 2.5% PRPr, dark blue to CM + 10% PRPr, and dark green to CM + 10% FBS.
MSC isolation and culture
Human MSCs (hMSCs) were commercially acquired (PT-2501; Lonza, Switzerland). Cells were expanded in culture using hMSC growth medium consisting of low-glucose Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 1% penicillin/streptomycin (Sigma-Aldrich) and 10% FBS and maintained in a tissue culture incubator at 37°C and 5% CO2. Cells were detached from culture flasks using trypsin EDTA (Sigma-Aldrich). Subsequently, cells were counted and resuspended at a cell density of 10 × 106 cells/mL of medium. For 3D culture, 9.5-mm diameter, 4-mm height scaffolds were pre-hydrated in phosphate buffered saline (PBS) for 15 min at room temperature. The excess PBS was removed from the scaffolds, which were then placed onto 24-well suspension plates (Sarstedt, Drinagh, Ireland). A total of 0.5 × 106 cells were seeded onto each scaffold (0.25 × 106 cells on each side of the scaffold). The scaffolds were subsequently incubated for 15 min before addition of medium. The medium was changed every 3 days from the scaffolds, and the study was carried out for 14 days. The medium was supplemented with either PRPr alone (2.5% or 10%) or a combination of PRPr (2.5% or 10%) and chondrogenic factors or a combination of 10% FBS and chondrogenic factors (control condition). In one condition, cells were previously expanded on 2.5% PRPr supplemented hMSC growth medium in substitution to 10% FBS prior to seeding onto the scaffolds. The chondrogenic factors were 20 ng/mL human TGF-β3 (Prospec, Rehovot, Israel), 50 µg/mL ascorbic acid (Sigma-Aldrich), 40 µg/mL proline (Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich), 1× ITS (Insulin, Transferrin, Selenium) (BD Biosciences, Oxford, UK), and 0.11 mg/mL sodium pyruvate (Sigma-Aldrich). The culture medium supplemented with these chondrogenic factors was termed as chondrogenic medium (CM). The PRPr used in these experiments was pooled from four different donors. After 14 days, samples were separated for gene expression analysis (n = 3, for each condition), sulfated glycosaminoglycan (sGAG) quantification (n = 3, for each condition), and compressive modulus evaluation (n = 3, for each condition). The culture conditions for MSCs are summarized in a table of culture conditions (Table 1).
Cell proliferation assay
Cells were seeded in 96-well plates in quadruplicates at 1.2 × 104 cells/cm2. Cells were cultured for up to 7 days in IMDM (Gibco) supplemented with 10% FBS (control condition) or PRPr in different concentrations (from 1% to 10%). Medium was not changed until the end of treatment. For this experiment, three PRP samples were used as well as a mix of those samples in equal parts for each cell type. Neutral red uptake assay was performed to evaluate cells viability, due to its higher economic advantages and sensibility than other cytotoxicity tests.33 Briefly, 0.005% neutral red (Sigma) solution was incubated for 3 h. A solution containing 50% ethanol, 1% acetic acid, and 49% water was added to elute the red staining, and absorbance was measured at 490 nm (Spectramax M5; Molecular Devices, Sunnyvale, CA, USA). Since the number of cells seeded was similar in every condition, the increase in the number of viable cells over time indicates cell proliferation.
Histology
Samples were fixed in 10% formalin for 1 h and using a Histokinette tissue processor (Leica TP1050), were dehydrated and paraffin wax embedded. Blocks of wax embedded samples were serially sectioned using a microtoming machine (Leica RM2255) which sliced the samples at 5-µm-thick sections. The sections were successively mounted on polylysine-coated glass slides (Fisher-Scientific, Ireland). The slides were deparaffinized and hydrated before they were stained with alcian blue 1% at pH 1.0 (w/v, pH 1.0) (Sigma), to demonstrate sGAG deposition. Then, slides were counterstained with neutral red 0.5% (w/v) (Sigma). The sections were subsequently dehydrated and mounted with cover slides using distyrene–plasticizer– xylene (DPX). Images were obtained with a microscope (Leica DMI 6000 B) equipped with a digital camera (Leica DFC 500). Alcian blue pH 1.0 staining is routinely used on in vitro chondrogenic induction protocols.34–39 At low pH, all carboxylic and phosphoric acid groups are neutralized, and at a high ionic strength, this tetravalent cation strongly binds to some negatively charged polymers, as glycosaminoglycans (GAGs), but not others, as nucleic acids.40 The analyses of stained sections focused on the determination of the presence of hyaline cartilage characteristics, as an ECM rich in sGAG, where a more intense blue-colored ECM is an indicator of higher sGAG deposition, cells with a round chondrocyte morphology, and an outer fibroblastic perichondrium-like zone.
Pellet area measurement
After samples were fixed, but before dehydration for paraffin embedment, images were obtained with a microscope (Leica DMI 6000 B) equipped with a digital camera (Leica DFC 500). Low magnification was used in order for the entire sample to be captured. The measurement of the pellet area in those images was then performed using the Fiji Is Just ImageJ 1.49a software.41
Quantitative polymerase chain reaction
For gene expression analysis, total RNA was isolated using an RNeasy kit (Qiagen, Crawley, UK) as previously described (Duffy et al., 2011). A total of 200 ng of total RNA was reverse transcribed to complementary DNA (cDNA) using a QuantiTect reverse transcription kit (Qiagen) on an authorized thermal cycler (Mastercycler Personal; Eppendorf, Stevenage, UK). Real-time polymerase chain reactions (PCRs) were run on 7500 real-time PCR System (Applied Biosystems, Paisley, UK) using a QuantiTect SYBR Green PCR Kit (Qiagen). The relative expression of messenger RNA (mRNA) was calculated by delta–delta Ct (ΔΔCt) method, where the fold increase of gene expression was 2−(ΔΔCt).42 Target mRNA analyzed were SOX9, COL2A1, ACAN, and COL10A1, with GAPDH used as a housekeeping gene.
sGAG quantification
For sGAG quantification, scaffolds were digested in papain enzyme solution containing 0.5 M EDTA, cysteine-HCl, and 1 mg/mL papain enzyme (Carica papaya; Sigma-Aldrich), and sGAG quantification was performed with Blyscan™ (Biocolor Life Science Assays, Carrickfergus, UK) according to the manufacturer’s instructions. Readings were taken from a photometric plate reader (Wallac 1420 Victor2 D; Perkin Elmer, MA, Waltham, USA) at 656 nm.
Mechanical testing: compressive modulus analysis
For compressive modulus evaluation, unconfined compressive testing was carried out on seeded scaffold samples incubated in PBS using a mechanical testing machine (Zwick-Roell, Rehovot and Ulm, Germany). Briefly, the mechanical testing machine (Z050; Zwick-Roell, Germany) was fitted with a 5-N load cell and tests were conducted at a strain rate of 10% per minute with each sample being tested three times. Stress was calculated from scaffold surface area and applied force, while strain was calculated from displacement of the scaffolds in relation to the original thickness. The compressive modulus was defined based on the slope of a linear fit to the stress–strain curve over 2%–5% strain.
Statistical analysis
Results are expressed as mean ± standard deviation for at least three replicates. One-way nonparametric analysis of variance followed by the Tukey post-hoc test (to compare all pairs in group) was assessed to verify statistical significance; p < 0.05 was considered statistically significant. Statistical analysis was performed using Prism 5.00 software (GraphPad Software Inc., San Diego, CA, USA).
Results
PRPr formulation
Platelet concentration may differ according to the method used to obtain PRP. Herein, we have tested two different centrifugation methods to concentrate platelets and no statistical difference was observed between them. Out of 13 patients, platelet numbers varied from 0.92 to 3.76 × 106 µL−1. These concentrations were further considered to prepare PRPr.
PRPr effect on NCCs
NCC proliferation
To evaluate PRPr activity on cell expansion in vitro, NCCs were cultured in 1%, 2.5%, 5%, and 10% of PRPr supplemented culture medium (Figure 1(a)). Cells cultured in 2.5% of PRPr presented a similar proliferation rate compared to 10% FBS and maintained similar phenotype (Figure 1(b) and (c)). However, the proliferation rate increased when cells were cultured in 5% and 10% of PRPr, and NCCs changed to small polygonal-shaped cells when cultured in 10% PRPr (Figure 1(d)). After these observations, further experiments were achieved only with 2.5% and 10% PRPr. All experiments were achieved using 10% FBS as reference.
Figure 1.
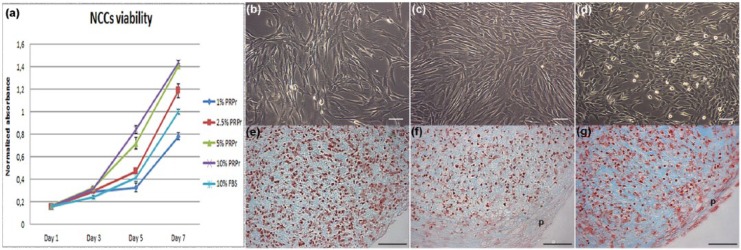
Direct use of PRPr on NCCs expansion and chondrogenic differentiation. (a) NCCs viability curves in the presence of PRPr samples in different concentrations compared to 10% FBS. All values regard to absorbance normalized to 10% FBS neutral red assay absorbance on day 7. The data are presented as a mean and the error bars correspond to standard deviation (SD); (b) 10% FBS–, (c) 2.5% PRPr–, and (d) 10% PRPr–treated cells in monolayer culture in passage 2. Pellets of cells in passage 3 in the presence of (e) 10% FBS, (f) 2.5% PRPr, and (g) 10% PRPr for 21 days. Sulfated GAGs evidenced by alcian blue pH1.0 staining (blue) and cells nuclei by neutral red (red). Outer layer of fibroblastic cells, which regards a perichondrium layer (p). Scale bars: 50 µm.
PRPr: platelet-rich plasma releasate; NCCs: nasoseptal chondrogenic cells; FBS: fetal bovine serum; GAGs: glycosaminoglycans.
PRPr onto NCC pellets
To evaluate PRPr capacity to induce chondrogenic differentiation, third passage NCCs were cultured in 3D pellet system for 21 days in different concentrations of PRPr, as described. PRPr direct administration onto the pellet culture failed to promote full chondrogenic differentiation of NCCs in vitro (Figure 1(e)–(g)). However, pellets cultured in 10% PRPr (Figure 1(g)) presented more intense staining for synthesized ECM, as well as some round-shaped chondrocyte-like cells. Of note, all PRPr-treated pellets presented an outer layer of cells circulating the pellets, in a perichondrium-like structure.
PRPr pre-treatment on low-passage NCCs
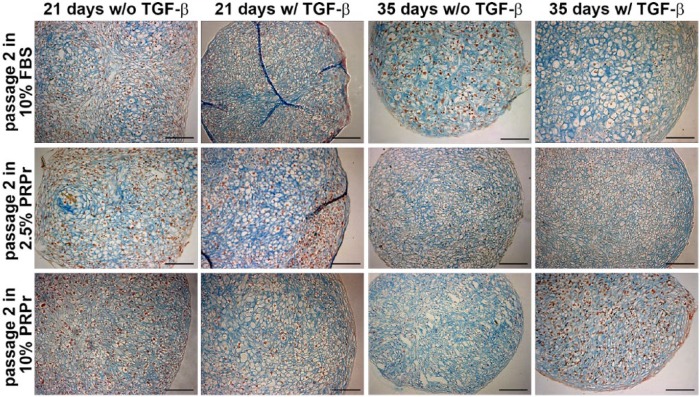
Hereafter, NCCs were cultured in two-dimensions (2D) in 2.5% PRPr, 10% PRPr, and 10% FBS in passage 2 and then transferred to 3D pellet culture in passage 3 with or without TGF-β, to evaluate cartilage-like formation (Figure 2). Similar cartilage-like pellets were formed in all three conditions. However, it can be noticed that, after 21 days of culture, 2.5% PRPr treatment resulted in pellets that best resemble a hyaline cartilage–like structure, with intense ECM staining and more cells with chondrocyte-like morphology. Although no statistical significance was observed, PRPr-treated pellets, in special 10% PRPr, were also larger than 10% FBS–treated pellets (Figure 4(a)). Only after 35 days, 10% FBS treatment resulted in pellets with intense ECM staining, high number of cells with chondrocyte morphology and as large as PRPr-treated ones (Figures 2 and 4(b)).
Figure 2.
Effect of PRPr pre-treatment on passage 3 pellet culture system. Pellets of cells that were pre-treated in 2D in passage 2 in the presence of 10% FBS, 2.5% PRPr, and 10% PRPr for 21 or 35 days. Pellets were cultured in serum-free medium with (w/) or without (w/o) TGF-β. Sulfated GAGs evidenced by alcian blue pH 1.0 staining (blue) and cells nuclei by neutral red (red). Scale bars: 100 µm.
PRPr: platelet-rich plasma releasate; FBS: fetal bovine serum; GAGs: glycosaminoglycans.
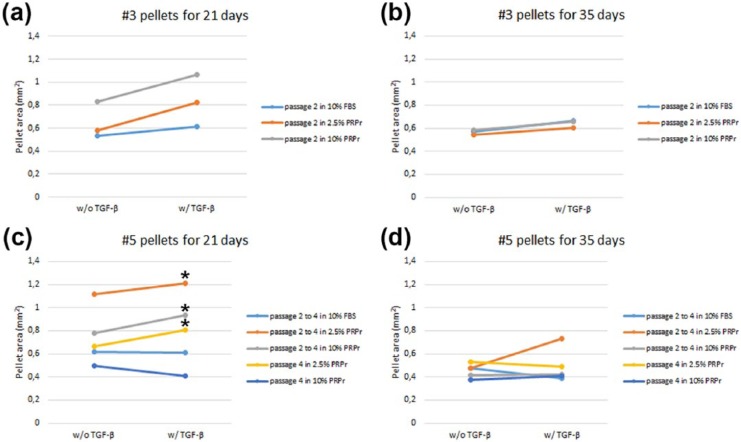
Figure 4.
Quantification of NCCs pellet areas. Areas (mm2) of (a) passage 3 pellets cultured for 21 days, (b) passage 3 pellets cultured for 35 days, (c) passage 5 pellets cultured for 21 days, (d) and passage 5 pellets cultured for 35 days, in medium without (w/o) or with (w/) TGF-β. Asterisks represent statistical significance compared to 10% FBS pre-treated pellets (p < 0.05).
NCCs: nasoseptal chondrogenic cells; FBS: fetal bovine serum.
PRPr pre-treatment on high-passage NCCs
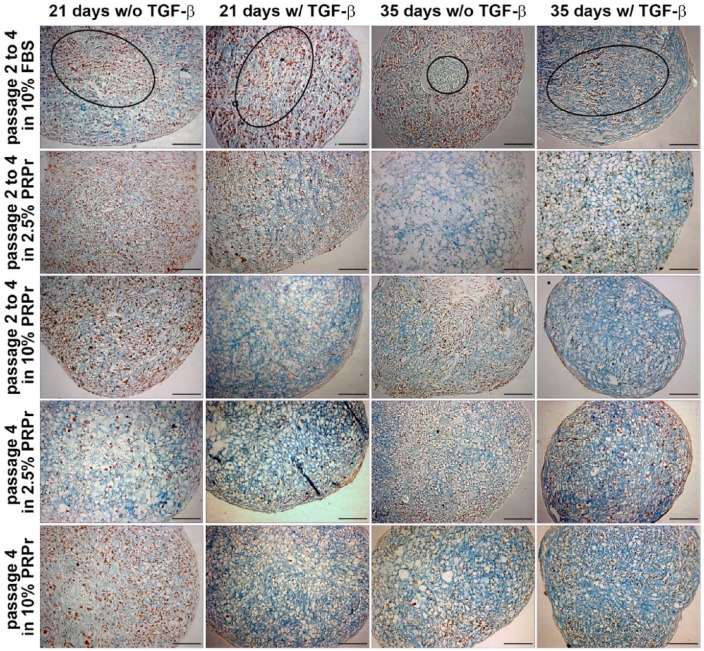
As observed (Figure 2), NCCs can originate a cartilage-like pellet up to the third passage when pre-treated with FBS or PRPr. However, higher passage expansion with FBS is associated with chondrocyte dedifferentiation and loss of chondrogenic capacity.7,8 To test whether a similar process would happen with NCCs and how PRPr could affect it, NCCs were expanded in 2D cultures from passage 2 to passage 4 in the presence of 2.5% and 10% PRPr and then changed to 3D pellet cultures on the fifth passage. Expansion in 10% FBS was used as control (Figure 3). Expansion in 10% FBS clearly hampered NCCs cartilage-like pellet formation. A fibrous inner spot with poor cartilage-like histological characteristics was observed. Only in a few spots of the pellet, cells from the periphery demonstrated mild chondrogenic differentiation. Conversely, PRPr pre-treatment resulted in pellets with intense sGAG production and mostly cells with typical chondrocyte morphology. Moreover, both 2.5% and 10% PRPr pre-treated pellets were significantly larger than 10% FBS pre-treated pellets, all cultured under TGF-β induction, after 21 days of culture. No difference was observed between the two concentrations of PRPr whatsoever (Figure 4(c)). After 35 days of culture under TGF-β induction, 2.5% PRPr pre-treated pellets were again larger than the other groups, although with no statistical significance (Figure 4(d)).
Figure 3.
Effect of PRP pre-treatment on passage 5 pellet culture system. Pellets of cells which were pre-treated in 2D from passage 2 to passage 4 in the presence of 10% FBS, 2.5% PRPr, and 10% PRPr, or until passage 3 in 10% FBS and only in passage 4 in the presence of 2.5% PRPr and 10% PRPr, for 21 or 35 days. Pellets were cultured in serum-free medium with (w/) or without (w/o) TGF-β. Sulfated GAGs evidenced by alcian blue pH 1.0 staining (blue) and cells nuclei by neutral red (red). Inner fibrous layers of 10% FBS pre-treated pellets are highlighted in ellipses. Scale bars: 100 µm.
PRP: platelet-rich plasma; FBS: fetal bovine serum; PRPr: platelet-rich plasma releasate; GAGs: glycosaminoglycans.
PRPr and NCC chondrogenic recommitment
The data presented above indicate that PRPr could prevent NCCs loss of chondrogenic potential after in vitro expansion, at least up to the fifth passage, even at low concentrations. To test whether the releasate would stimulate phenotype “recovery” after NCCs expansion, fourth-passage NCCs were cultured in the presence of PRPr only one passage prior to 3D pellet culture. Our results showed that cells recommitted and differentiated toward the chondrogenic lineage when pre-treated with PRPr (Figure 3). Pellets cultured in these conditions showed evidence of chondrogenesis with more intensively stained and a large area of synthesized sGAG-rich matrix, and more clear round chondrocyte-like cells, than those that were cultured in 10% FBS until passage 4 (Figure 3). Although those remarkable chondrogenic histological characteristics could be found both in 2.5% PRPr and 10% PRPr pre-treated on passage 4 pellets, the 2.5% PRPr pre-treated ones were significantly larger than 10% FBS pre-treated pellets from passage 2 to passage 4 under TGF-β induction and after 21 days of culture (Figure 4(c)).
PRPr effect on MSCs
PRPr effect on MSC proliferation and chondrogenic gene expression
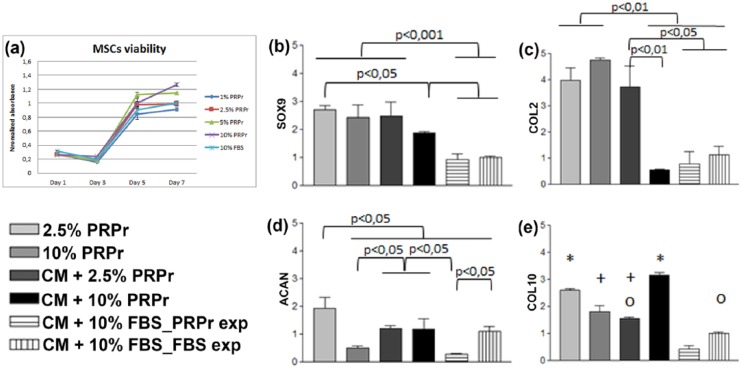
As compared to NCCs, 2.5% PRPr also presented the most similar effects on MSCs proliferation compared to 10% FBS (Figure 5(a)). To verify their chondrogenic differentiation, passage 7 MSCs were seeded on CHyA scaffolds for 14 days in the presence of 2.5% and 10% PRPr alone or in CM supplemented with 2.5% PRPr and 10% PRPr. CM supplemented with 10% FBS was used as reference. FBS group was divided into two subgroups, as MSCs were pre-treated on passage 6 with 10% FBS or 2.5% PRPr. A higher SOX9 expression was observed on PRPr-based conditions (Figure 5(b)), as well as COL2A1 (Figure 5(c)), with the exception of CM supplemented with 10% PRPr. Low concentrations of PRPr led to the highest ACAN expression (Figure 5(d)). CM supplemented with 2.5% PRPr presented COL10A1 expression statistically similar to the control group, which was the CM supplemented with 10% FBS, with cells expanded in 10% FBS (Figure 5(e)).
Figure 5.
PRPr effects on MSC expansion and gene expression. (a) MSC viability curves in the presence of PRPr samples in different concentrations compared to 10% FBS. All values regard to absorbance normalized to 10% FBS neutral red assay absorbance on day 7. The data are presented as a mean and the error bars correspond to standard deviation (SD). MSCs were cultured on CHyA scaffolds supplemented with 2.5% PRPr, 10% PRPr, chondrogenic medium with 2.5% PRPr (CM + 2.5% PRPr), chondrogenic medium with 10% PRPr (CM + 10% PRPr), chondrogenic medium with 10% FBS in cells expanded in 2.5% PRPr (CM + 10% FBS_PRPr exp), and chondrogenic medium with 10% FBS in cells expanded in 10% FBS (CM + 10% FBS_FBS exp), which was considered the control condition, and by which the delta Ct results were normalized. (b) SOX9, (c) COL2A1, (d) ACAN, and (e) COL10A1 were analyzed. The results were normalized to the control condition and expressed as relative expression. For (e), similar symbols between groups represent statistic similarity among them (p > 0.05). The data are presented as a mean, and the error bars correspond to SD.
PRPr: platelet-rich plasma releasate; MSC: mesenchymal stromal cell; FBS: fetal bovine serum; CHyA: collagen-hyaluronic acid; CM: chondrogenic medium.
PRPr effect on MSC sGAG production and CHyA seeded biomechanical properties
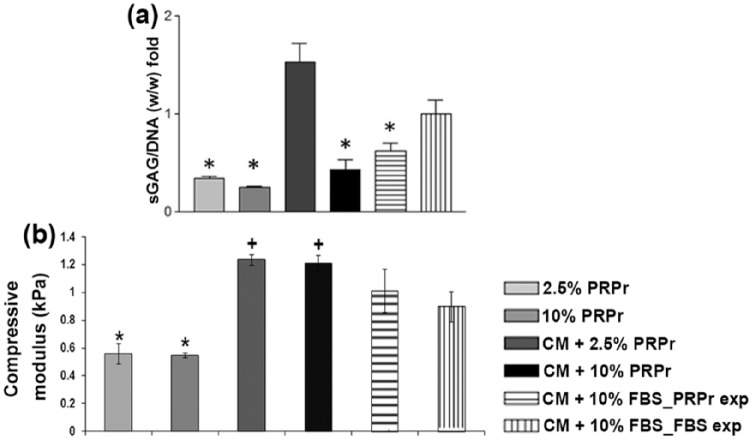
To verify whether higher chondrogenic gene expression on PRPr groups resulted in higher biochemical and biomechanical improvement, sGAG production and compressive modulus evaluation were performed. Biochemical analyses evidenced that higher sGAG production was observed in the CM supplemented with 2.5% PRPr. On the other hand, other conditions showed lower amount of sGAG production than the control group (Figure 6(a)). The groups in which PRPr replaced FBS in the CM presented higher mechanical properties than those maintained in FBS. In contrast, PRPr alone, that is, without the addition of CM, was not enough to induce higher levels of compressive modulus, with values remaining even lower than in CM groups supplemented with 10% FBS (Figure 6(b)).
Figure 6.
Biochemical and biomechanical effects of PRP on CHyA seeded scaffolds following 14 days of culture. sGAG synthesis was evaluated by biochemical analysis and the values were normalized by DNA content. Cultures were supplemented with 2.5% PRPr, 10% PRPr, chondrogenic medium with 2.5% PRPr (CM + 2.5% PRPr), chondrogenic medium with 10% PRPr (CM + 10% PRPr), chondrogenic medium with 10% FBS in cells expanded in 2.5% PRPr (CM + 10% FBS_PRP exp), and chondrogenic medium with 10% FBS in cells expanded in 10% FBS (CM + 10% FBS_FBS exp), which was considered the control condition, by which all results were normalized. (a) Asterisks and cross represent p < 0.05 compared to 10% FBS, and similar symbols between groups represent statistic similarity among them (p > 0.05). Compressive modulus of CHyA seeded scaffolds. (b) Asterisks and cross represent p < 0.05 compared to 10% FBS, and similar symbols between groups represent statistic similarity among them (p > 0.05). The data are presented as a mean and the error bars correspond to standard deviation (SD).
PRP: platelet-rich plasma; CHyA: collagen-hyaluronic acid; sGAG: sulfated glycosaminoglycan; PRPr: platelet-rich plasma releasate; CM: chondrogenic medium; FBS: fetal bovine serum.
Discussion
In this study, using two different cell culture models, we observed that PRPr efficiently modulated cells proliferation, chondrogenic commitment, and differentiation, in a concentration-dependent manner.
PRP concentration apparently has a great impact on clinical outcomes. For bone regeneration, platelet concentration in PRP has an optimal limited concentration range, approximately 106 µL−1 (five times higher than the 200 × 103 µL−1 average platelet concentration in peripheral blood). Lower concentrations present suboptimal effects; on the contrary, higher platelet concentration seems to inhibit bone growth.43 When we cultured cells in 2.5% PRPr, the releasate induced cell proliferation similarly to 10% FBS, both NCCs and MSCs. Other studies pointed PRP concentration around 1% in cells culture medium to be similar to the control group regarding cell proliferation.44,45 On the other hand, human platelet lysate used to expand adipose-derived mesenchymal progenitor cells at the concentration of 2.5% has been previously shown to maintain cells phenotype and ability to differentiate toward adipogenic lineage.46 Our data also showed that no significant increase in cell proliferation was induced when PRPr concentration was changed to 5% and 10%, probably due to the reach of an optimal induction of proliferation already at the concentration of 5%. This phenomenon has been reported previously by another group using articular chondrocytes.47 For meniscus cell culture, no difference in proliferation results has been reported when increasing from 10% to 20% PRP.48For fibroblasts, the plasma rich in growth factors (PRGF) had similar effects on tendon and skin-derived, while a dose-dependent effect was seen on synovium-derived, according to platelet concentration.49
NCCs changed to possess a small polygonal-shape when cultured in higher concentrations of PRPr, compared to the fibroblast-like morphology in lower concentrations of PRPr and the FBS. This change in cell morphology may be correlated to their proliferation rate, where 10% FBS and 2.5% PRPr are similar and 10% PRPr is higher than both. Probably, the lower proliferation rate allows cells to elongate and acquire a fusiform fibroblast-like morphology in culture, which does not occur when cells proliferate more rapidly. To assess the effects of PRPr on cells chondrogenic commitment, a pellet culture system was chosen for NCCs to readily verify through histological analysis this already committed cell population chondrogenic capacity. On the other hand, a scaffold system was chosen for MSCs, as it represents a model for future tissue engineering therapy approach with undifferentiated cells extensively used for this purpose. In this case, gene expression and biochemical and biomechanical analyses were done to evaluate chondrogenic differentiation. When PRPr or FBS were directly incorporated within the NCCs pellets cultures, it was not possible to achieve a chondrogenic differentiation as expected. Similar to our findings, synovium-derived MSC pellets cultured directly in the presence of PRP did not present chondrogenic differentiation, which was only successfully achieved in the presence of a CM supplemented with BMP-2 and TGF-β1.50 In fact, pellet culture system is usually performed under serum-free conditions.32,51 Because of this, instead of adding PRPr directly to pellets, the cells were expanded in the presence of PRPr and subsequently cultured in 3D pellets at the following passage. This approach has previously been utilized for canine chondrocytes, by priming cells with TGF-β1, basic fibroblast growth factor (bFGF), and PDGF-BB, which are also present in PRP.30 In that study, although cell priming during monolayer expansion led to a dedifferentiated phenotype, later pellet culture demonstrated their higher chondrogenic feature compared to control group, where those growth factors were not present.52
At low passage, 2.5% PRPr was enough to further induce NCCs chondrogenic differentiation after a shorter period than cells expanded in 10% FBS. This could be interpreted as the ability of cells to more promptly respond to chondrogenic induction when treated with a low concentration of PRPr compared to 10% FBS. At high passage, cells which have been cultured on PRPr showed pellets with a more similar morphology to hyaline cartilage than those from cells previously cultured on 10% FBS. In vitro culture can alter cell physiology and lead to a loss of chondrogenic capacity,11 and PRPr was able to prevent and reverse it. Taken together with the pellet area measurement results, it is possible to conclude that the use of PRPr at the concentration of 2.5% was enough to maintain NCCs chondrogenic commitment and to induce its recommitment when lost after FBS expansion. Although other in vitro studies point PRP as a dedifferentiation/fibrogenic inducer,44,53 our results are in accordance with studies that demonstrate PRP in vitro chondrogenic induction on chondrocytes.47,54
PRPr supported SOX9, COL2A1, and ACAN gene expression, markers of chondrogenic lineage, and COL10A1 expression, marker of chondrocytes hypertrophy on MSCs seeded on CHyA scaffolds. However, the use of CM supplemented with 2.5% PRPr showed the most remarkable characteristics regarding chondrogenic induction, as well as basal levels of COL10A1 expression. Interestingly, CM supplemented with 10% PRPr did not show increase in COL2A1, as well as presented high levels of COL10A1, which could be an indicator of hypertrophy. However, further evaluation to support the hypothesis of a higher amount of PRPr being related to MSCs hypertrophy is still needed. Although PRPr alone was sufficient to increase some chondrogenic genes, probably due to post-transcription factors, it was not able to induce greater sGAG production nor biomechanical properties, only achieved when CM was also present. The highest sGAG content was observed in the samples cultured with CM supplemented with 2.5% PRPr, while in CM supplemented with 10% PRPr it was lower than the control group. The highest compressive modulus was achieved in CM supplemented with PRPr groups, followed by CM supplemented with FBS groups and finally by PRPr alone groups. Interestingly, the compressive modulus did not present significant difference between 2.5% and 10% PRPr alone or in the presence of CM. In addition, although CM supplemented with 10% FBS had smaller effect on chondrogenic gene expression, the compressive modulus of these scaffolds was higher than of the ones treated with PRPr alone. These could be explained by the fact that the increase in compressive modulus is correlated to various factors. For example, sGAG content has a modest correlation to compressive modulus on tissue-engineered constructs. Other ECM components and the remodeling of the construct throughout culture also affect its mechanical properties.55 The pre-expansion of MSCs on the passage prior to scaffold culture, in a similar way that it was investigated for NCCs, was not possible to induce a higher chondrogenic capacity than those pre-treated with 10% FBS.
Taken together, these results point that the presence of CM for MSC chondrogenesis in this model is highly important. Moreover, the substitution of the traditionally used 10% FBS supplementation in the CM by 2.5% PRPr showed to be an interesting strategy, as evidenced by gene expression, sGAG synthesis, and compressive modulus measurement, while by 10% PRPr could represent hypertrophy. A low concentration of PRPr enhanced the capacity of MSCs to differentiate to the chondrogenic lineage compared to 10% FBS, and although in a different model, in a similar way of the NCCs findings. Nevertheless, as in an undifferentiated state, a higher PRPr concentration could induce MSCs to a different phenotype. It has been shown that when PRP clot is added directly to culture, it presented an inhibition toward chondrogenesis both for chondrocytes and MSCs.56 Probably, the differences in PRP concentration in medium could explain this opposing result compared to our study. On the other hand, it has been recently reviewed that PRP showed to enhance, or at least did not hinder, MSCs chondrogenic phenotype in vitro.57 Also, the role of PRP as a valid augmentation for cartilage scaffolds has been investigated in a number of preclinical studies with the majority confirming promising results for scaffolds combined with PRP.17
Finally, the different effects of low and high concentrations of PRPr on cells chondrogenesis can also be discussed under the light of cell proliferation. It has been shown that between day 0 and day 7 of pellet culture system, the number of MSCs is increased in 30%, thus indicating cell proliferation. From day 7, the number of cells decreases, and collagens, proteoglycans, and other matrix components’ gene expression is generally upregulated.58 Proliferation is indeed a requirement for in vitro chondrogenesis of MSCs. Treatment with mitomycin C, which blocks proliferation, hampered MSC chondrogenic differentiation. On the other hand, little proliferation is observed after 21 days in pellet culture system in areas with strong matrix deposition.34 Those in vitro studies seem to mimic embryonic chondrogenesis, where cells initially undergo extensive proliferation, which eventually stops prior to matrix deposition and cartilaginous nodules formation.59,60 Thus, the results in this work showing greater enhancement of chondrogenic phenotype under 2.5% PRPr treatment rather than 10% PRPr may be correlated to the more intensive effect on cell proliferation by 10% PRPr than 2.5% PRPr.
Conclusion
In the work presented in this article, it was possible to produce a PRPr capable of eliciting an enhanced chondrogenic effect in vitro, which when used as a substitute to FBS prevented NCCs dedifferentiation as well as promoted their redifferentiation toward the chondrogenic lineage. In the case of MSCs, PRPr induced the expression of chondrogenic markers, production of sGAG, and increase in biomechanical properties in a scaffold culture system. Collectively, we can conclude that PRPr can be used as a substitute for FBS for in vitro 2D expansion of chondrogenic cells, with special regard to its concentration, cell type, and passage. Further work is required to confirm whether cellular expansion with PRPr will enhance cartilage repair in vivo.
Footnotes
Declaration of conflicting interests: The authors declare no conflict of interest.
Funding: The authors would like to thank the financial support from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Science Foundation Ireland, International Strategic Cooperation Award (12/ISCA/2494), and the Health Research Board of Ireland (HRA_POR/2011/27).
References
- 1. Bora FW, Miller G. Joint physiology, cartilage metabolism, and the etiology of osteoarthritis. Hand Clin 1987; 3(3): 325–336, http://www.ncbi.nlm.nih.gov/pubmed/3308909 (accessed 9 June 2014). [PubMed] [Google Scholar]
- 2. Nelson L, Fairclough J, Archer CW. Use of stem cells in the biological repair of articular cartilage. Expert Opin Biol Ther 2010; 10(1): 43–55, http://www.ncbi.nlm.nih.gov/pubmed/20420516 [DOI] [PubMed] [Google Scholar]
- 3. Ahmed TAE, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev 2010; 16(3): 305–329, http://www.ncbi.nlm.nih.gov/pubmed/20025455 [DOI] [PubMed] [Google Scholar]
- 4. Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994; 331(14): 889–895, http://www.ncbi.nlm.nih.gov/pubmed/8078550 (accessed 25 May 2014). [DOI] [PubMed] [Google Scholar]
- 5. Brittberg M. Autologous chondrocyte implantation–technique and long-term follow-up. Injury 2008; 39(Suppl. 1): S40–S49, http://www.ncbi.nlm.nih.gov/pubmed/18313471 (accessed 28 May 2014). [DOI] [PubMed] [Google Scholar]
- 6. Jacobi M, Villa V, Magnussen RA, et al. MACI—a new era? Sports Med Arthrosc Rehabil Ther Technol 2011;3(1): 10, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3117745&tool=pmcentrez&rendertype=abstract (accessed 9 June 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tew SR, Murdoch AD, Rauchenberg RP, et al. Cellular methods in cartilage research: primary human chondrocytes in culture and chondrogenesis in human bone marrow stem cells. Methods 2008; 45(1): 2–9, http://www.ncbi.nlm.nih.gov/pubmed/18442700 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 8. Lin Z, Fitzgerald JB, Xu J, et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J Orthop Res 2008; 26(9): 1230–1237, http://www.ncbi.nlm.nih.gov/pubmed/18404652 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 9. Jakob M, Démarteau O, Schäfer D, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem 2001; 81(2): 368–377, http://www.ncbi.nlm.nih.gov/pubmed/11241676 [DOI] [PubMed] [Google Scholar]
- 10. Yaeger PC, Masi TL, de Ortiz JL, et al. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res 1997; 237(2): 318–325, http://www.ncbi.nlm.nih.gov/pubmed/9434627 [DOI] [PubMed] [Google Scholar]
- 11. Barbero A, Ploegert S, Heberer M, et al. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum 2003; 48(5): 1315–1325, http://www.ncbi.nlm.nih.gov/pubmed/12746904 (accessed 5 June 2014). [DOI] [PubMed] [Google Scholar]
- 12. Kim H, Im G. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A 2008; 15(7), http://online.liebertpub.com/doi/abs/10.1089/ten.tea.2008.0368 (accessed 30 July 2014). [DOI] [PubMed] [Google Scholar]
- 13. Amano O, Koshimizu U, Nakamura T, et al. Enhancement by hepatocyte growth factor of bone and cartilage formation during embryonic mouse mandibular development in vitro. Arch Oral Biol 1999; 44(11): 935–946, http://www.ncbi.nlm.nih.gov/pubmed/10580541 [DOI] [PubMed] [Google Scholar]
- 14. Tuan RS. Cellular signaling in developmental chondrogenesis: n-cadherin, Wnts, and BMP-2. J Bone Joint Surg Am 2003; 85(Suppl.): 137–141, http://www.ncbi.nlm.nih.gov/pubmed/12721357 (accessed 30 July 2014). [DOI] [PubMed] [Google Scholar]
- 15. Nurden A, Nurden P, Sanchez M. Platelets and wound healing. Front Biosci 2007; 2: 3532–3548, http://europepmc.org/abstract/MED/18508453 (accessed 10 June 2014). [DOI] [PubMed] [Google Scholar]
- 16. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent 2001; 10(4): 225–228, http://www.ncbi.nlm.nih.gov/pubmed/15085519 [DOI] [PubMed] [Google Scholar]
- 17. Perdisa F, Filardo G, Di Matteo B, et al. Platelet rich plasma: a valid augmentation for cartilage scaffolds? A systematic review. Histol Histopathol 2014, http://www.ncbi.nlm.nih.gov/pubmed/24458849 [DOI] [PubMed]
- 18. Marcacci M, Filardo G, Kon E. Treatment of cartilage lesions: what works and why? Injury 2013; 44(Suppl. 1): S11–S15, http://www.ncbi.nlm.nih.gov/pubmed/23351863 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 19. Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage 2013; 21(11): 1627–1637, http://www.ncbi.nlm.nih.gov/pubmed/23933379 (accessed 2 June 2014). [DOI] [PubMed] [Google Scholar]
- 20. Zhang L, Spector M. Comparison of three types of chondrocytes in collagen scaffolds for cartilage tissue engineering. Biomed Mater 2009; 4(4): 045012, http://www.ncbi.nlm.nih.gov/pubmed/19636108 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 21. Koga H, Muneta T, Nagase T, et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res 2008; 333(2): 207–215, http://www.ncbi.nlm.nih.gov/pubmed/18560897 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 22. Pittenger MF. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411): 143–147, http://www.sciencemag.org/cgi/doi/10.1126/science.284.5411.143 (accessed 25 May 2014). [DOI] [PubMed] [Google Scholar]
- 23. Caplan A. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007; 213: 341–347, http://onlinelibrary.wiley.com/doi/10.1002/jcp.21200/full (accessed 9 July 2014). [DOI] [PubMed] [Google Scholar]
- 24. Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 2005; 52(8): 2521–2529, http://www.ncbi.nlm.nih.gov/pubmed/16052568 (accessed 3 June 2014). [DOI] [PubMed] [Google Scholar]
- 25. Puetzer J. Comparative review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng Part B Rev 2010; 16(4), http://online.liebertpub.com/doi/abs/10.1089/ten.TEB.2009.0705 (accessed 10 June 2014). [DOI] [PubMed] [Google Scholar]
- 26. Do Amaral RJFC, Pedrosa CDSG, Kochem MCL, et al. Isolation of human nasoseptal chondrogenic cells: a promise for cartilage engineering. Stem Cell Res 2012; 8(2): 292–299, http://www.ncbi.nlm.nih.gov/pubmed/22099383 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 27. Van Osch GJ, Van Der Veen SW, Burger EH, et al. Chondrogenic potential of in vitro multiplied rabbit perichondrium cells cultured in alginate beads in defined medium. Tissue Eng 2000; 6(4): 321–330, http://www.ncbi.nlm.nih.gov/pubmed/10992429 [DOI] [PubMed] [Google Scholar]
- 28. Shafiee A, Kabiri M, Ahmadbeigi N, et al. Nasal septum-derived multipotent progenitors: a potent source for stem cell-based regenerative medicine. Stem Cells Dev 2011; 20(12): 2077–2091, http://www.ncbi.nlm.nih.gov/pubmed/21401444 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 29. Shafiee A, Seyedjafari E, Sadat Taherzadeh E, et al. Enhanced chondrogenesis of human nasal septum derived progenitors on nanofibrous scaffolds. Mater Sci Eng C Mater Biol Appl 2014; 40: 445–454, http://www.ncbi.nlm.nih.gov/pubmed/24857513 (accessed 9 July 2014). [DOI] [PubMed] [Google Scholar]
- 30. Amable PR, Carias RBV, Teixeira MVT, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther 2013; 4(3): 67, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3706762&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsiko A, Levingstone TJ, O’Brien FJ, et al. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J Mech Behav Biomed Mater 2012; 11: 41–52, http://www.ncbi.nlm.nih.gov/pubmed/22658153 (accessed 10 June 2014). [DOI] [PubMed] [Google Scholar]
- 32. Johnstone B, Hering TM, Caplan AI, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 1998; 238(1): 265–272, http://www.ncbi.nlm.nih.gov/pubmed/12589731 [DOI] [PubMed] [Google Scholar]
- 33. Repetto G, del Peso A, Zurita JL. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 2008; 3(7): 1125–1131, http://www.ncbi.nlm.nih.gov/pubmed/18600217 (accessed 26 May 2014). [DOI] [PubMed] [Google Scholar]
- 34. Dexheimer V, Frank S, Richter W. Proliferation as a requirement for in vitro chondrogenesis of human mesenchymal stem cells. Stem Cells Dev 2012; 21(12): 2160–2169, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3411365&tool=pmcentrez&rendertype=abstract (accessed 9 June 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahearne M, Liu Y, Kelly DJ. Combining freshly isolated chondroprogenitor cells from the infrapatellar fat pad with a growth factor delivery hydrogel as a putative single stage therapy for articular cartilage repair. Tissue Eng Part A 2014; 20(5–6): 930–939, http://www.ncbi.nlm.nih.gov/pubmed/24090441 (accessed 26 December 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bernstein P, Dong M. Pellet culture elicits superior chondrogenic redifferentiation than alginate-based systems. Biotechnol Prog 2009; 4–10, http://onlinelibrary.wiley.com/doi/10.1002/btpr.186/full (accessed 26 December 2014). [DOI] [PubMed]
- 37. Jung Y, Chung Y-I, Kim SH, et al. In situ chondrogenic differentiation of human adipose tissue-derived stem cells in a TGF-beta1 loaded fibrin-poly(lactide-caprolactone) nanoparticulate complex. Biomaterials 2009; 30(27): 4657–4664, http://www.ncbi.nlm.nih.gov/pubmed/19520426 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 38. Dai J, Wang J, Lu J, et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials 2012; 33(31): 7699–7711, http://www.ncbi.nlm.nih.gov/pubmed/22841919 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 39. Ando W, Tateishi K, Katakai D, et al. In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A 2008; 14(12): 2041–2049, http://www.ncbi.nlm.nih.gov/pubmed/18636944 [DOI] [PubMed] [Google Scholar]
- 40. Björnsson S. Quantitation of proteoglycans as glycosaminoglycans in biological fluids using an alcian blue dot blot analysis. Anal Biochem 1998; 256: 229–237. [DOI] [PubMed] [Google Scholar]
- 41. Schindelin J, Arganda-Carreras I, Frise E. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9(7), http://www.nature.com/nmeth/journal/v9/n7/full/nmeth.2019.html%3FWT.ec_id%3DNMETH-201207 (accessed 10 June 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25(4): 402–408, http://www.ncbi.nlm.nih.gov/pubmed/11846609 [DOI] [PubMed] [Google Scholar]
- 43. Weibrich G, Hansen T, Kleis W, et al. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004; 34(4): 665–671, http://www.ncbi.nlm.nih.gov/pubmed/15050897 (accessed 28 May 2014). [DOI] [PubMed] [Google Scholar]
- 44. Gaissmaier C, Fritz J, Krackhardt T, et al. Effect of human platelet supernatant on proliferation and matrix synthesis of human articular chondrocytes in monolayer and three-dimensional alginate cultures. Biomaterials 2005; 26(14): 1953–1960, http://www.ncbi.nlm.nih.gov/pubmed/15576169 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 45. Murphy MB, Blashki D, Buchanan RM, et al. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials 2012; 33(21): 5308–5316, http://www.ncbi.nlm.nih.gov/pubmed/22542609 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 46. Blande IS, Bassaneze V, Lavini-Ramos C, et al. Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion 2009; 49(12): 2680–2685, http://www.ncbi.nlm.nih.gov/pubmed/19694997 (accessed 10 June 2014). [DOI] [PubMed] [Google Scholar]
- 47. Spreafico A, Chellini F, Frediani B, et al. Biochemical investigation of the effects of human platelet releasates on human articular chondrocytes. J Cell Biochem 2009; 108(5): 1153–1165, http://www.ncbi.nlm.nih.gov/pubmed/19731249 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 48. Gonzales VK, de Mulder ELW, de Boer T, et al. Platelet-rich plasma can replace fetal bovine serum in human meniscus cell cultures. Tissue Eng Part C Methods 2013; 19(11): 892–899, http://www.ncbi.nlm.nih.gov/pubmed/23621108 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 49. Anitua E, Sánchez M, Zalduendo MM, et al. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif 2009; 42(2): 162–170, http://www.ncbi.nlm.nih.gov/pubmed/19250293 (accessed 9 June 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee JK, Lee S, Han SA, et al. The effect of platelet-rich plasma on the differentiation of synovium-derived mesenchymal stem cells. J Orthop Res 2014; 32: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 51. Zhang L, Su P, Xu C, et al. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett 2010; 32(9): 1339–1346, http://www.ncbi.nlm.nih.gov/pubmed/20464452 (accessed 24 May 2014). [DOI] [PubMed] [Google Scholar]
- 52. Alegre-Aguarón E, Sampat SR, Xiong JC, et al. Growth factor priming differentially modulates components of the extracellular matrix proteome in chondrocytes and synovium-derived stem cells. PLoS One 2014; 9(2): e88053, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3917883&tool=pmcentrez&rendertype=abstract (accessed 9 June 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaps C, Loch A, Haisch A, et al. Human platelet supernatant promotes proliferation but not differentiation of articular chondrocytes. Med Biol Eng Comput 2002; 40(4): 485–490, http://www.ncbi.nlm.nih.gov/pubmed/12227637 [DOI] [PubMed] [Google Scholar]
- 54. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage 2006; 14(12): 1272–1280, http://www.ncbi.nlm.nih.gov/pubmed/16820306 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 55. Pfeiffer E, Vickers SM, Frank E, et al. The effects of glycosaminoglycan content on the compressive modulus of cartilage engineered in type II collagen scaffolds. Osteoarthritis Cartilage 2008; 16(10): 1237–1244. [DOI] [PubMed] [Google Scholar]
- 56. Drengk A, Zapf A, Stürmer EK, et al. Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs 2009; 189(5): 317–326, http://www.ncbi.nlm.nih.gov/pubmed/18689989 (accessed 9 June 2014). [DOI] [PubMed] [Google Scholar]
- 57. Rubio-Azpeitia E, Andia I. Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J 2014; 4(1): 52–62, http://www.mltj.org/index.php?PAGE=articolo_dett&ID_ISSUE=744&id_article=6420 (accessed 13 August 2014). [PMC free article] [PubMed] [Google Scholar]
- 58. Sekiya I, Vuoristo JT, Larson BL, et al. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A 2002; 99(7): 4397–402, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=123659&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem 2006; 97(1): 33–44, http://www.ncbi.nlm.nih.gov/pubmed/16215986 (accessed 23 May 2014). [DOI] [PubMed] [Google Scholar]
- 60. Ten Berge D, Brugmann SA, Helms JA, et al. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development 2008; 135(19): 3247–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]