Abstract
A 59-year-old woman with hypertrophic obstructive cardiomyopathy was admitted to our institution with worsening heart failure. She developed mechanical intracardiac hemolysis due to left ventricular outflow tract (LVOT) obstruction. Despite surgical myectomy, the LVOT pressure gradient (PG) remained high and hemolytic anemia recurred. A dual-chamber implantable cardioverter defibrillator was then implanted, which decreased the LVOT-PG and improved hemolysis.
Keywords: Hypertrophic obstructive cardiomyopathy, Mechanical hemolytic anemia, Dual-chamber pacing
1. Introduction
Anemia worsens obstructions in hypertrophic obstructive cardiomyopathy (HOCM). Mechanical intracardiac hemolysis has been proposed as a rare cause of anemia in patients with HOCM [1]. Here we present the case of a patient with HOCM complicated by intracardiac mechanical hemolysis that was dramatically controlled by dual-chamber pacing.
2. Case report
A 59-year-old woman with HOCM was admitted to our institution with worsening heart failure (New York Heart Association [NYHA] class IV). She developed pulmonary hypertension and normocytic and normochromic anemia with a hemoglobin level of 8.6 g/dL. Lactate dehydrogenase level was slightly increased, and the indirect bilirubin level was increased more than the direct bilirubin level. A peripheral blood smear revealed increased numbers of reticulocytes and slightly increased numbers of fragmented red blood cells (Fig. 1). Coombs test was negative. Based on the laboratory findings (Table 1), the cause of the anemia was thought to be mechanical intracardiac hemolysis due to a left ventricular outflow tract (LVOT) obstruction.
Fig. 1.
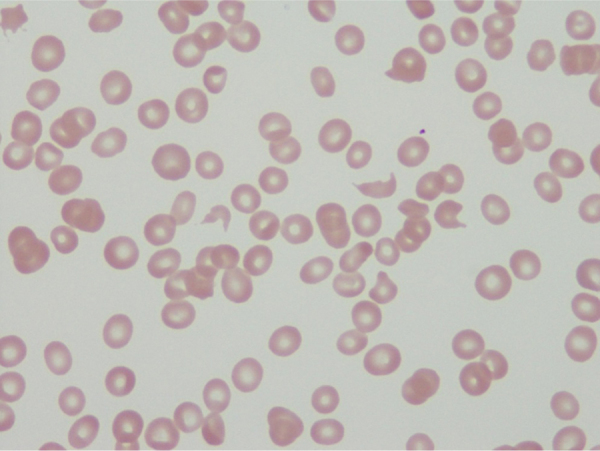
Increased number of fragmented red blood cells on a peripheral blood smear.
Table 1.
Time course of the laboratory data. Serum folate and vitamin B12 concentrations are also within the normal range (479 pg/mL and 4.3 ng/mL, respectively).
| Post-myectomy | Pre-pacing | 1 week later | 3 weeks later | |
|---|---|---|---|---|
| Hemoglobin, g/dL | 7.9 | 8.6 | 12.2 | 13.3 |
| Hematocrit, % | 24.0 | 25.7 | 37.1 | 38.7 |
| Red blood cells, ×104/μL | 240 | 260 | 371 | 373 |
| Total indirect bilirubin, mg/dL | 1.6/1.0 | 1.3/0.9 | 0.4/0.1 | 0.4/0.1 |
| Lactate dehydrogenase, IU/L | 280 | 264 | 215 | 213 |
| Haptoglobin, mg/dL | 6 | 7 | 94 | 117 |
| Serum iron concentration, μg/dL | 39 | 34 | 36 | 59 |
| Ferritin, ng/mL | 42 | 38 | 39 | 39 |
| Total iron binding capacity, μg/dL | 382 | 367 | 313 | 320 |
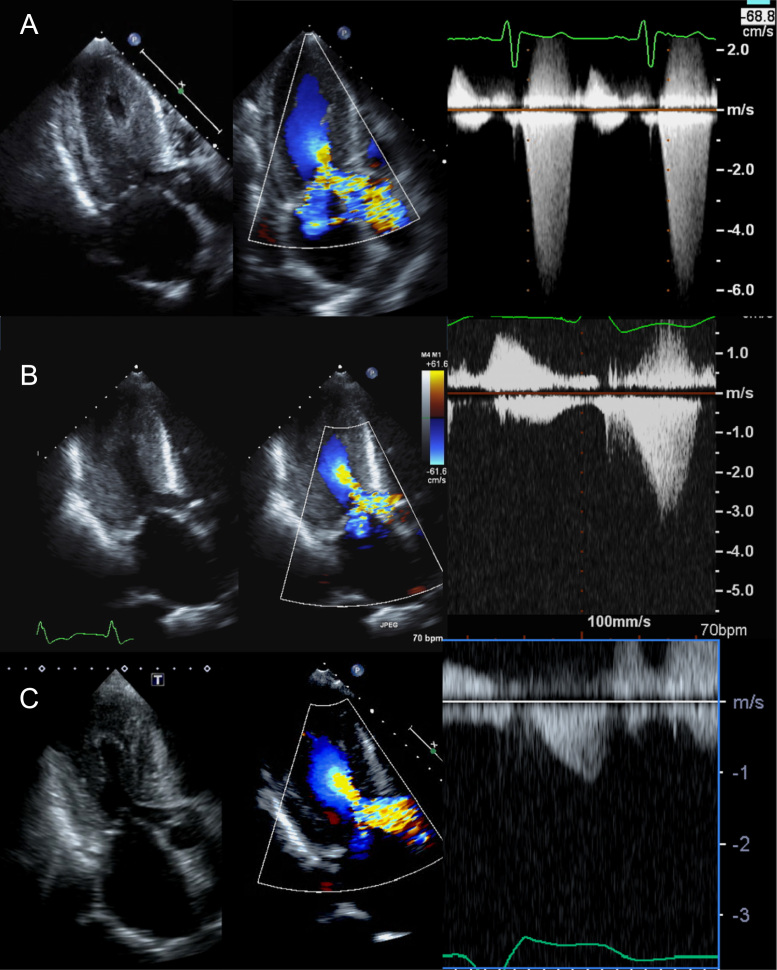
The patient was initially treated with carvedilol 10 mg daily, verapamil 120 mg daily, and cibenzoline 300 mg daily according to the Japanese Circulation Society guideline [2]. Her heart failure was refractory to medical therapy. Myectomy was chosen because repeated blood transfusions were required to improve her anemia and heart failure, and the LVOT pressure gradient (LVOT-PG) was >150 mmHg. A thin, 2×5 mm2 section of the myocardium was removed. During the surgery, a transient decrease in LVOT-PG was observed on transesophageal echocardiography and low blood pressure was noted. After inotropic support was discontinued, no decrease in LVOT-PG was noted, rather, it remained at 156 mmHg just before implantable cardioverter defibrillator (ICD) implantation after myectomy (Fig. 2A). The ICD was indicated by the following findings: (1) documentation of nonsustained VT, (2) abnormal blood pressure response during exercise stress, (3) positive late ventricular potential, and (4) late gadolinium enhancement on cardiac magnetic resonance imaging.
Fig. 2.
Time course of the left ventricular outflow tract (LVOT) obstruction on ultrasound echocardiography. (A) The LVOT obstruction had a pressure gradient of 156 mmHg after optimal medical therapy prior to dual-chamber pacing. (B) After 1 week of dual-chamber pacing, the LVOT pressure gradient decreased to 68 mmHg. (C) After 3 weeks of dual-chamber pacing, the LVOT pressure gradient decreased to 26 mmHg.
A dual-chamber ICD was then implanted and the optimal atrioventricular (AV) interval to obtain the lowest PG was adjusted to a sensed PV interval of 70 ms/AV pace of 60 ms using echocardiography. After 1 week, the LVOT-PG had decreased to 68 mmHg (Fig. 2B); by 3 weeks, it had decreased to 26 mmHg (Fig. 2C). The hemolysis was well controlled because of the decreased PG, and the patient was stabilized and discharged.
After 3 months of treatment, her hemoglobin level remained at 12.3 g/dL and her congestive heart failure improved from NYHA class IV to class II.
3. Discussion
Mechanical intracardiac hemolysis has been reported as a rare cause of anemia in patients with HOCM [1]. LVOT obstruction causes hemolytic anemia, which then increases the left ventricular contractility and exacerbates LVOT obstruction and hemolytic anemia. This results in a vicious obstruction–anemia cycle.
Studies have shown that dual-chamber pacing significantly reduces the LVOT-PG in patients with HOCM. The rationale for dual-chamber pacing in HOCM is that pre-excitation of the left ventricular apex results in a paradoxical septal motion, decreased ejection velocity, amelioration of any systolic anterior motion of the mitral valve, and a reduced LVOT gradient. The M-PATHY study by Maron et al. [3] showed that the LVOT-PG decreased in 40% of the study population. Megevand et al. [4] also reported that the LVOT gradient was significantly reduced and that the reduction remained significant during the late follow-up period. All patients had an improved LVOT-PG during the late follow-up compared with that noted prior to dual-chamber pacemaker implantation.
In this case, dual-chamber pacing not only decreased LVOT-PG, but also improved hemolytic anemia. The LVOT-PG reduction likely decreased the mechanical red cell shear stress. Dual-chamber pacing could be an effective therapy in patients with HOCM and refractory anemia due to mechanical hemolysis.
4. Conclusion
To our knowledge, this is the first report of a patient with HOCM in whom mechanical hemolysis improved following dual-chamber pacing, which subsequently resolved the vicious obstruction–anemia cycle.
Conflict of interest
None.
References
- 1.Palaez-Dominguez S., Perez-Benito L., Sanz Moreno J. Severe hemolytic anemia in hypertrophic cardiomyopathy. Am Heart J. 1992;124:1082–1083. doi: 10.1016/0002-8703(92)90998-b. [DOI] [PubMed] [Google Scholar]
- 2.Doi Y, Ichida F, Kawai H, et al. Guidelines for diagnosis and treatment of patients with hypertrophic cardiomyopathy. The Japanese Circulation Society 2012. Available from: 〈http://www.j-circ.or.jp/guideline/pdf/JCS2012_doi_h.pdf〉. [DOI] [PubMed]
- 3.Maron B.J., Nishimura R.A., McKenna W.J. Assessment of permanent dual-chamber pacing as a treatment for drug-refractory symptomatic patients with obstructive hypertrophic cardiomyopathy. A randomized, double-blind, crossover study (M-PATHY) Circulation. 1999;99:2927–2933. doi: 10.1161/01.cir.99.22.2927. [DOI] [PubMed] [Google Scholar]
- 4.Megevand A., Ingles J., Richmond D.R. Long-term follow-up of patients with obstructive hypertrophic cardiomyopathy treated with dual-chamber pacing. Am J Cardiol. 2005;95:991–993. doi: 10.1016/j.amjcard.2004.12.045. [DOI] [PubMed] [Google Scholar]