The cytosolic reducing agent glutathione can reverse thiol modification of cysteine residues inside the pores of connexins and other channels permeable to large molecules.
Abstract
Cysteine-scanning mutagenesis combined with thiol reagent modification is a powerful method with which to define the pore-lining elements of channels and the changes in structure that accompany channel gating. Using the Xenopus laevis oocyte expression system and two-electrode voltage clamp, we performed cysteine-scanning mutagenesis of several pore-lining residues of connexin 26 (Cx26) hemichannels, followed by chemical modification using a methanethiosulfonate (MTS) reagent, to help identify the position of the gate. Unexpectedly, we observed that the effect of MTS modification on the currents was reversed within minutes of washout. Such a reversal should not occur unless reducing agents, which can break the disulfide thiol–MTS linkage, have access to the site of modification. Given the permeability to large metabolites of connexin channels, we tested whether cytosolic glutathione (GSH), the primary cell reducing agent, was reaching the modified sites through the connexin pore. Inhibition of gamma-glutamylcysteine synthetase by buthionine sulfoximine decreased the cytosolic GSH concentration in Xenopus oocytes and reduced reversibility of MTS modification, as did acute treatment with tert-butyl hydroperoxide, which oxidizes GSH. Cysteine modification based on thioether linkages (e.g., maleimides) cannot be reversed by reducing agents and did not reverse with washout. Using reconstituted hemichannels in a liposome-based transport-specific fractionation assay, we confirmed that homomeric Cx26 and Cx32 and heteromeric Cx26/Cx32 are permeable to GSH and other endogenous reductants. These results show that, for wide pores, accessibility of cytosolic reductants can lead to reversal of MTS-based thiol modifications. This potential for reversibility of thiol modification applies to on-cell accessibility studies of connexin channels and other channels that are permeable to large molecules, such as pannexin, CALHM, and VRAC.
INTRODUCTION
Cysteine substitution followed by thiol modification has long been used to explore the structure and topology of ion channels and transporters. Recently, wider structural insights have been achieved by combining chemical modification with data from crystal structures. New thiol derivatives have provided novel resources to determine near-neighbor interactions, including salt bridges and hydrophobic bonds, state-dependent accessibility via cross-linking that traps specific conformational changes, protein dynamics via fluorescent derivatives, etc. (Karlin and Akabas, 1998; Newell and Czajkowski, 2007; Zhu and Casey, 2007; Gandhi and Olcese, 2009). These data provide experimental constraints that can be used in molecular modeling of ion channels and transporters, including those for which little is known structurally because of the lack of high resolution structures. The chemical modification strategy can serve to test, validate, and refine the models provided by crystal structures in specific conformational states.
The most commonly used thiol reagents are those derived from MTS. The MTS reaction with thiols is reversible upon treatment with reducing agents such as DTT and TCEP, a property that serves as an experimental control to show specificity of the modification. Ideally, to assess MTS accessibility to the channel pore, one would like to use the inside-out or outside-out patch configuration to avoid modification of other cellular targets that could affect interpretation of the results. However, for many ion channels, the excised patch configuration is problematic because of rundown and instability over the time course of the experiments. Chemical modification studies can be profitably performed in whole cells (e.g., Xenopus laevis oocytes) for channels and transporters where excised patches are not feasible, including P2X, STIM, and Hv1 channels (Li et al., 2008; Gonzalez et al., 2010; Allsopp et al., 2011; Kawate et al., 2011; Amcheslavsky et al., 2014). In this configuration, chemical accessibility can be achieved only from the extracellular side yet can yield novel insights regarding the structural and functional properties of the domains that gate the channels.
Here, we apply two classes of thiol-reactive reagents to explore pore accessibility of specific residues in the connexin 26 (Cx26) channel. The results are informative about channel-gating residues and molecular permeability of the channels, and highlight a caution about the use of reducible thiol-linkage chemistries when studying wide pores. We observed that glutathione (GSH) and other reducers permeate through the connexin channel pore, which may have important implications in cellular physiology.
MATERIALS AND METHODS
Channel expression and molecular biology
cDNA for hCx26 was purchased from OriGene. Wild-type Cx26 was subcloned in the pGEM-HA vector (Promega) for expression in Xenopus oocytes. Cysteine substitutions in hCx26 were produced with QuikChange II Site-Directed Mutagenesis kits (Agilent Technologies). DNA sequencing performed at the NJMS Molecular Resource Facility confirmed the amino acid substitutions. Nhe1-linearized hCx26 wild-type and mutant DNAs were transcribed in vitro to cRNAs using the T7 Message Machine kit (Ambion).
Electrophysiology
Electrophysiological data were collected using the two-electrode voltage-clamp technique. All recordings were made at room temperature (20–22°C). The recording solutions contained (mM): 118 NaCl, 2 KCl, 0.25 Ca2+, and 5 HEPES, pH 7.4. Currents from oocytes expressing Cx26 and cysteine mutants were recorded 1–3 d after cRNA injection using an oocyte clamp (OC-725C; Warner Instruments). Antisense oligonucleotide against the endogenous connexin, Cx38 (1 mg/ml), was always coinjected along with hCx26 or mutant mRNA. Currents were sampled at 2 kHz and low pass filtered at 0.2 kHz. Microelectrode resistances were between 0.1 and 1.2 MΩ when filled with 3 M KCl. All recordings were performed using agar bridges connecting bath and ground chambers.
Measurement of chemical modification
Hemichannel currents from oocytes expressing wild-type Cx26 or cysteine mutations were obtained in a Ringer’s solution containing low Ca2+ (0.25 mM), which facilitates opening of connexin hemichannels. Chemical modification by membrane-impermeable 2-sulfonatoethyl MTS (MTSES) or N-2-sulfoethyl maleimide (maleimide ES) was performed by superfusion of the oocyte under TEVC. The solution containing the reagent was freshly prepared by directly dissolving the compound in the external solution to a final concentration of 100–500 µM. Chemical modification was assessed by measuring the tail current peaks after reaching current saturation during a depolarizing pulse from −80 to 0 mV. Because of the slow kinetics of activation and deactivation of Cx26 hemichannels, large depolarizing pulses were used to reach steady state. MTSES and maleimide ES were purchased from Toronto Research Chemicals Inc. To reduce reduced GSH (rGSH) levels using tert-butyl hydroperoxide (TBHO2), oocytes containing Cx26 cysteine mutants were perfused in the low Ca2+ Ringer’s solution with 2 mM TBHO2 for 15 min before the application of MTSES. TBHO2 was not applied in the presence MTSES, but washing off was performed in its presence. To reduce intracellular levels of GSH, we used l-buthionine-S,R-sulfoximine (BSO), an inhibitor of gamma-glutamylcysteine synthetase. Freshly isolated Xenopus oocytes were incubated in a low Ca2+ Ringer’s solution containing 20 mM BSO. After 24 h, the oocytes were injected with mRNA for hCx26 wild type or mutants. Chemical modification with MTSES was performed between 60 and 72 h after BSO treatment. The oocyte solution was changed daily with freshly prepared BSO.
Measurement of GSH levels
The total intracellular GSH concentration from Xenopus oocytes was determined by using the GSH assay kit from Sigma-Aldrich. For intracellular measurements, selected oocytes were kept in ND96 solution with low Ca2+ (0.25 mM) containing or not 20 mM BSO. After 48 and 72 h, the collected oocytes first were deproteinized with 5% 5-sulfosalicylic acid solution, followed by centrifugation to remove the precipitated protein, and then assayed for GSH as per the manufacturer’s instructions. For measuring extracellular GSH release, 30 oocytes per condition were incubated in 100 µl of low Ca2+ (0.25 mM) solution 1 d after injection or not with hCx26 mRNA. The extracellular medium was collected 6 and 24 h after incubation. The measurement of GSH was achieved by using a kinetic assay in which catalytic amounts (nmol) of GSH cause a continuous reduction of 5,5′-dithiobis(2-nitrobenzoic acid) to TNB, and the oxidized form of GSH (GSSG) formed is recycled by GSH reductase and NADPH. The GSSG present will also react to give a positive value in this reaction. The assay uses a standard curve of rGSH to determine the amount of GSH in the biological sample. A plate reader was set to 412 nm with kinetic read at 1-min intervals for 5 min to calculate the nanomoles of GSH in the sample, as suggested by the manufacturer.
Protein purification and transport-specific fractionation (TSF)
Permeability of several cytosolic reductants through connexin channels was tested using a liposome-based TSF system used previously to define the permeability of specific molecules through connexin channels (Bevans et al., 1998; Koreen et al., 2004; Ayad et al., 2006). In brief, homomeric Cx32, Cx26, and heteromeric Cx26/Cx32 channels were immunopurified from a heterologous expression system (Koreen et al., 2004), and the purified hemichannels were reconstituted into unilamellar rhodamine–labeled phospholipid liposomes. The molecular tracer to be tested is entrapped in the liposomes during reconstitution. By design, a significant fraction of the liposomes does not contain functional channels (average channel to liposome ratio, ∼0.7). The liposomes are centrifuged through an iso-osmolar density gradient that fractionates the liposomes into two populations. The liposomes that are permeable to sucrose (e.g., have functional sucrose-permeable channels) become more dense and move to a lower position in the gradient, whereas those that are not remain in the upper part of the gradient. The result is two populations of channels: an upper band of liposomes that are not permeable to sucrose (containing no functional channels), and a lower band of liposomes that are permeable to sucrose (containing functional, sucrose-permeable channels). The two bands are recovered, and the tracer to liposome ratio in each population is determined. The tracer to lipid ratio in the upper band serves as an internal control for tracer loading and nonspecific leakage from the liposomes. If a test molecule is permeable through the channels, it will be lost from the lower population of liposomes. If it is impermeable, the tracer to lipid ratio in the lower band is the same as in the upper band. Typically, 30–50% of the liposomes are in the lower band. If a purification/reconstitution is unsuccessful, there is no lower band (i.e., no functional channels), so there is nothing to measure; the key to the permeability measurement is comparison of the tracer to lipid ratio in the population of liposomes. This method has been used to assess permeability to a variety of molecules, including fluorescent tracers, cyclic nucleotides, and inositol phosphates (Bevans et al., 1998; Koreen et al., 2004; Locke et al., 2004; Ayad et al., 2006). It is used here to assess permeability of several cellular reductants.
GSH, NADH, and NADPH levels in each population of liposomes (upper and lower bands) were assessed. The GSH was assayed by the method described above. The NADH and NADPH were assayed as described in Graeff and Lee (2002). The retention or release of the nucleotides from the liposomes was assessed as the relative amount of nucleotide per liposome. Data are tracer/liposome ratios in lower TSF band as percentage of ratio in upper-band liposomes in the same gradient, corrected for nonspecific binding. As a positive internal control, a molecule that is not permeable through the Cx26 and Cx26/Cx32 channels but is permeable through the Cx32 channel was used (3-PA, a derivatized trisaccharide; Locke et al., 2004).
Online supplemental material
Fig. S1 shows that the application of MTSES and maleimide does not modify tail current wild-type hCx26 hemichannels expressed in Xenopus oocytes. Fig. S2 shows that introduced cysteines are repeatedly modified with MTSES after washing off the reagent. Fig. S3 demonstrates that Cx46 is also reversely modified by MTSES at an introduced cysteine in the pore. Fig. S4 shows that treatment with BSO significantly reduces rGSH in Xenopus oocytes. Fig. S5 shows higher extracellular levels of GSH in oocytes expressing hCx26 hemichannels when compared with noninjected oocytes.
RESULTS
Reversible chemical modification by MTS at cysteine residues lining the connexin pore
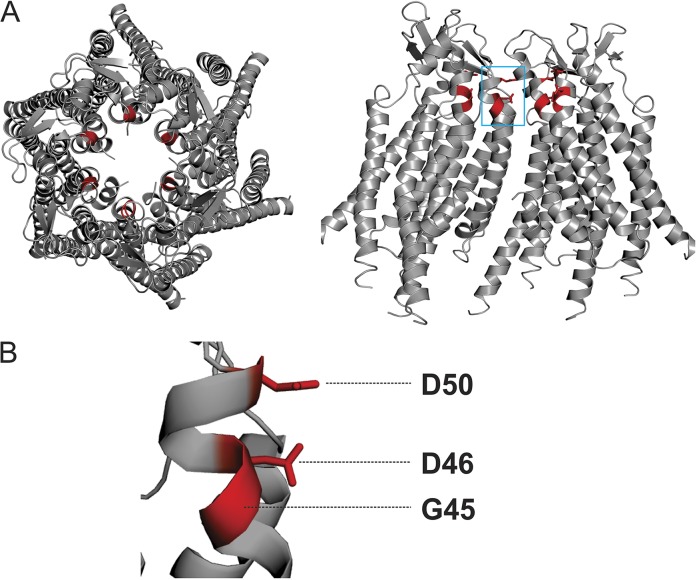
Cysteine substitution followed by modification by MTS is a useful tool to define the roles of pore-lining and charged residues in the gating and conductance of connexin channels (Kronengold et al., 2003a; Tang et al., 2009; Verselis et al., 2009; Lopez et al., 2013; Sánchez et al., 2013). Here, this technique was applied to explore the functional roles of three residues (D50, D46, and G45) that line the extracellular side of the pore and have been shown to be involved in electrostatic stabilization of the open state and/or regulation of the channels by extracellular calcium ion. Previous reports from us and other groups have shown that hemichannel currents significantly increase when cysteines substituted at these positions are exposed to and modified by MTSES, a negatively charged MTS reagent (Sánchez et al., 2010, 2013; Lopez et al., 2013; MTSES modification of D50C and D46C reestablishes the native negative charge at this position that stabilizes the open state). The locations of these residues are depicted in Fig. 1.
Figure 1.
Residues selected for chemical modification in the Cx26 hemichannel. (A) Top (left) and side view (right) of the human Cx26 hemichannel crystal structure. Four subunits are shown in the side view to show the pore-lining region. (B) Magnification of residues D50, D46, and G45, all highlighted in red.
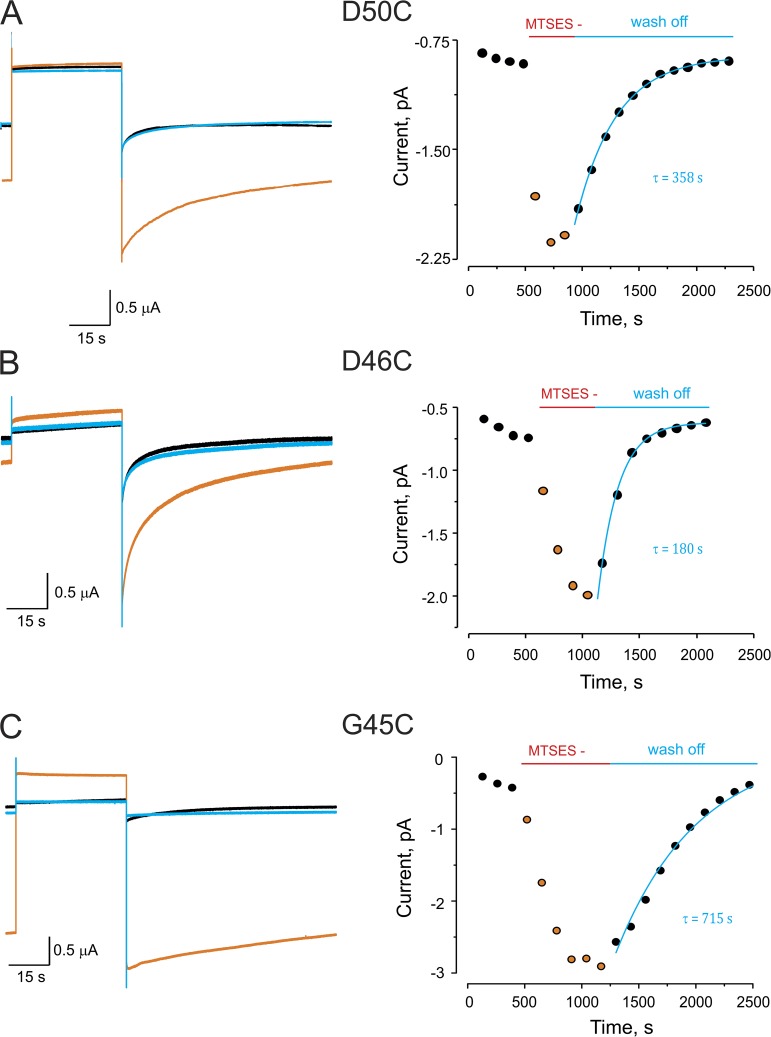
An example of the effect of MTSES modification is shown in Fig. 2 A (left), which depicts representative current traces in response to a depolarizing pulse in an oocyte expressing D50C mutant hemichannels. The current traces were obtained at 0.25 mM Ca2+ to enhance hemichannel opening (low Ca2+ facilitates Cx26 hemichannel currents). As expected, the holding and tail currents significantly increased in the presence of 500 µM MTSES (Fig. 2 A, left, orange trace), compared with the currents before MTSES exposure (black trace). Strikingly, within 5 min after wash off of MTSES, the holding and tail currents returned to levels similar to those observed before MTSES exposure (blue trace). The time course of the MTSES effect on the peak tail currents and its (unexpected) reversal after MTSES washout were determined (Fig. 2 A, right). Tail currents increased after the addition of MTSES, but after washout, the currents returned to the original levels, with an approximate time constant of 358 s. Similar effects were seen for MTSES exposure to D46C and G45C channels, with reversal of the MTSES effects having time constants of ∼180 and ∼715 s, respectively (Fig. 2, B and C). No modifications were observed in wild-type Cx26 currents in the presence, or after wash off, of 500 µM MTSES (Fig. S1 A). The reversibility of the effect of MTSES on the currents mediated by cysteine mutants suggested that MTS modification was being chemically reversed, as a second application of MTSES has a similar effect to the first application (Fig. S2). The MTS–thiol linkage can only be broken by reducing agents, raising the intriguing possibility that endogenous cytosolic reducing agents permeate the connexin pore to reverse the MTS modification. A recent study showed slow permeation of GSH through Cx46 hemichannels (Slavi et al., 2014). We examined whether MTSES modification was reversible in Cx46 hemichannels containing a substituted cysteine at pore-lining position 43 (E43C). Although Cx46 E43C channels showed slower MTSES modification than that observed for cysteine mutants of Cx26 hemichannels, there was a sustained recovery from the chemical modification after washing off MTSES (Fig. S3), further supporting slow permeation of a reducing agent through connexin channels.
Figure 2.
Reversible chemical modification by MTS at cysteine residues lining the Cx26 pore. (A) MTSES modification at D50C. (Left) Current traces from an oocyte expressing Cx26 D50C hemichannels in response to a depolarizing pulse from −80 to 0 mV. Black trace is the current before modification. Orange trace is a representative trace in the presence of MTSES after reaching the maximal effect of the modification. Blue trace is the current after wash off of MTSES. (Right) Time course of the maximal tail currents in the absence, the presence, and after wash off of MTSES. Solid blue line represents single-exponential fit for recovery from modification. (B and C) The corresponding data for MTSES modification at positions D46C and G45C, respectively. Experimental procedure as described in A. All traces were obtained in the presence of 0.25 mM of extracellular Ca2+.
Decrease of cellular rGSH decelerates and attenuates reversibility of MTSES effects
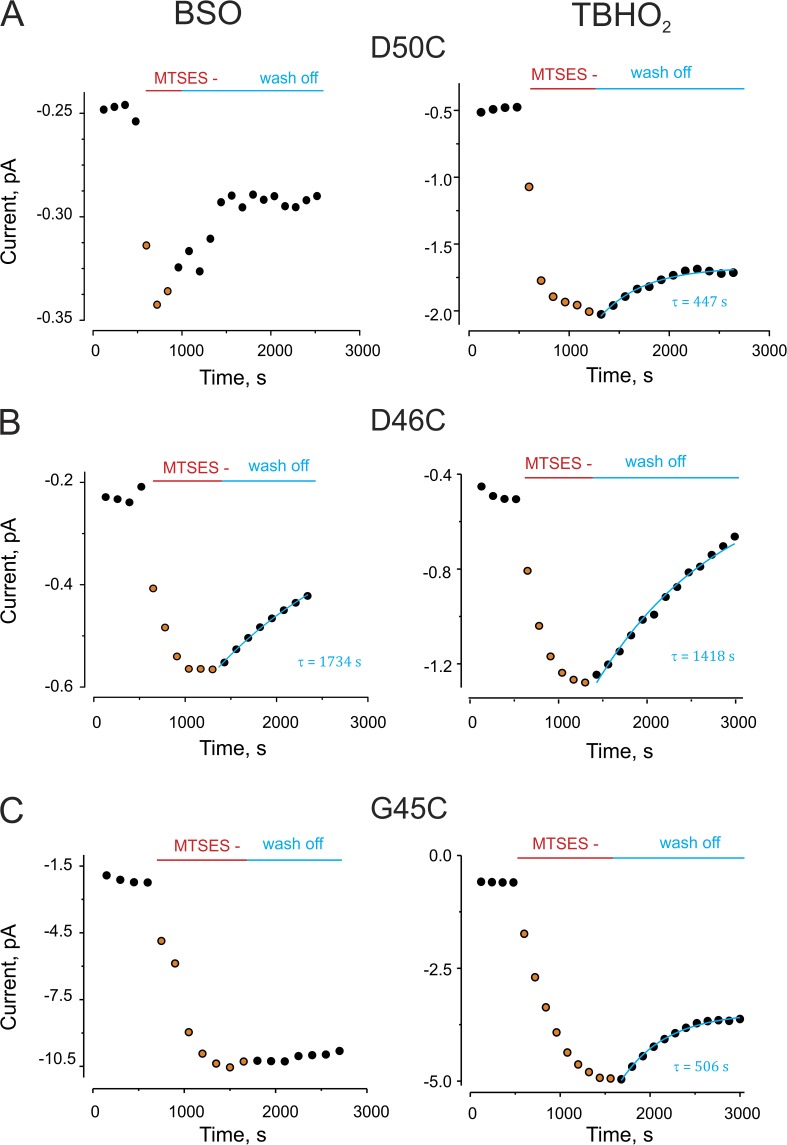
To explore whether a cytoplasmic reducing agent was reversing the MTSES modification, we reasoned that the reversibility would be attenuated if cellular rGSH, the primary cytoplasmic reducing agent, were decreased in the oocytes. Two reagents were used to manipulate the levels of rGSH: TBHO2, which increases the oxidized GSH (GSSG) level by oxidizing rGSH (Ochi, 1992; de la Peña et al., 2011) and BSO, an inhibitor of GSH biosynthesis (Griffith and Meister, 1979; Övey and Naziroğlu, 2015). Thus, BSO treatment decreases total GSH, and TBHO2 decreases rGSH specifically. Fig. S2 shows that BSO treatment significantly decreased the levels of cellular rGSH. Fig. 3 shows representative traces of the results of oocyte treatment with these reagents on reversal of MTSES effects. In each case, the reversibility of the MTSES effect on the currents after washout was substantially delayed and/or attenuated. Note that there was no significant effect of either reagent on resting current levels.
Figure 3.
Decreased levels of cellular rGSH decelerate or diminish reversibility of MTS modification. BSO and TBHO2, which decrease cytoplasmic rGSH levels, decelerate and/or diminish reversibility of the MTSES modification. (A) A representative experiment for MTSES modification at position D50C. (Left) Dots show the time course of maximal tail current for an oocyte pretreated with 2 mM TBHO2, which was then exposed to MTSES. Wash off of MTSES was in the presence of TBHO2. (Right) Same experiment, but with 24–48-h preexposure to BSO. The tail currents were measured in the presence of 0.25 mM of extracellular Ca2+ after a depolarizing pulse from −80 to 0 mV. (B and C) Representative experiments for MTS modification at positions D46C and G45C, respectively, as described in A.
Fig. 4 shows the percentage of reversibility from MTSES modification (after 5-min wash off) for D50C, D46C, and G45C mutants. In the absence of BSO or TBHO2, all three mutants showed almost full recovery from MTS modification (Fig. 4, white bars); however, in the presence of the BSO or TBHO2, only partial recovery is observed. Overall, the effect of the treatment with TBHO2 showed higher effectiveness than BSO. This could be explained by the modest reduction of rGSH by BSO treatment (Fig. S4), which relies on enzymatic inhibition, compared with the direct oxidizing effect of TBHO2. These results indicate that cellular depletion of rGSH enhances the stability of the MTSES modification of Cx26 at these residues, consistent with the idea of cellular reductants playing a role in reversal of MTSES effects.
Figure 4.
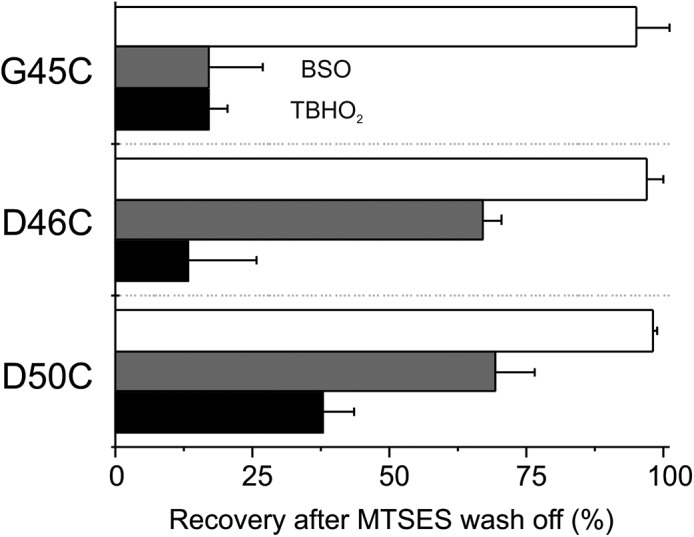
BSO or TBHO2 treatment significantly decreases recovery after MTSES modification. Percentage of recovery from MTSES modification after BSO (gray bars) or TBHO2 (black bars) treatment in oocytes expressing D50C, D46C, or G45C. White bars correspond to recovery from MTSES modification without any treatment. The values were normalized at steady state or after 300 s of MTSES wash off. Error bars represent the mean ± SEM of at least eight independent measurements.
Connexin hemichannels are permeable to cytosolic reducers
Connexin channels are generally thought to be permeable to molecules with diameters below 12 Å, and a variety of studies on several connexin isoforms suggests that molecules of the size of cellular reducing agents, such as GSH, NADH, and NADPH, are permeable (Harris, 2007). There is indirect evidence for permeability of GSH and NAD+ through Cx43 hemichannels (Barhoumi et al., 1993; Bruzzone et al., 2001; Stridh et al., 2008), and for permeability of GSH though Cx46 gap junctions (Slavi et al., 2014). Consistently, we found that extracellular levels of GSH were higher in the external media from oocytes injected with hCx26 when compared with noninjected oocytes after 6 and 24 h of incubation in Ringer’s solution containing 0.25 mM Ca2+ (Fig. S5).
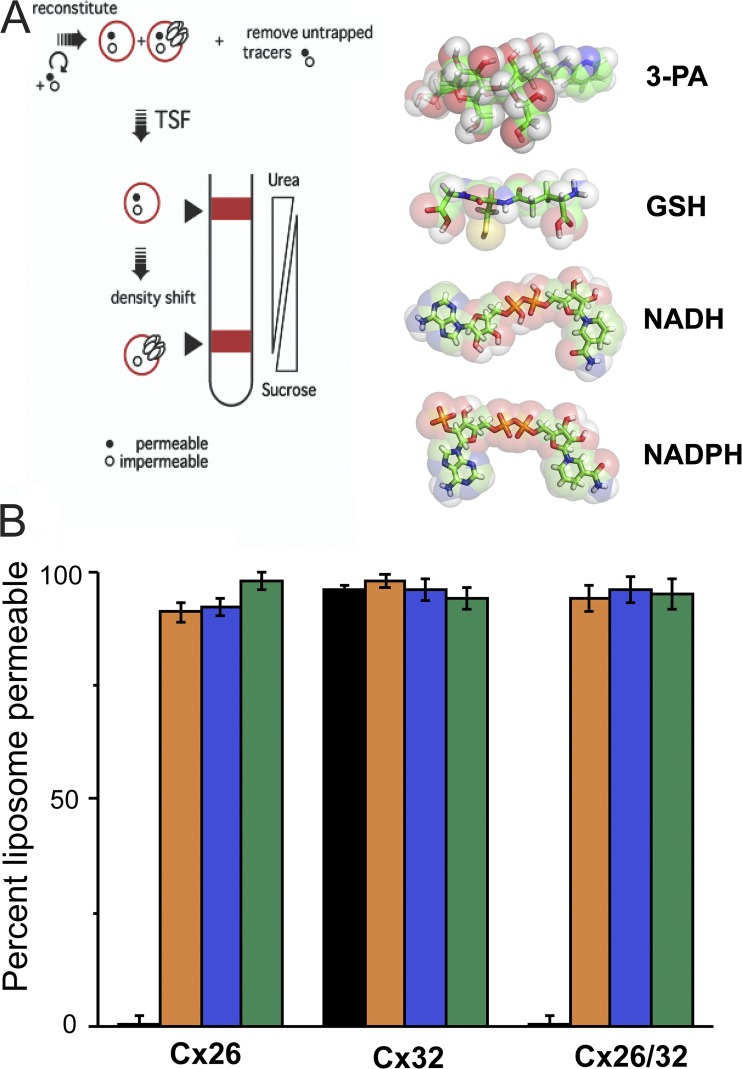
To directly demonstrate permeability of cellular reductants through connexin hemichannels, we used a liposome-based assay of molecule permeability of connexin channels, previously used to demonstrate connexin channel permeability to inositol phosphates (Ayad et al., 2006). In this all-or-none assay of permeation, retention/leakage of test compounds through hemichannel-containing liposomes is compared with that of liposomes that do not contain functional connexin channels. As shown in Fig. 5, all of the tested cellular reductants (GSH, NADH, and NADPH) were permeable through all the connexin channels tested (homomeric Cx26 and Cx32, and heteromeric Cx26/Cx32). 3-PA, which is a trisaccharide derivative, permeates Cx32 channels but not Cx26-containing channels, as reported previously (Bevans et al., 1998; Locke et al., 2004). The results confirm that several cellular reductants freely permeate a variety of connexin channels and thereby have access to pore-lining residues.
Figure 5.
Connexin hemichannels are permeable to cytosolic reductants. (A; left) Scheme of TSF method. (Right) Chemical structure of 3-PA and endogenous cellular reductants. (B) Percentage of liposomes permeable to cytosolic reducing agents for liposomes containing either homomeric or heteromeric hemichannels formed by Cx32, Cx26, and Cx32/26. Orange bars, GSH; blue bars, NADH; green bars, NADPH. Homomeric and heteromeric hemichannels were fully permeable through all tested reducers. 3-PA (black bars), a trisaccharide derivative known to be permeable through Cx32 channels but not Cx26-containing channels, was used as control. Error bars represent the mean ± SEM of at least three independent measurements.
Irreversible cysteine modification in the pore
If cysteine modification by MTSES is being reversed by exposure to cellular reducing agents, then cysteine modification by an irreducible linkage should result in maintained modification of the connexin currents. Thioether linkages formed by maleimides are not reversed by reducing agents.
The experiments shown in Fig. 2 were repeated using a negatively charged maleimide (maleimide ES) (Fig. 6 A). Maleimide treatment of the cysteine mutants decreased the currents (Fig. 6, B and C) rather than increasing them as had MTSES. We attribute the opposite effect of this modification on the currents to the larger size of the maleimide moiety, which, after reaction with the cysteine thiols, could sterically occlude the pore or affect gating in a way that the MTS reagent could not (e.g., by stabilizing a closed state of hemichannels). No current modification was observed in oocytes expressing D46C mutants, likely because of the inability of maleimide ES to interact with the cysteine at this position.
Figure 6.
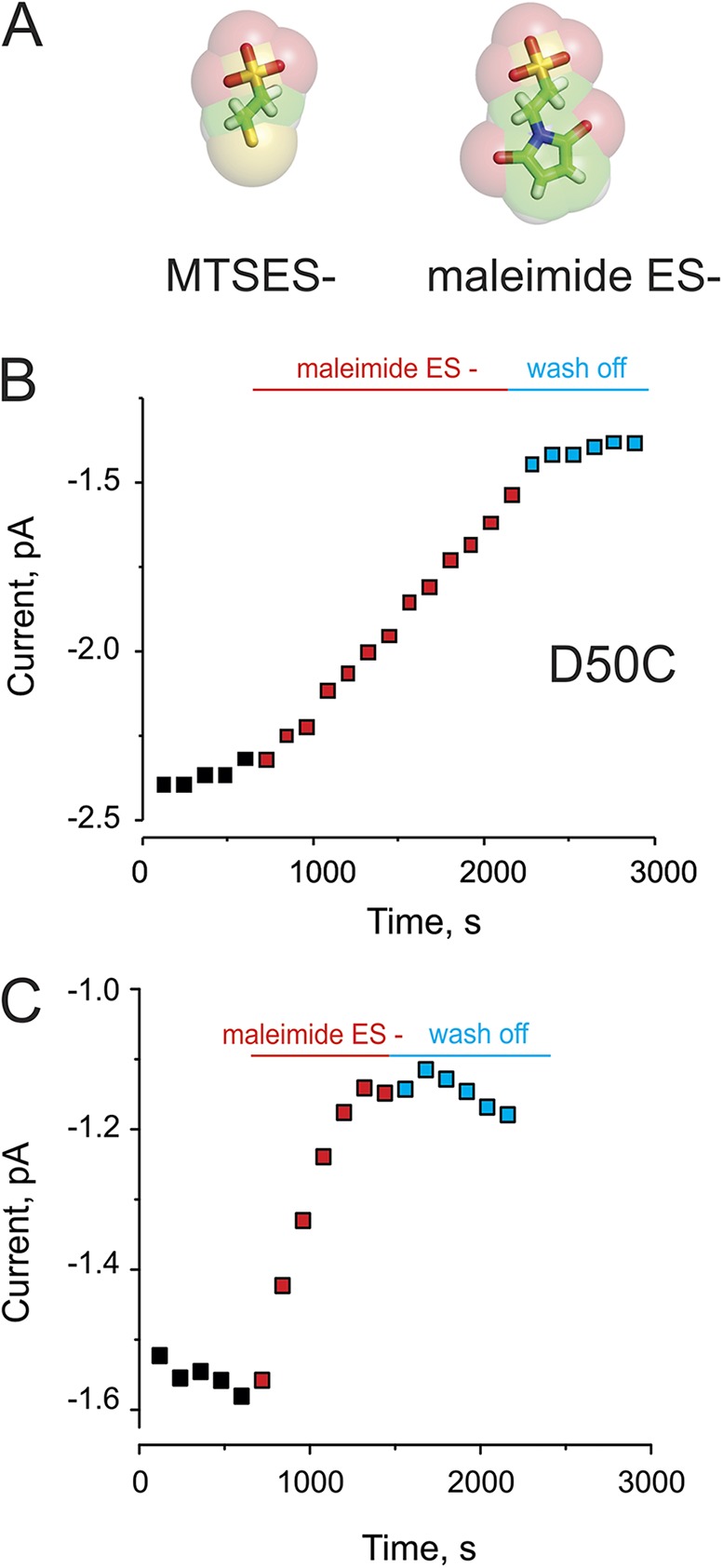
Irreversible maleimide modification at cysteine residues lining the pore. (A) Comparison of the chemical structures of MTSES and maleimide ES. (B) Time course of the peak tail current in the absence (black), the presence (red), or after wash off (blue) of maleimide ES for modification of D50C hemichannels expressed in Xenopus oocytes. (C) Maleimide ES modification at positions G45C, as described in A.
Importantly, the effect of maleimide modification on the connexin currents, in contrast to MTSES modification, did not reverse after washout (Fig. 6, B and C). Collectively with the previous data, this confirms that the reversal of the MTSES effects is caused by exposure of cell-generated reducing agents accessing the sites via the connexin pore itself.
DISCUSSION
The use of MTS reagents to identify accessible cysteines introduced by mutagenesis minimally requires that the reagents have no effect on channel/transporter function in the absence of the cysteine substitution, and that the functional change seen upon MTS exposure persists after MTS washout. Although MTS reagents have been used profitably in studies of connexin channels, the potential for reversibility and its implications for rates of reaction have not been explored. In this work, we found that the effects of MTS exposure reversed upon washout with a time course roughly similar to that required to achieve maximal modification. In the absence of an MTS effect on channels that did not contain a cysteine substitution, one possibility was that each of the three cysteine substitutions led to a conformational alteration that allowed the MTS to act as a pore blocker. MTSES has been shown to be a pore blocker in a (cysteine-less) CFTR mutant (Li et al., 2009). This action seems unlikely in the present work, as the effect of MTS exposure to the cysteine mutants was to increase, not decrease, the currents. The alternative was that the covalent MTS modification of the targeted cysteine was being reversed by reducing agents. If so, then both the degree and kinetics of the functional changes would be contaminated by the reverse reaction. The data show that the reversibility is decelerated and/or lessened when cellular levels of GSH or rGSH are reduced. These results, along with demonstration that GSH and other cellular reductants can permeate Cx26 (and other connexin) channels, indicate that the MTS effect on the connexins containing the substituted cysteines was GSH sensitive, a nominal criterion for MTS–thiol modification, and that cellular GSH had access from the cytosol to the targeted sites within the connexin pore. The subsequent experiments with non-reducible thiol modification by maleimide confirmed the accessibility of the substituted cysteines from the extracellular side of the pore and validated the initial MTS effects.
This finding suggests that for pore channels permeable to small metabolites (e.g., connexins, pannexins, CALHM channels, VRAC), the use of chemical modification to structure–function studies is problematic when using thiol reagents; such studies are likely to be compromised by a reverse reaction caused by GSH permeation. This has not been reported before for classical ion channels that are not permeable to molecules. Previous work has used cysteine-scanning mutagenesis and chemical modification with MTS derivatives to infer permeability or conformational changes upon gating of connexin hemichannels (Kronengold et al., 2003b; Tang et al., 2009; Verselis et al., 2009; Sánchez et al., 2010). None of the studies reported modification rates of the state-dependent accessibility for thiol reagents because of technical limitations and perhaps slow modification as a result of reversibility. Some of these studies, however, have elegantly shown changes in unitary hemichannel conductance by MTS reagents when using the excised inside-out patch configuration in which cellular GSH is not a factor (Kronengold et al., 2003b; Verselis et al., 2009); unfortunately, quantitative measurements of modification rates are not possible because of profound channel rundown, rapid flickering gating, and/or lack of open probability control in the excised patch configuration.
In an attempt to avoid MTS reversibility by GSH in oocytes where the relative open probability for Cx26 hemichannels is easily measured by electrophysiological methods, we tested two reagents (BSO and TbHO2) to decrease the levels of rGSH. BSO or TbHO2 treatment did not affect Cx26 wild-type hemichannel currents, as evidenced in the holding currents in Figs. 2 and 3. Treatment with BSO has experimental disadvantages compared with TBHO2 because of the long treatment time required to deplete cellular GSH (48–72 h). Chronic depletion of GSH affects the intracellular redox environment as well as several cell-signaling pathways, which can have downstream effects and affect cellular viability. On the other hand, treatment with the membrane-permeable oxidizing agent TbHO2 rapidly converts rGSH into GSSG (the oxidized form of GSH) and therefore is less likely to have pleiotropic effects or to affect viability. In this work, both agents reduce the reversal of MTS modification of Cx26 currents, with TbHO2 being more effective (Fig. 4).
In addition, we used maleimide because the thiol modification by this reagent is a highly specific reaction at neutral pH (at alkaline pH, glycine and proline can be modified by maleimides; Lundblad, 2005). The reaction with cysteine involves the addition of the thiol across the double bond of the maleimide to yield a thioether, which produces an irreducible linkage. The absence of changes in wild-type Cx26 hemichannel currents (Fig. S1 B) after maleimide exposure indicates that the changes observed for D50C and G45C mutants are caused by specific modification of the introduced cysteines. The maleimide modifications are slow and essentially irreversible, as shown in Fig. 6. The slow reaction rates observed for maleimide can be explained by low diffusional accessibility to the external pore and/or low velocity of the chemical reaction between maleimide and sulfhydryl groups, which is in the range of 1 to 5 × 103 M−1s−1 in aqueous solutions for free l-cysteines (Gorin et al., 1966). This is substantially less than that of MTS reagents, which for reaction with free l-cysteines is on the order of 105 M−1s−1 (Lundblad, 2005). The slow modification may limit the applicability of maleimide reagents to study channel structure and function of connexin channels, as it would be difficult to distinguish whether the rate-limiting reaction is due to the intrinsic lower rate of the reaction or poor diffusional accessibility to cysteine within the pore. Yet certain studies, including fluorescence tagging of substituted cysteine residues, would be more reliable using maleimide than MTS derivatives to prevent reversibility.
Physiological implications
In various contexts, connexin hemichannels have been shown to be permeable to a variety of cellular molecules. Recently, Cx46 gap junctions have been proposed as a pathway for the delivery of GSH to the lens nucleus, which is thought to be critical to keep lens transparency and for protection from oxidative stress (Slavi et al., 2014). The results of the present study highlight permeability of rGSH though Cx26 hemichannels (one may infer permeability through Cx26 junctional channels as well). There is a large concentration gradient of GSH across plasma membrane; cytosolic concentrations are typically 1–10 mM, and plasma concentrations are in the low micromolar range (Ballatori et al., 2009). Therefore, opening of an unapposed Cx26 hemichannel will passively release substantial GSH. Cellular release of GSH is required for operation of the gamma-glutamyl cycle, in which GSH conjugated to extracellular amino acids plays a key role in amino acid uptake and metabolism (Meister and Powers, 1978; Viña et al., 1989) and protection against oxidative damage (Vargas and Johnson, 2009). Members of the VRAC family of channels and MDR transporters have been implicated in GSH release (Marchan et al., 2008; Sabirov et al., 2013). Flux of GSH through Cx26 hemichannels to the extracellular environment, driven by its ∼1,000-fold concentration gradient, may have important physiological implications in the inner ear, liver, and skin. In addition, intercellular movement of GSH via junctional channels may also play significant roles in maintaining intracellular redox homeostasis.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of General Medical Sciences (grants RO1-GM099490 to J.E. Contreras, RO1-R01GM101950 to A.L. Harris and J.E. Contreras, and RO1GM36044 to A.L. Harris).
The authors declare no competing financial interests.
Angus C. Nairn served as editor.
Footnotes
Abbreviations used in this paper:
- BSO
- l-buthionine-S,R-sulfoximine
- Cx26
- connexin 26
- GSH
- glutathione
- maleimide ES
- N-2-sulfoethyl maleimide
- MTSES
- 2-sulfonatoethyl MTS
- rGSH
- reduced GSH
- TBHO2
- tert-Butyl hydroperoxide
- TSF
- transport-specific fractionation
References
- Allsopp R.C., El Ajouz S., Schmid R., and Evans R.J.. 2011. Cysteine scanning mutagenesis (residues Glu52-Gly96) of the human P2X1 receptor for ATP: Mapping agonist binding and channel gating. J. Biol. Chem. 286:29207–29217. 10.1074/jbc.M111.260364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A., Safrina O., and Cahalan M.D.. 2014. State-dependent block of Orai3 TM1 and TM3 cysteine mutants: Insights into 2-APB activation. J. Gen. Physiol. 143:621–631. 10.1085/jgp.201411171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad W.A., Locke D., Koreen I.V., and Harris A.L.. 2006. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J. Biol. Chem. 281:16727–16739. 10.1074/jbc.M600136200 [DOI] [PubMed] [Google Scholar]
- Ballatori N., Krance S.M., Marchan R., and Hammond C.L.. 2009. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Aspects Med. 30:13–28. 10.1016/j.mam.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhoumi R., Bowen J.A., Stein L.S., Echols J., and Burghardt R.C.. 1993. Concurrent analysis of intracellular glutathione content and gap junctional intercellular communication. Cytometry. 14:747–756. 10.1002/cyto.990140707 [DOI] [PubMed] [Google Scholar]
- Bevans C.G., Kordel M., Rhee S.K., and Harris A.L.. 1998. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J. Biol. Chem. 273:2808–2816. 10.1074/jbc.273.5.2808 [DOI] [PubMed] [Google Scholar]
- Bruzzone S., Guida L., Zocchi E., Franco L., and De Flora A.. 2001. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 15:10–12. [DOI] [PubMed] [Google Scholar]
- de la Peña P., Alonso-Ron C., Machín A., Fernández-Trillo J., Carretero L., Domínguez P., and Barros F.. 2011. Demonstration of physical proximity between the N terminus and the S4-S5 linker of the human ether-a-go-go-related gene (hERG) potassium channel. J. Biol. Chem. 286:19065–19075. 10.1074/jbc.M111.238899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi C., and Olcese R.. 2009. The Voltage-Clamp Fluorometry Technique. Potassium Channels. Vol. 491 Lippiat J., editor Humana Press, New York: 213–231. 10.1007/978-1-59745-526-8_17 [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Koch H.P., Drum B.M., and Larsson H.P.. 2010. Strong cooperativity between subunits in voltage-gated proton channels. Nat. Struct. Mol. Biol. 17:51–56. 10.1038/nsmb.1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin G., Martic P.A., and Doughty G.. 1966. Kinetics of the reaction of N-ethylmaleimide with cysteine and some congeners. Arch. Biochem. Biophys. 115:593–597. 10.1016/0003-9861(66)90079-8 [DOI] [PubMed] [Google Scholar]
- Graeff R., and Lee H.C.. 2002. A novel cycling assay for nicotinic acid-adenine dinucleotide phosphate with nanomolar sensitivity. Biochem. J. 367:163–168. 10.1042/bj20020644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O.W., and Meister A.. 1979. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 254:7558–7560. [PubMed] [Google Scholar]
- Harris A.L. 2007. Connexin channel permeability to cytoplasmic molecules. Prog. Biophys. Mol. Biol. 94:120–143. 10.1016/j.pbiomolbio.2007.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., and Akabas M.H.. 1998. Substituted-cysteine accessibility method. Methods Enzymol. 293:123–145. 10.1016/S0076-6879(98)93011-7 [DOI] [PubMed] [Google Scholar]
- Kawate T., Robertson J.L., Li M., Silberberg S.D., and Swartz K.J.. 2011. Ion access pathway to the transmembrane pore in P2X receptor channels. J. Gen. Physiol. 137:579–590. 10.1085/jgp.201010593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreen I.V., Elsayed W.A., Liu Y.J., and Harris A.L.. 2004. Tetracycline-regulated expression enables purification and functional analysis of recombinant connexin channels from mammalian cells. Biochem. J. 383:111–119. 10.1042/BJ20040806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronengold J., Trexler E.B., Bukauskas F.F., Bargiello T.A., and Verselis V.K.. 2003a. Pore-lining residues identified by single channel SCAM studies in Cx46 hemichannels. Cell Commun. Adhes. 10:193–199. 10.1080/cac.10.4-6.193.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronengold J., Trexler E.B., Bukauskas F.F., Bargiello T.A., and Verselis V.K.. 2003b. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J. Gen. Physiol. 122:389–405. 10.1085/jgp.200308861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Chang T.H., Silberberg S.D., and Swartz K.J.. 2008. Gating the pore of P2X receptor channels. Nat. Neurosci. 11:883–887. 10.1038/nn.2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.S., Demsey A.F., Qi J., and Linsdell P.. 2009. Cysteine-independent inhibition of the CFTR chloride channel by the cysteine-reactive reagent sodium (2-sulphonatoethyl) methanethiosulphonate. Br. J. Pharmacol. 157:1065–1071. 10.1111/j.1476-5381.2009.00258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke D., Koreen I.V., Liu J.Y., and Harris A.L.. 2004. Reversible pore block of connexin channels by cyclodextrins. J. Biol. Chem. 279:22883–22892. 10.1074/jbc.M401980200 [DOI] [PubMed] [Google Scholar]
- Lopez W., Gonzalez J., Liu Y., Harris A.L., and Contreras J.E.. 2013. Insights on the mechanisms of Ca2+ regulation of connexin26 hemichannels revealed by human pathogenic mutations (D50N/Y). J. Gen. Physiol. 142:23–35. 10.1085/jgp.201210893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad R.L. 2005. Chemical Reagents for Protein Modification. Third edition CRC Press, Boca Raton, FL: 352 pp. [Google Scholar]
- Marchan R., Hammond C.L., and Ballatori N.. 2008. Multidrug resistance-associated protein 1 as a major mediator of basal and apoptotic glutathione release. Biochim. Biophys. Acta. 1778:2413–2420. 10.1016/j.bbamem.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., and Powers S.G.. 1978. Glutamine-dependent carbamyl phosphate synthetase: catalysis and regulation. Adv. Enzyme Regul. 16:289–315. 10.1016/0065-2571(78)90079-1 [DOI] [PubMed] [Google Scholar]
- Newell J.G., and Czajkowski C.. 2007. Cysteine Scanning Mutagenesis: Mapping Binding Sites of Ligand-Gated Ion Channels. Handbook of Neurochemistry and Molecular Neurobiology. Lajtha A., Baker G., Dunn S., and Holt A., Springer, New York: 439–454. 10.1007/978-0-387-30401-4_21 [DOI] [Google Scholar]
- Ochi T. 1992. Inhibition of the activity of glutathione peroxidase by tertiary-butylhydroperoxide in cultured Chinese hamster cells and the role of cellular glutathione in the recovery of the activity. Toxicology. 71:119–127. 10.1016/0300-483X(92)90058-M [DOI] [PubMed] [Google Scholar]
- Övey I.S., and Naziroğlu M.. 2015. Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: Involvement of TRPM2 and TRPV1 channels. Neuroscience. 284:225–233. 10.1016/j.neuroscience.2014.09.078 [DOI] [PubMed] [Google Scholar]
- Sabirov R.Z., Kurbannazarova R.S., Melanova N.R., and Okada Y.. 2013. Volume-sensitive anion channels mediate osmosensitive glutathione release from rat thymocytes. PLoS ONE. 8:e55646 10.1371/journal.pone.0055646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez H.A., Mese G., Srinivas M., White T.W., and Verselis V.K.. 2010. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J. Gen. Physiol. 136:47–62. 10.1085/jgp.201010433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez H.A., Villone K., Srinivas M., and Verselis V.K.. 2013. The D50N mutation and syndromic deafness: Altered Cx26 hemichannel properties caused by effects on the pore and intersubunit interactions. J. Gen. Physiol. 142:3–22. 10.1085/jgp.201310962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavi N., Rubinos C., Li L., Sellitto C., White T.W., Mathias R., and Srinivas M.. 2014. Connexin 46 (cx46) gap junctions provide a pathway for the delivery of glutathione to the lens nucleus. J. Biol. Chem. 289:32694–32702. 10.1074/jbc.M114.597898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stridh M.H., Tranberg M., Weber S.G., Blomstrand F., and Sandberg M.. 2008. Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: Likely involvement of connexin hemichannels. J. Biol. Chem. 283:10347–10356. 10.1074/jbc.M704153200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Dowd T.L., Verselis V.K., and Bargiello T.A.. 2009. Conformational changes in a pore-forming region underlie voltage-dependent “loop gating” of an unapposed connexin hemichannel. J. Gen. Physiol. 133:555–570. 10.1085/jgp.200910207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M.R., and Johnson J.A.. 2009. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev. Mol. Med. 11:e17 10.1017/S1462399409001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verselis V.K., Trelles M.P., Rubinos C., Bargiello T.A., and Srinivas M.. 2009. Loop gating of connexin hemichannels involves movement of pore-lining residues in the first extracellular loop domain. J. Biol. Chem. 284:4484–4493. 10.1074/jbc.M807430200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viña J., Perez C., Furukawa T., Palacin M., and Viña J.R.. 1989. Effect of oral glutathione on hepatic glutathione levels in rats and mice. Br. J. Nutr. 62:683–691. 10.1079/BJN19890068 [DOI] [PubMed] [Google Scholar]
- Zhu Q., and Casey J.R.. 2007. Topology of transmembrane proteins by scanning cysteine accessibility mutagenesis methodology. Methods. 41:439–450. 10.1016/j.ymeth.2006.08.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.