Integrins mediate endothelial NO production and vasodilation in response to the compression of muscle arterioles.
Abstract
Cardiac and skeletal muscle contraction lead to compression of intramuscular arterioles, which, in turn, leads to their vasodilation (a process that may enhance blood flow during muscle activity). Although endothelium-derived nitric oxide (NO) has been implicated in compression-induced vasodilation, the mechanism whereby arterial compression elicits NO production is unclear. We cannulated isolated swine (n = 39) myocardial (n = 69) and skeletal muscle (n = 60) arteriole segments and exposed them to cyclic transmural pressure generated by either intraluminal or extraluminal pressure pulses to simulate compression in contracting muscle. We found that the vasodilation elicited by internal or external pressure pulses was equivalent; moreover, vasodilation in response to pressure depended on changes in arteriole diameter. Agonist-induced endothelium-dependent and -independent vasodilation was used to verify endothelial and vascular smooth muscle cell viability. Vasodilation in response to cyclic changes in transmural pressure was smaller than that elicited by pharmacological activation of the NO signaling pathway. It was attenuated by inhibition of NO synthase and by mechanical removal of the endothelium. Stemming from previous observations that endothelial integrin is implicated in vasodilation in response to shear stress, we found that function-blocking integrin α5β1 or αvβ3 antibodies attenuated cyclic compression–induced vasodilation and NOx (NO−2 and NO−3) production, as did an RGD peptide that competitively inhibits ligand binding to some integrins. We therefore conclude that integrin plays a role in cyclic compression–induced endothelial NO production and thereby in the vasodilation of small arteries during cyclic transmural pressure loading.
INTRODUCTION
The vascular tone in myocardium and skeletal muscle circulation is not only regulated by hemodynamics (Kuo et al., 1990; Goto et al., 1996; Sorop et al., 2002; Chiu and Chien, 2011), but it is also affected by external muscle contraction, which compresses the embedded blood vessels (Spaan, 1985; Hoffman, 1987; Goto et al., 1991; Clifford et al., 2006). It is well established that flow shear stress acting on the endothelium regulates nitric oxide (NO) and plays a key role in vascular biology (Kuo et al., 1990; Goto et al., 1996; Sorop et al., 2002, 2003; Chiu and Chien, 2011). The external compression on the blood vessel wall during muscle contraction is also recognized as an independent regulator of vascular tone (Buckwalter et al., 1998; Naik et al., 1999; Clifford et al., 2006; VanTeeffelen and Segal, 2006). Muscle contraction may generate ∼600 mmHg of extravascular pressure (Sejersted et al., 1984). Therefore, the intramuscular pressure may exceed intravascular pressure. Although there is evidence that endothelial NO mediates compression-elicited vasodilation in myocardium and skeletal muscle (Sun et al., 2001, 2004), the involvement of integrin in mechanotransduction is unclear.
The extraluminal compression changes the transmural pressure (equal to intraluminal minus the extraluminal pressure) and in turns changes the lumen diameter and hence the circumferential deformation of the blood vessel wall. Moreover, extraluminal compression causes radial compression, which may result in radial deformation. Because cyclic stretch plays an important role in the regulation of endothelial NO in cell culture (Awolesi et al., 1994, 1995; Ziegler et al., 1998; Kuebler et al., 2003; Takeda et al., 2006), we can presume that the circumferential deformation induced by transmural pressure may mediate vasodilation.
Integrins are well-established mechanosensors that convert mechanical and chemical stimulation to cellular signaling (Muller et al., 1997; Davis et al., 2001; Martinez-Lemus et al., 2003). Endothelial integrin mediates blood flow shear stress–elicited biological response (Muller et al., 1997; Yano et al., 1997; Shyy and Chien, 2002). PI3K (phosphoinositide 3-kinase)/Akt (protein kinase B) mediates integrin activation-induced endothelial NO synthase (eNOS) phosphorylation to produce NO (Morello et al., 2009). The mechanosensory role of integrins in stretch stimulus has been extensively investigated in the myogenic response of vascular smooth muscle (VSM) cells (Williams, 1998; Davis et al., 2001; Martinez-Lemus et al., 2003). It is unclear whether integrins play a role in compression-induced vasodilation.
Here, we hypothesize that endothelial integrins are implicated in the compression-induced vasodilation during muscle contraction through cyclic circumferential deformation. To test this hypothesis, we used in vitro coronary and skeletal muscle small arteries (inner diameter of 300–400 µm). Pressure myography equipped with an extraluminal pressure generator was used to determine the compression-induced vascular vasodilation. To verify the role of circumferential deformation, isovolumic myography (Lu and Kassab, 2011; Lu et al., 2013) was used to monitor vascular vasodilation while the circumferential deformation was inhibited (i.e., no change in strain but change in stress) during cyclic compression.
MATERIALS AND METHODS
The swine were provided by Michigan State University and housed at Indiana University School of Medicine Facilities (Laboratory Animal Resource Center). The swine had ad libitum access to water and food. A room temperature of 20–22°C and humidity of 30–70% were maintained. The animals were given a physical evaluation and acclimated for at least 3 d before the surgical procedure. The animal experiments were performed in accordance with national and local ethical guidelines, including the Principles of Laboratory Animal Care, the Guide for the Care and Use of Laboratory Animals, and the National Society for Medical Research, and an approved Indiana University School of Medicine Institutional Animal Care and Use Committee protocol regarding the use of animals in research.
39 male Duroc swine weighing 52 ± 14 kg (33–69 kg) were used in this study. The pigs were fasted overnight. Surgical anesthesia was induced with TKX (10 mg/kg Telazol, 5 mg/kg ketamine, and 5 mg/kg xylazine) and maintained with isoflurane 1–2%. Ventilation was provided with a respirator to maintain physiological PCO2 and PO2 at ∼35 and 100 mmHg, respectively. Electrocardiographic leads were attached to the animals’ limbs, and cardiac electrical signals were monitored on a defibrillator (Lifepak 9P; Physio-Control, Inc.). Body temperature was maintained at 37–38°C and blood pH at 7.4 ± 0.1. The anesthesia was monitored and recorded once per 10 min during the procedure. The adequacy of anesthesia was confirmed by stability of respiration and heart rate, absence of palpebral reflex and jaw tone, pink mucous membrane, and no limb withdrawal reflex. Two to three branches of artery (∼330 µm in diameter) within vastus intermedius muscle of the left leg was harvested and immediately stored in 4°C physiological saline solution (PSS; in mmol/L: 142 NaCl, 4.7 KCl, 2.7 sodium HEPES, 3 HEPES acid, 1.17 MgSO4, 2.79 CaCl2, and 5.5 glucose). The chemicals in this study were purchased from Sigma-Aldrich unless mentioned specifically. The chest cavity was exposed, and the heart was excised and immediately stored in 4°C PSS. Two to four branches of the left anterior descending (LAD) coronary artery (∼350 µm in diameter) were excised from the subendocardium. 60 small branches of artery in vastus intermedius muscle and 69 small branches of LAD coronary arteries were measured. The distribution of the vessel segments in the various groups is denoted in the legend to Fig. 6.
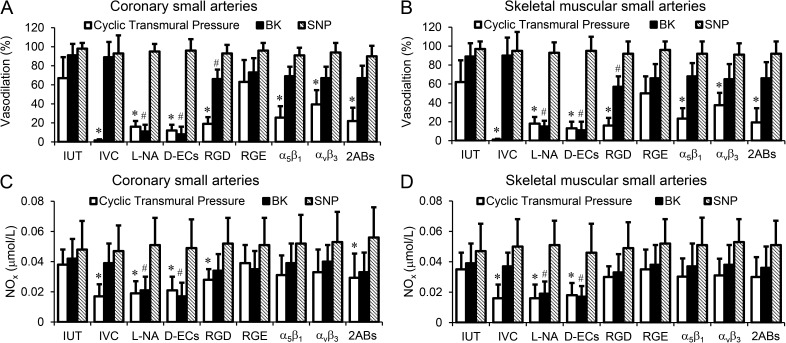
Figure 6.
Vasodilation and NOx (nitrate/nitrite) production of coronary and skeletal muscular small arteries were induced by the mechanical or pharmacological stimulations. (A) Vasodilation of coronary small arteries was induced by the cyclic transmural pressure or stimulated by BK, ACh, or SNP after the treatments. (B) Vasodilation of skeletal muscular small arteries was induced by the cyclic transmural pressure or stimulated by BK, ACh, or SNP after the treatments. (C) NOx production of coronary small arteries was exposed to stimulations after treatments. (D) NOx production of skeletal muscular small arteries was exposed to stimulations after treatments. The treatments included intact and untreated vessel (IUT), isovolumic condition (IVC) to maintain the diameter constant during cyclic transmural pressure, L-NAME incubation (L-NA), endothelial cells mechanically removed using a wire (D-ECs), GRGDSP peptide incubation (RGD), GRGESP peptide incubation (RGE), incubation with function-blocking integrin α5β1 antibody (α5β1), incubation with function-blocking integrin αvβ3 antibody (αvβ3), and incubation with function-blocking integrin α5β1 and αvβ3 antibodies (2ABs). The number of the vessel segments in the various groups was as follows: coronary segments: n = 15 for IUT, n = 6 for L-NA, n = 5 for D-ECs, n = 6 for RGD, n = 6 for RGE, n = 5 for IVC, n = 6 for α5β1, n = 5 for αvβ3, and n = 11 for 2ABs; skeletal muscular vessel segments: n = 10 for IUT, n = 5 for L-NA, n = 5 for D-ECs, n = 6 for RGD, n = 5 for RGE, n = 4 for IVC, n = 6 for α5β1, n = 6 for αvβ3, and n = 10 for 2ABs. *, P < 0.05 versus incubations; #, P < 0.05 versus stimulations. Error bars represent mean ± SD.
Experimental setup
To provide external pressure, the setup was assembled to create an isovolumic system within a sealable transparent box. The isovolumic system consisted of a chamber with two connectors, which bridge the blood vessel and rigid tubes. One tube connected to a 50-ml flask with PSS, and the flask was pressurized with a pressure regulator to inflate the vessel at the desired intraluminal pressure. Another tube connected with a Tuohy-Borst adapter (Cook Medical) mounted with a solid-state pressure transducer (SPR-524; Mikro-Tip catheter transducer; Millar, Inc.) to monitor the intraluminal pressure. The PSS contained 1% dialyzed albumin aerated with mixed gas (22% O2, 5% CO2, and balanced with 73% N2) that filled the chamber and tubes before vessel cannulation. A CCD camera on a microscope transferred the image of vessel to computer that digitized the external diameter of the vessel. Because the sample rate of digital conversion (200/s) was higher than the rate of change in the vessel dimension, the diameter was tracked in real time. The sealable transparent box was a larger container to accommodate the chamber of the isovolumic system and allowed the tubes of the isovolumic system to channel through the wall of the container to connect the blood vessel segment in the chamber with pressure source and pressure transducer. The channels for the tubes crossing the wall of the container were sealed using silicone sealant. The container was sealed using a gastight cover so that an extraluminal pressure could be applied to simulate the compression of the arteries embedded in contracting myocardium or skeletal muscle. Another pressure regulator was used to control the pressure in the container. A pule pressure generator connected with pressure regulators was used to generate either intraluminal or extraluminal pulse pressures. The transmural pressure of the vessel segment was defined as intraluminal pressure minus extraluminal pressure.
The vessel was inflated to physiological pressure while the outlet was closed off. Hence, the vessel was merely pressurized with no flow (no axial shear stress). Isobaric and isovolumic procedures were performed during either intraluminal or extraluminal pressure variation. The vascular contraction or relaxation under isobaric state (pressure myography) was characterized by the decrease or increase in diameter of the vessel segment. Under isovolumic conditions, the contraction or relaxation was characterized by the increase or decrease in intraluminal pressure during constant lumen volume (Lu and Kassab, 2011; Lu et al., 2013).
Experimental procedures
The vessel segment was cannulated in the chamber and warmed up to 37°C slowly (20–25 min) and equilibrated for 40 min at a transmural pressure of 15 mmHg at in situ length. The coronary or skeletal muscle arterial segments, inflated to physiological pressure (∼100 mmHg), were precontracted to an approximate 60% diameter with endothelin-1 at a concentration of 10−8–10−7 mol/L or phenylephrine at 10−7–10−6 mol/L, respectively. The vessel segments were then exposed to cyclic transmural pressure from 100 to 0 mmHg, which was generated either by pulse extraluminal pressure from 0 to 100 mmHg at 1 Hz while the intraluminal pressure was maintained at 100 mmHg or pulse intraluminal pressure from 0 to 100 mmHg while the extraluminal pressure was maintained at 0 mmHg. The standard period of pulse transmural pressure acting on vessel segment was 2 min. After stimulation of pulse transmural pressure, the endothelium-dependent vasodilation of coronary small arteries was induced with bradykinin (BK; 10−7–10−6 mol/L) or skeletal muscular small arteries with acetylcholine (ACh; 10−7–10−6 mol/L), respectively. The endothelium-independent vasodilation was induced with sodium nitroprusside (SNP; 10−10–10−5 mol/L) to verify the sensitivity of VSM to NO. To assess the role of endothelium in cyclic transmural pressure–induced vasodilation, the vessel segments with intact endothelium were incubated with L-NAME (Nω-nitro-l-arginine methyl ester hydrochloride, a nonselective NOS inhibitor; 10−6 mol/L) for 30 min (Rees et al., 1990), or the endothelium were denuded using an inner diameter–matched wire (Lu et al., 2004). The role of integrins was evaluated by intraluminally incubating the vessel segments with either RGD peptide (10−6 mol/L; GRGDSP; EZBiolab) or control RGE peptide (10−6 mol/L; GRGESP; EZBiolab) for 60 min where RGD peptide inhibited mechanical stimuli–induced integrin activation (Mogford et al., 1997; Schlaepfer and Hunter, 1997; Yip and Marsh, 1997; Platts et al., 1998). In additional experiments to further confirm the involvement of integrins in the cyclic transmural pressure–induced vasodilation, the vessel segments were intraluminally incubated with mouse anti–human integrin α5β1 monoclonal antibody (25 µg/ml; clone name, JBS5; EMD Millipore) for 60 min or mouse anti–human integrin αvβ3 monoclonal antibody (35 µg/ml; clone name, LM609; EMD Millipore) for 60 min. A 50-µl microsyringe driven by a microsyringe pump (UMP3; World Precision Instruments) and controller (Micro4; World Precision Instruments) were used to continuously administrate the antibodies to the lumen of vessel segments at the rate of 0.5 µl/min. The agonist-induced endothelium-dependent and -independent vasodilation were verified. At the end of each experiment, the calcium free–induced vasorelaxation was used to verify VSM relaxation.
To identify the change of the diameter (cyclic stretch) in the compression-induced vasodilation as the major stimulus, an isovolumic myography was used to maintain constant vessel diameter during cyclic transmural pressure induced by pulse extraluminal pressure. A constant intraluminal volume and pressure (100 mmHg) were maintained by closing the inlet and outlet Tygon tubing (ID, 0.6 mm, OD, 1.2 mm; Fisher Scientific) using screw-driving clamps and a microsyringe to compensate for the fluid filtration through the arterial wall (Lu and Kassab, 2011; Lu et al., 2013). The pulse extraluminal pressure was varied from 0 to 100 mmHg, which simulated the compression of the arteries within contractile myocardium, whereas the isovolumic myography maintained a nearly constant diameter and allowed the change of stresses in the vessel segment.
Nitrite/nitrate (NOx) analysis
To evaluate endothelial NO during vasodilation, we analyzed the variation of NOx, which is a metabolite of NO in aqueous solution. Approximately 200 µl PSS in the bath was collected before and after the vasodilation induced by either cyclic transmural pressure or after the application of various vasodilators, and NOx was analyzed using the ENO-20 NOx Analyzer (Eicom). This method is based on a combination of the Griess reaction and high performance liquid chromatography to detect <5-nM concentration in pH-neutral solution (Lu and Kassab, 2004).
Western blot analysis
The small coronary and skeletal muscle arterial segments were grouped in line with the various treatments and homogenized in lysis buffer (50 mM glycerophosphate, 100 µM sodium orthovanadate, 2 mM magnesium chloride, 1 mM EGTA, 0.5% Triton X-100, 1 mM DL-dithiothreitol, 20 µM pepstatin, 20 µM leupeptin, 0.1 U/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride). Total protein concentration was measured by a BCA kit (Bio-Rad Laboratories). 50 µg of proteins was electrophoresed on 4–20% Tris-glycine gel (Invitrogen) and transferred to a polyvinylidene difluoride membrane (EMD Millipore). Immunoblotting was performed with anti-Akt (a 1:300 dilution; Santa Cruz Biotechnology, Inc.) or anti-phosphorylated Akt (1:200; Santa Cruz Biotechnology, Inc.) in blocking buffer. The membrane was then rinsed and incubated with horseradish peroxidase–conjugated secondary antibody (1:5,000; Santa Cruz Biotechnology, Inc.). The membranes were developed with chemiluminescence detection reagent (PerkinElmer). The densitometric analysis was implemented, and the ratio of phosphorylated Akt and total Akt was calculated.
Fluorescence microscopy
The vessel segments for immunohistological analysis were fixed in 4% paraformaldehyde for 4 h and transferred to 20% sucrose for 30 min and 30% sucrose for overnight. The vessel segments were embedded with tissue-freezing medium (Neg 50; Richard-Allen Scientific) and sectioned with a cryostat (CM 1850; Leica). The sections were proceeded to immunofluorescence procedures, i.e., blocking, permeabilization, anti-phosphorylated Akt (1:200; Santa Cruz Biotechnology, Inc.), and fluorescence secondary antibodies (Alexa Fluor 546; Life Technologies) incubation. The nuclei were stained with a fluorescent probe (Hoechst 33342; Life Technologies). The internal elastic lamina of vessel segments were visualized by fluorescence (excitation/emission: 470/540 nm) microscope (Eclipse TE300; Nikon).
Data analysis and statistics
The degree of vasodilation to reflect the recovery of diameter from pre-constriction was calculated as follows: Vasodilation (%) = (ΔDvd/ΔDvc) × 100. ΔDvd was equal to the difference of the peak diameter during vasodilation induced by cyclic transmural pressure or vasodilators to the diameter of the pre-constriction induced by vasoconstrictors. ΔDvc was equal to the difference of the diameter at 1 min before the administration of the vasoconstrictors for pre-constriction to the stable diameter during pre-constriction (Fig. 2). The preparation of vessel segments was standardized so that the maximal endothelium-dependent relaxation in response to the endothelium-dependent vasodilator was >85%. The data were discarded when the segments did not meet these criteria. The data were presented as mean ± SD, and significant differences between two groups were determined by Student’s t test. A probability of P < 0.05 was considered to be indicative of a statistically significant difference.
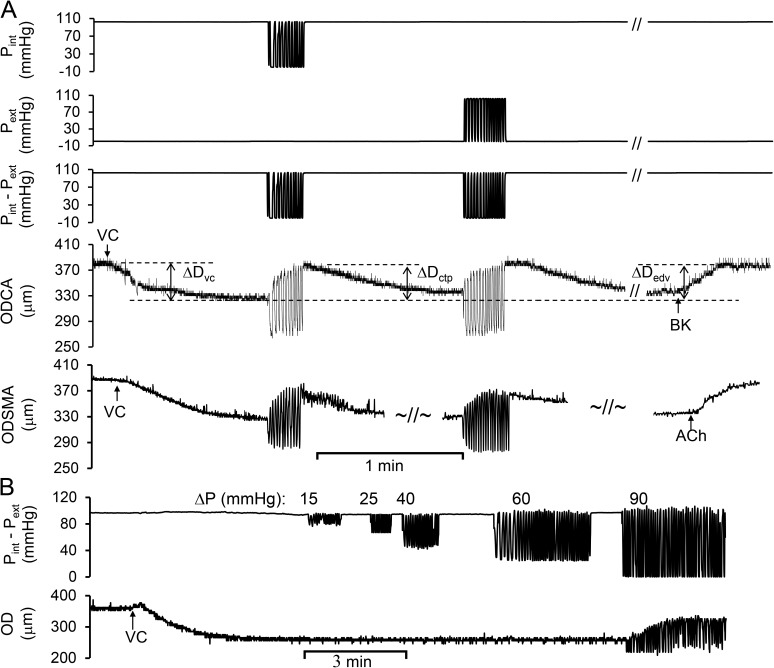
Figure 2.
The cyclic transmural pressure induced the vasodilation in the precontracted coronary and skeletal muscular small arterial segments. Pint was cyclically varied from ∼90 to 0 mmHg and later maintained constant at ∼90 mmHg. Pext was maintained constant at 0 mmHg and later cyclically varied from 0 to ∼90 mmHg. Pint–Pext was similar regardless of intraluminal or extraluminal variations. (A) The vasodilation in coronary and skeletal muscle arteries. ODCA was the OD of coronary arterial segment, which was tracked in real time during variations of transmural pressure. VC, vasoconstrictor (endothelin-1); ΔDvc, diameter change by vasoconstrictor; ΔDctp, peak diameter change by cyclic transluminal pressure; ΔDedv, peak diameter change by endothelium-dependent vasodilator; BK and ACh, bradykinin and acetylcholine (endothelium-dependent vasodilator). ODSMA was the OD of skeletal muscular arterial segment, which was tracked in real time during variations of transmural pressure. Vasodilation (%) = ΔDctp/ΔDvc. The defined abbreviations here are applied in all subsequent figures. (B) The vasodilation in response to the magnitude of the cyclic transmural pressure.
RESULTS
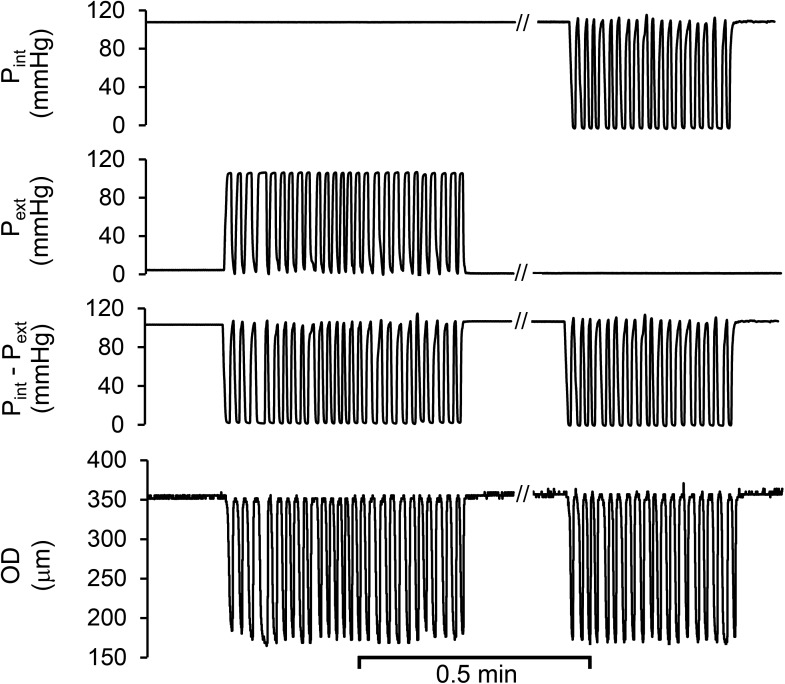
We verified the passive diameter change of an arterial segment in response to changes in transmural pressure (Fig. 1) by either intraluminal or extraluminal pressure, as the diameter was dictated by the transmural pressure in this isolated vessel preparation. As expected, the passive changes of peak diameter were essentially identical when transmural pressure was imposed by either cyclic intraluminal or extraluminal pressure (Fig. 1). We also found that the vasodilation induced by either cyclic intraluminal or extraluminal pressure in a precontracted vessel segment was determined by the transmural pressure (Fig. 2 A). As shown in Fig. 2 A, the diameter of coronary or skeletal muscle arterial segment decreased via contractile response to endothelin-1 (10−7 mol/L). After the diameter of contractile vessel reached a stable value, the cyclic intraluminal pressure (∼90 to 0 mmHg) was applied during a constant extraluminal pressure (0 mmHg) to generate cyclic transmural pressure. The diameter shows an oscillatory change during the cyclic transmural pressure, which significantly increased over time; i.e., the vessel vasodilated. When the transmural pressure remained constant at 100 mmHg (no pulsatility), the diameter gradually decreased toward the level before the cyclic transmural pressure was applied, i.e., vasoconstriction. While the cyclic extraluminal pressure (0 to ∼90 mmHg) was then applied during a constant intraluminal pressure (∼90 mmHg) to generate cyclic transmural pressure, the diameter showed an oscillatory change during the cyclic transmural pressure with transient vasodilation. The two ways to generate cyclic transmural pressure, constant extraluminal with cyclic intraluminal pressure or constant intraluminal pressure with cyclic extraluminal pressure, resulted in similar vasodilation (Fig. 2 A). At the end of study, the endothelial function was verified by endothelium-dependent vasodilator BK (10−7 mol/L). In fact, the progressive vasoconstriction during the cessation of cyclic loading underscored its role in vasodilation. We varied the magnitude of the cyclic transmural pressure (Fig. 2 B). We found that the magnitude of transmural pressure must be ∼86.3 ± 11.6 mmHg (n = 3) to induce the vasodilation. The in vivo magnitude of pulse pressure caused by cardiac systole and diastole is below the threshold to induce the vasodilation. We also found that the frequency of the cyclic transmural pressure from 0.5 to 1.5 did not change the vasodilation.
Figure 1.
Typical diameter variation of coronary arterial segment without pre-constriction was caused by cyclic transmural pressures that were generated by either intraluminal or extraluminal pressure variation. Pint, the intraluminal pressure constant at ∼90 mmHg and later cyclically varied from ∼90 to 0 mmHg; Pext, extraluminal pressure cyclically varied from 0 to ∼90 mmHg and later maintained constant at 0 mmHg; Pint–Pext, the cyclic transmural pressure calculated by the difference of Pint and Pext. OD, outer diameter of the segment was automatically tracked during cyclic transmural pressure. The defined abbreviations here are applied in all subsequent figures.
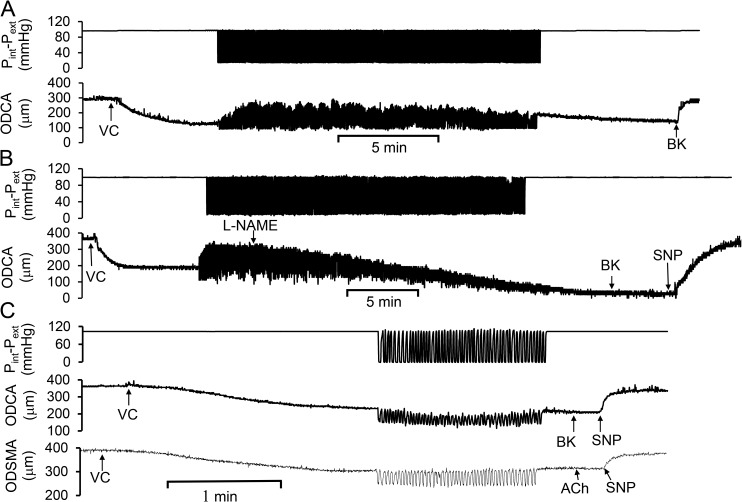
After a transient change of the vasodilation for ∼20 min, we verified that L-NAME was sufficient to inhibit the cyclic transmural pressure–induced endothelium-dependent vasodilation. Fig. 3 A shows a dynamic vasodilation for 20 min induced by cyclic transmural pressure. Fig. 3 B shows that the cyclic transmural pressure–induced vasodilation was gradually attenuated by L-NAME administration; i.e., L-NAME gradually replaced l-arginine, which is the substrate for eNOS to produce NO. The agonist-induced endothelium-dependent or -independent vasodilation was also verified after cyclic transmural pressure by use of BK, ACh, or SNP. The observations suggest that NO mediates cyclic transmural pressure–induced vasodilation. Because L-NAME also inhibits iNOS and nNOS (Pou et al., 1992; Haul et al., 1999), we further determined that the cyclic transmural pressure–induced vasodilation was eliminated after the endothelium was mechanically denuded by a diameter-matched wire (Fig. 3 C); i.e., endothelial NO mediates the cyclic transmural pressure–induced vasodilation.
Figure 3.
Endothelial role in the cyclic transmural pressure–induced vasodilation. The cyclic transmural pressure induced the vasodilation in precontracted coronary and skeletal muscular small arterial segments, and the vasodilation was inhibited by nonselective NOS inhibitor or denuded endothelium. (A) Cyclic transmural pressure–induced vasodilation persisted for at least 20 min. (B) Administration of L-NAME caused a dynamic vasoconstriction during cyclic transmural pressure. SNP, sodium nitroprusside (a NO donor). (C) Cyclic transmural pressure–induced vasodilation was inhibited by the mechanical removal of endothelium by a vessel diameter–matched wire.
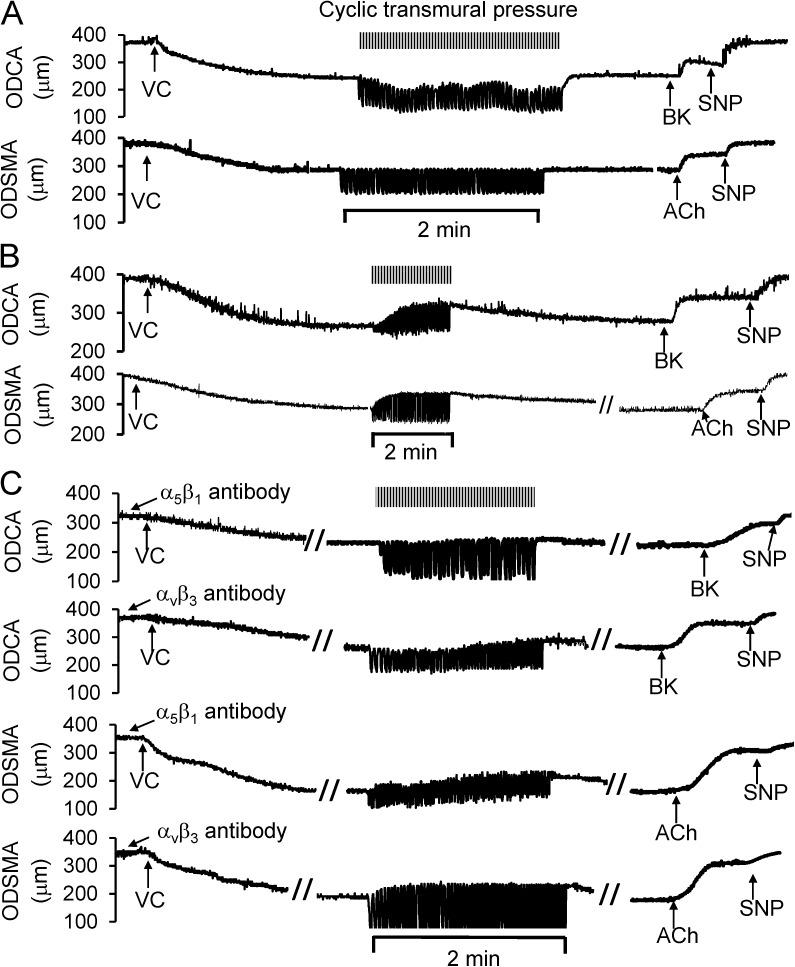
To assess the role of integrins, we investigated the effect of RGD peptide and the function-blocking integrin α5β1 and αvβ3 antibodies on the cyclic transmural pressure–induced vasodilation. RGD peptides competitively combine with integrin to attenuate mechanical stimuli–induced integrin activation (Mogford et al., 1997; Schlaepfer and Hunter, 1997; Yip and Marsh, 1997; Platts et al., 1998). In Fig. 4 A, using a typical sample of diameter tracings, we show that the preincubation of the vessel segments with RGD peptide inhibited the cyclic transmural pressure–induced vasodilation. GRGESP (a control RGE peptide) did not attenuate the cyclic transmural pressure-induced vasodilation (Fig. 4 B). The diameter tracings of the vessel segments preincubated with the function-blocking integrin α5β1 or αvβ3 antibodies also show that the antibodies attenuated the cyclic transmural pressure–induced vasodilation (Fig. 4 C).
Figure 4.
Role of integrins in the cyclic transmural pressure–induced vasodilation. (A) RGD peptide (GRGDSP) incubated with the vessel segments for 60 min prevented the vasodilation induced by the cyclic transmural pressure. (B) Control RGE peptide (GRGESP) did not affect the cyclic transmural pressure–induced vasodilation. (C) Function-blocking integrin α5β1 or αvβ3 antibodies compromised the cyclic transmural pressure–induced vasodilation. The top two panels represent the coronary arteries, and bottom two panels represent the skeletal muscle arteries. The vessel segments were incubated with function-blocking integrin α5β1 and αvβ3 antibodies, which were continuously administrated into lumen with a microsyringe pump.
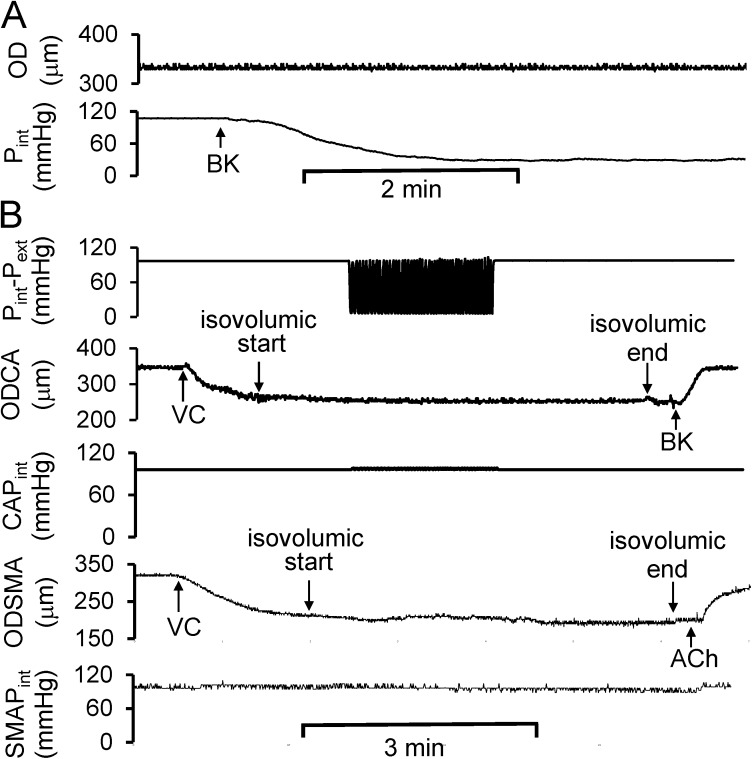
We used isovolumic myography to identify the role of circumferential deformation, as the vasodilation in isovolumic myography is reflected by a decrease in intraluminal pressure at a constant diameter (Fig. 5 A). Isovolumic myography restricts the change of diameter by maintaining constant fluid volume in the vessel segment, and the cyclic transmural pressure can only be generated by extraluminal pulse pressure. We found that the cyclic transmural pressure–induced vasodilation was eliminated under constant diameter condition (Fig. 5 B).
Figure 5.
Role of circumferential stretch in the cyclic transmural pressure–induced vasodilation. (A) The representation of the decrease in Pint during BK-induced endothelium-dependent vasodilation. Pext was equal to zero. (B) At isovolumic condition, e.g., approximately constant diameter during cyclic transmural pressure, the cyclic transmural pressure failed to elicit integrin-mediated endothelium-dependent vasodilation in both coronary and skeletal muscular small arterial segments. CAPint, intraluminal pressure of coronary small arterial segment; SMAPint, intraluminal pressure of skeletal muscular small arterial segment.
Fig. 6 summarizes the vasodilation and NOx production of coronary and skeletal muscle arteries exposed to various treatments. We verified that the changes of the vessel diameter during the cyclic transmural pressure were the stimuli to elicit a statistically significant endothelium-dependent vasodilation. The vasodilation attenuated by RGD peptide but not the control RGD peptide implicates the role of integrin. Furthermore, we verified the involvement of integrins in the cyclic transmural pressure–induced vasodilation using function-blocking integrin α5β1 and αvβ3 antibodies. Interestingly, function-blocking integrin α5β1 or αvβ3 antibody alone did not attenuate the vasodilation to an equivalent level of RGD peptide (Fig. 6, A and B). The vasodilation was attenuated similar to RGD peptide when the vessel segment was incubated with both integrin α5β1 and αvβ3 antibodies (Fig. 6, A and B).
We also verified that the cyclic transmural pressure–induced vasodilation was significantly smaller than agonist-induced vasodilation in both coronary and skeletal muscular small arteries (P < 0.05). The agonists-induced endothelium-dependent vasodilation was also attenuated by the RGD peptide (Fig. 6). Endothelium-independent vasodilation did not change significantly under various treatments. The NOx production in the vessel segments exposed to various treatments showed a similar response to vasodilation, which confirms the role of NO in the cyclic transmural pressure (Fig. 6, C and D).
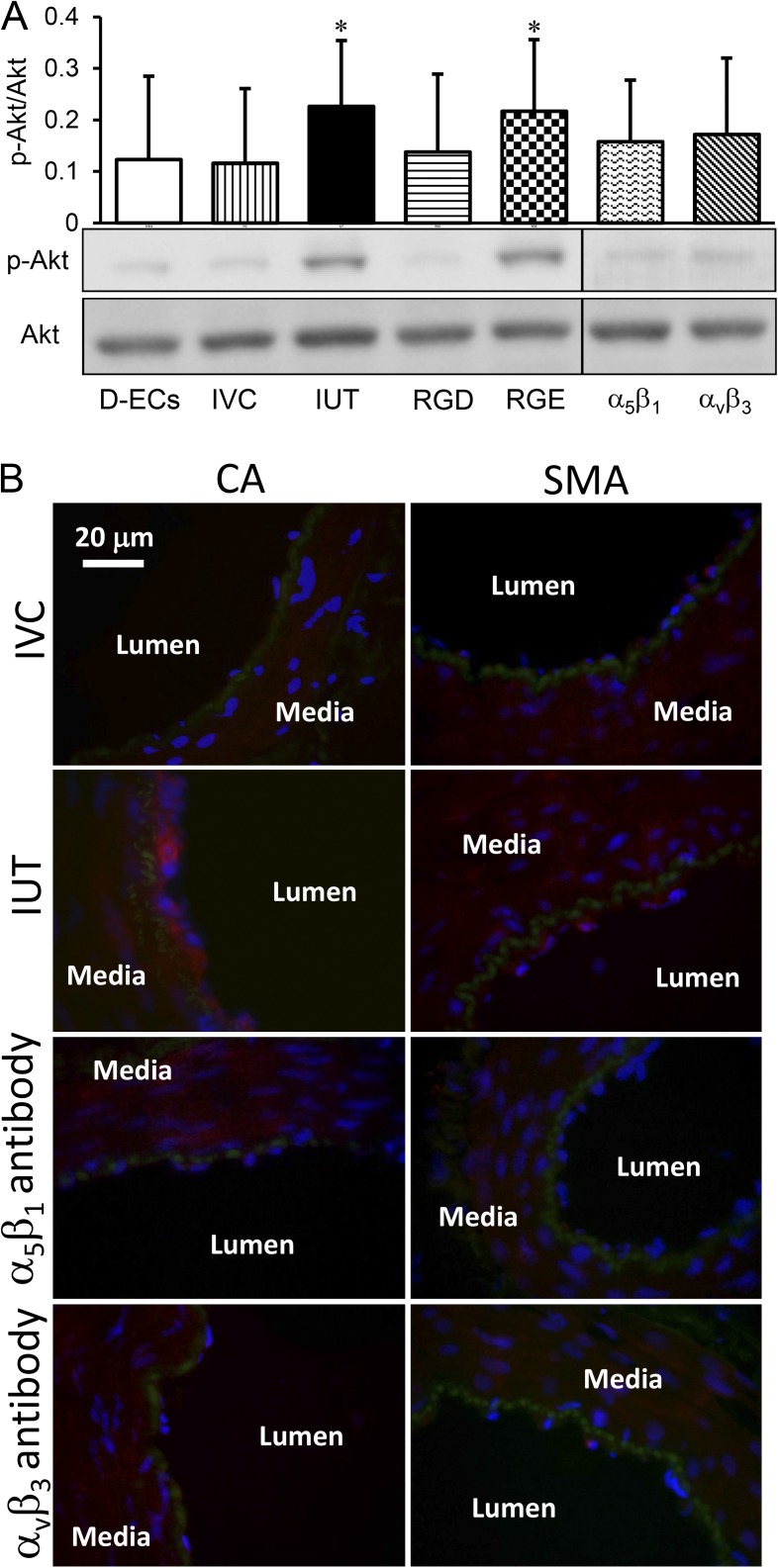
The phosphorylated Akt of the vessel segments was measured to provide further evidence of integrin signaling pathway activated by the cyclic transmural pressure (Fig. 7 A). The group of denuded vessel segments served as control. The cyclic transmural pressure up-regulated Akt phosphorylation. The constant diameter (isovolumic) condition, RGD peptide, and function-blocking integrin α5β1/αvβ3 antibodies prevented up-regulation of Akt phosphorylation in the vessel segments exposed to cyclic transmural pressure. To verify the endothelial Akt phosphorylation in response to the cyclic transmural pressure, we used immunofluorescence to visualize the distribution of Akt phosphorylation across the vessel wall. A dense fluorescence (red) was observed in the endothelial layer in the stimulation of the cyclic transmural pressure (Fig. 7 B), which indicates higher activation of Akt phosphorylation in the endothelial layer in response to cyclic transmural pressure.
Figure 7.
Akt phosphorylation of the small arterial segments stimulated by the cyclic transmural pressure after treatments. (A) Expression of Akt phosphorylation. Akt, protein kinase B; p-Akt, phosphorylated Akt. The treatments included the endothelial cells mechanically denuded using a wire (D-ECs), intact and untreated vessel segment (IUT), isovolumic condition (IVC) to maintain diameter constant during the cyclic transmural pressure, GRGDSP peptide incubation (RGD), GRGESP peptide incubation (RGE), incubation with function-blocking integrin α5β1 antibody (α5β1), and incubation with function-blocking integrin αvβ3 antibody (αvβ3). *, P < 0.05 versus IVC. Error bars represent mean ± SEM. (B) Immunofluorescence to verify Akt phosphorylation in endothelial cells. Fluorescent secondary antibody (Alexa Fluor 546; red) indicated that the signal of Akt phosphorylation was stronger in endothelial cells than that in the medial layer of both coronary small arteries (CA) and skeletal muscular small arteries (SMA) in IUT, and no difference between endothelial cells and the medial layer in both CA and SMA at IVC, α5β1 antibody inhibition, or αvβ3 antibody inhibition. Internal elastic lamina was indicated by green and the nucleus by blue.
DISCUSSION
We applied cyclic extraluminal pressure on vessel segments to induce vasodilation, which simulates compression-induced vasodilation in arteries embedded in myocardium or skeletal muscle. The major finding of this study is that cyclic circumferential deformation is the mechanical stimulus of compression-induced vasodilation of coronary and skeletal muscular small arteries, which is mediated through endothelial NO. We also found that integrins, likely integrins α5β1 and αvβ3, are involved in the mechanotransduction of the compression-induced endothelium-dependent vasodilation.
Physiologically, the blood vessel embedded in skeletal muscle or myocardium may be exposed to pulse extraluminal pressure caused by contraction of muscle. Contraction of myocardium or muscle can generate high pressure within the muscle (intramuscular pressure), which compresses the external surface of the embedded blood vessel, e.g., ∼600 mmHg in skeletal muscle contraction or ∼120 mmHg during cardiac systole (Sejersted et al., 1984; Heineman and Grayson, 1985; Spaan, 1985; Hoffman, 1987; Goto et al., 1991). The compression of muscular contraction can result in vasodilation of arterioles in the muscle, which is one mechanism of increase in peripheral perfusion during exercise (Buckwalter et al., 1998; Naik et al., 1999; Clifford et al., 2006; VanTeeffelen and Segal, 2006). In myocardium, the compression-induced vasodilation may enhance perfusion of subendocardium under physiological conditions (Spaan, 1985; Hoffman, 1987; Goto et al., 1991, 1996; Sorop et al., 2002). Because endothelial NO has been implicated in the compression-induced vasodilation (Sun et al., 2001, 2004), we focused on endothelial mechanotransduction of compression-induced vasodilation in this study.
Mechanical compression caused by muscular contraction may decrease the transmural pressure of blood vessel within the muscle or myocardium. Although compression is an important determinant of blood vessel diameter, the muscle–vessel mechanical interaction during contraction also includes potential tethering between muscle and blood vessel and axial bending, both of which can also affect the dimension of blood vessel (Hamza et al., 2003). Our experiments focus on the representation of blood vessel exposed to external pressure, i.e., isotropic external loading. We confirmed that the dynamic peak changes of diameter are equivalent during either pulse intraluminal or extraluminal pressures at the same transmural pressures (Fig. 1). The vasodilation induced by either pulse intraluminal or extraluminal is also equivalent (Fig. 2 A); i.e., the pulse transmural pressure also induces vasodilation. Furthermore, constant diameter during pulse transmural pressure eliminates the compression-induced vasodilation (Fig. 5). Therefore, change of diameter or cyclic circumferential deformation is likely the actual mechanical stimulus in the compression-induced vasodilation. In fact, it is well established that cyclic deformation stimulates an increase in NO production in cultured endothelial cells (Awolesi et al., 1994, 1995; Ziegler et al., 1998; Kuebler et al., 2003; Takeda et al., 2006). Our observations on cyclic deformation–induced endothelium-dependent vasodilation underscores a physiological role of cyclic deformation on endothelial NO production in the blood vessel.
Generally, integrin mediates extracellular remodeling and intracellular signal transduction in response to both chemical and mechanical stimulation. Integrins convert mechanical stimuli to cytoplasmic signaling and therefore play a pivotal role in mechanotransduction of vascular biology (Davis et al., 2001; Martinez-Lemus et al., 2003). Without intrinsic enzymatic activity, integrin clustering stimulates signal transduction via the recruitment of crucial intracellular signaling molecules, such as FAK, c-Src, Fyn, etc. (Schlaepfer and Hunter, 1997; Shyy and Chien, 2002; Tucker, 2002). In endothelial cells, cyclic stretch can induce integrin reorganization, which may activate the PI3K/Akt signaling pathway, and in turn, its signaling promotes NO release through eNOS phosphorylation (Yano et al., 1997; Morello et al., 2009). Here, we used RGD peptide and function-blocking integrin antibodies to investigate the role of integrins in compression-induced vasodilation. The RGD peptide may inhibit mechanical stimulus–elicited integrin activation (Muller et al., 1997). Our result indicates that the RGD peptide attenuates compression-induced vasodilation (Figs. 4 A and 6, A and B).
The specificity of integrins involved in the compression-induced vasodilation cannot be determined solely by the treatment of RGD peptide, as several vascular integrins recognize the RGD integrin-binding motif (Mogford et al., 1997; Yip and Marsh, 1997; Platts et al., 1998; Martinez-Lemus et al., 2003). Therefore, we used function-blocking integrin α5β1 and αvβ3 antibodies to verify the involvement of endothelial integrin in the cyclic transmural pressure–induced vasodilation (Figs. 4 C and 6, A and B), as integrins α5β1 and αvβ3 play a dominant role in endothelial mechanotransduction. We also investigated the effect of obtustatin (ligand of integrin α1β1), Bio1211 (the inhibitor of integrin α4β1), and shikonin on the compression-induced vasodilation (not depicted). Obtustatin and Bio1211 failed to attenuate the compression-induced vasodilation in this study. Because shikonin acutely activates protein kinase B (Nigorikawa et al., 2006; Wang et al., 2013), even though it has been suggested to inhibit integrin αvβ3 expression (Hisa et al., 1998), the attenuation of the vasodilation by shikonin in this study may not be attributed to integrin. The endothelial Akt phosphorylation in the compression-induced vasodilation (Fig. 7, A and B) confirms the involvement of integrins. We also washed out the RDG peptide to determine the reversibility of the compression-induced vasodilation (not depicted). We found that the vasodilation only recovered by ∼20% over the 4 h of washing, which suggests that integrin mechanotransduction in the vasodilation may have been prevented by the previous bindings. Future studies are needed to identify the precise mechanistic pathways connecting integrin to eNOS.
The wall shear stress on the vessel wall is a well-known regulator of endothelial NO (Kuo et al., 1990; Sorop et al., 2003; Takeda et al., 2006; Chiu and Chien, 2011). Although our experimental setup did not establish axial fluid flow in the artery segment during pulse pressures, wall shear stress may not be zero because of radial flow or filtration through the wall (Lu et al., 2013). We estimated the maximal wall shear stress to be <0.3 dynes/cm2 by calculating the ratio of volume change of arteriole segment during cyclic stretch. This shear stress is far below the physiological level in artery (12 dynes/cm2) and hence not likely to affect endothelial NO. The hydrostatic pressure is also thought to regulate cellular function and signaling pathway. A hydrostatic pressure (80–160 mmHg) was suggested to reduce basal NO release in cultured endothelial cells for 8 h but had no effect on the pressure-induced endothelin-1 increase when pretreated with a NOS inhibitor, L-NMMA (Hishikawa et al., 1995). Because we did not observe different vasodilation regardless of whether we varied internal or external pressure, it may be that the instantaneous effect of hydrostatic pressure is relatively small.
The pulse pressures may activate VSM myogenic response (Davis et al., 2001; Martinez-Lemus et al., 2003). Pulse transmural pressure can elicit coronary arteriole vasodilation, which is endothelium independent (Goto et al., 1996). Pulse transmural pressure also affects coronary arteriole sensitivity to endothelium-dependent vasodilator (Sorop et al., 2002). To limit the effect of myogenic response from VSM, we pre-constricted the small arterial segment using a vasoconstrictor to obtain a constant tone, which is a standard method for evaluation of endothelial function (Muller et al., 1997). The observation from L-NAME incubation and endothelium denudation determined that the vasodilation induced by pulse pressures was not the myogenic response of VSM (Fig. 3); i.e., endothelial NO removal drastically attenuated pulse pressure–induced vasodilation. Therefore, myogenic response is not the likely mechanism of the vasodilation in the current vessel preparation.
In this study, we did not quantify the magnitude of cyclic circumferential strain, as we used the variation of diameter at isobaric pressure to indicate vascular reactivity. For example, we can see that the cyclic diameter (or deformation) decreases during vasoconstriction (Fig. 3). To show that cyclic deformation (and not pressure) is the stimulus for vasodilation, we used the isovolumic method to maintain circumferential isometric state (Lu and Kassab, 2011). The results show that pulse pressure fails to elicit endothelial NO at the same magnitude and frequency of pulse pressure if diameter or cyclic stretch is maintained constantly under isovolumic conditions.
In summary, we showed that the diameter change (cyclic deformation) provides a compression-induced vasodilation in small intramural arteries of myocardium or skeletal muscle. We also found that integrins are implicated in the cyclic deformation–stimulated endothelial NO production and compression-induced vasodilation. This finding underscores the role of blood vessel–muscle interaction in the regulation of vasoreactivity.
Acknowledgments
We thank Ling Han for her contributions on protein expression and fluorescence microcopy in this study.
This research was supported in part by the National Institutes of Health (grant U01HL118738) and US-Israel Binational Science Foundation (grant 2009029).
The authors declare no competing financial interests.
Richard L. Moss served as editor.
Footnotes
Abbreviations used in this paper:
- ACh
- acetylcholine
- BK
- bradykinin
- eNOS
- endothelial nitric oxide synthase
- NO
- nitric oxide
- SNP
- sodium nitroprusside
- VSM
- vascular smooth muscle
References
- Awolesi M.A., Widmann M.D., Sessa W.C., and Sumpio B.E.. 1994. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery. 116:439–444, discussion:444–445. [PubMed] [Google Scholar]
- Awolesi M.A., Sessa W.C., and Sumpio B.E.. 1995. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J. Clin. Invest. 96:1449–1454. 10.1172/JCI118181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter J.B., Ruble S.B., Mueller P.J., and Clifford P.S.. 1998. Skeletal muscle vasodilation at the onset of exercise. J. Appl. Physiol. 85:1649–1654. [DOI] [PubMed] [Google Scholar]
- Chiu J.J., and Chien S.. 2011. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91:327–387. 10.1152/physrev.00047.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford P.S., Kluess H.A., Hamann J.J., Buckwalter J.B., and Jasperse J.L.. 2006. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J. Physiol. 572:561–567. 10.1113/jphysiol.2005.099507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.J., Wu X., Nurkiewicz T.R., Kawasaki J., Davis G.E., Hill M.A., and Meininger G.A.. 2001. Integrins and mechanotransduction of the vascular myogenic response. Am. J. Physiol. Heart Circ. Physiol. 280:H1427–H1433. [DOI] [PubMed] [Google Scholar]
- Goto M., Flynn A.E., Doucette J.W., Jansen C.M., Stork M.M., Coggins D.L., Muehrcke D.D., Husseini W.K., and Hoffman J.I.. 1991. Cardiac contraction affects deep myocardial vessels predominantly. Am. J. Physiol. 261:H1417–H1429. [DOI] [PubMed] [Google Scholar]
- Goto M., VanBavel E., Giezeman M.J., and Spaan J.A.. 1996. Vasodilatory effect of pulsatile pressure on coronary resistance vessels. Circ. Res. 79:1039–1045. 10.1161/01.RES.79.5.1039 [DOI] [PubMed] [Google Scholar]
- Hamza L.H., Dang Q., Lu X., Mian A., Molloi S., and Kassab G.S.. 2003. Effect of passive myocardium on the compliance of porcine coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 285:H653–H660. 10.1152/ajpheart.00090.2003 [DOI] [PubMed] [Google Scholar]
- Haul S., Gödecke A., Schrader J., Haas H.L., and Luhmann H.J.. 1999. Impairment of neocortical long-term potentiation in mice deficient of endothelial nitric oxide synthase. J. Neurophysiol. 81:494–497. [DOI] [PubMed] [Google Scholar]
- Heineman F.W., and Grayson J.. 1985. Transmural distribution of intramyocardial pressure measured by micropipette technique. Am. J. Physiol. 249:H1216–H1223. [DOI] [PubMed] [Google Scholar]
- Hisa T., Kimura Y., Takada K., Suzuki F., and Takigawa M.. 1998. Shikonin, an ingredient of Lithospermum erythrorhizon, inhibits angiogenesis in vivo and in vitro. Anticancer Res. 18:783–790. [PubMed] [Google Scholar]
- Hishikawa K., Nakaki T., Marumo T., Suzuki H., Kato R., and Saruta T.. 1995. Pressure enhances endothelin-1 release from cultured human endothelial cells. Hypertension. 25:449–452. 10.1161/01.HYP.25.3.449 [DOI] [PubMed] [Google Scholar]
- Hoffman J.I. 1987. Transmural myocardial perfusion. Prog. Cardiovasc. Dis. 29:429–464. 10.1016/0033-0620(87)90016-8 [DOI] [PubMed] [Google Scholar]
- Kuebler W.M., Uhlig U., Goldmann T., Schael G., Kerem A., Exner K., Martin C., Vollmer E., and Uhlig S.. 2003. Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am. J. Respir. Crit. Care Med. 168:1391–1398. 10.1164/rccm.200304-562OC [DOI] [PubMed] [Google Scholar]
- Kuo L., Davis M.J., and Chilian W.M.. 1990. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am. J. Physiol. 259:H1063–H1070. [DOI] [PubMed] [Google Scholar]
- Lu X., and Kassab G.S.. 2004. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J. Physiol. 561:575–582. 10.1113/jphysiol.2004.075218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., and Kassab G.S.. 2011. Assessment of endothelial function of large, medium, and small vessels: a unified myograph. Am. J. Physiol. Heart Circ. Physiol. 300:H94–H100. 10.1152/ajpheart.00708.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Guo X., Linares C., and Kassab G.S.. 2004. A new method to denude the endothelium without damage to media: structural, functional, and biomechanical validation. Am. J. Physiol. Heart Circ. Physiol. 286:H1889–H1894. 10.1152/ajpheart.00863.2003 [DOI] [PubMed] [Google Scholar]
- Lu X., Huxley V.H., and Kassab G.S.. 2013. Endothelial barrier dysfunction in diabetic conduit arteries: a novel method to quantify filtration. Am. J. Physiol. Heart Circ. Physiol. 304:H398–H405. 10.1152/ajpheart.00550.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lemus L.A., Wu X., Wilson E., Hill M.A., Davis G.E., Davis M.J., and Meininger G.A.. 2003. Integrins as unique receptors for vascular control. J. Vasc. Res. 40:211–233. 10.1159/000071886 [DOI] [PubMed] [Google Scholar]
- Mogford J.E., Davis G.E., and Meininger G.A.. 1997. RGDN peptide interaction with endothelial alpha5beta1 integrin causes sustained endothelin-dependent vasoconstriction of rat skeletal muscle arterioles. J. Clin. Invest. 100:1647–1653. 10.1172/JCI119689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello F., Perino A., and Hirsch E.. 2009. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc. Res. 82:261–271. 10.1093/cvr/cvn325 [DOI] [PubMed] [Google Scholar]
- Muller J.M., Chilian W.M., and Davis M.J.. 1997. Integrin signaling transduces shear stress-dependent vasodilation of coronary arterioles. Circ. Res. 80:320–326. 10.1161/01.RES.80.3.320 [DOI] [PubMed] [Google Scholar]
- Naik J.S., Valic Z., Buckwalter J.B., and Clifford P.S.. 1999. Rapid vasodilation in response to a brief tetanic muscle contraction. J. Appl. Physiol. 87:1741–1746. [DOI] [PubMed] [Google Scholar]
- Nigorikawa K., Yoshikawa K., Sasaki T., Iida E., Tsukamoto M., Murakami H., Maehama T., Hazeki K., and Hazeki O.. 2006. A naphthoquinone derivative, shikonin, has insulin-like actions by inhibiting both phosphatase and tensin homolog deleted on chromosome 10 and tyrosine phosphatases. Mol. Pharmacol. 70:1143–1149. 10.1124/mol.106.025809 [DOI] [PubMed] [Google Scholar]
- Platts S.H., Mogford J.E., Davis M.J., and Meininger G.A.. 1998. Role of K+ channels in arteriolar vasodilation mediated by integrin interaction with RGD-containing peptide. Am. J. Physiol. 275:H1449–H1454. [DOI] [PubMed] [Google Scholar]
- Pou S., Pou W.S., Bredt D.S., Snyder S.H., and Rosen G.M.. 1992. Generation of superoxide by purified brain nitric oxide synthase. J. Biol. Chem. 267:24173–24176. [PubMed] [Google Scholar]
- Rees D.D., Palmer R.M., Schulz R., Hodson H.F., and Moncada S.. 1990. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 101:746–752. 10.1111/j.1476-5381.1990.tb14151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D.D., and Hunter T.. 1997. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J. Biol. Chem. 272:13189–13195. 10.1074/jbc.272.20.13189 [DOI] [PubMed] [Google Scholar]
- Sejersted O.M., Hargens A.R., Kardel K.R., Blom P., Jensen O., and Hermansen L.. 1984. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J. Appl. Physiol. 56:287–295. [DOI] [PubMed] [Google Scholar]
- Shyy J.Y., and Chien S.. 2002. Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 91:769–775. 10.1161/01.RES.0000038487.19924.18 [DOI] [PubMed] [Google Scholar]
- Sorop O., Spaan J.A., and VanBavel E.. 2002. Pulsation-induced dilation of subendocardial and subepicardial arterioles: effect on vasodilator sensitivity. Am. J. Physiol. Heart Circ. Physiol. 282:H311–H319. [DOI] [PubMed] [Google Scholar]
- Sorop O., Spaan J.A., Sweeney T.E., and VanBavel E.. 2003. Effect of steady versus oscillating flow on porcine coronary arterioles: Involvement of NO and superoxide anion. Circ. Res. 92:1344–1351. 10.1161/01.RES.0000078604.47063.2B [DOI] [PubMed] [Google Scholar]
- Spaan J.A. 1985. Coronary diastolic pressure-flow relation and zero flow pressure explained on the basis of intramyocardial compliance. Circ. Res. 56:293–309. 10.1161/01.RES.56.3.293 [DOI] [PubMed] [Google Scholar]
- Sun D., Huang A., Recchia F.A., Cui Y., Messina E.J., Koller A., and Kaley G.. 2001. Nitric oxide-mediated arteriolar dilation after endothelial deformation. Am. J. Physiol. Heart Circ. Physiol. 280:H714–H721. [DOI] [PubMed] [Google Scholar]
- Sun D., Huang A., and Kaley G.. 2004. Mechanical compression elicits NO-dependent increases in coronary flow. Am. J. Physiol. Heart Circ. Physiol. 287:H2454–H2460. 10.1152/ajpheart.00364.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H., Komori K., Nishikimi N., Nimura Y., Sokabe M., and Naruse K.. 2006. Bi-phasic activation of eNOS in response to uni-axial cyclic stretch is mediated by differential mechanisms in BAECs. Life Sci. 79:233–239. 10.1016/j.lfs.2005.12.051 [DOI] [PubMed] [Google Scholar]
- Tucker G.C. 2002. Inhibitors of integrins. Curr. Opin. Pharmacol. 2:394–402. 10.1016/S1471-4892(02)00175-3 [DOI] [PubMed] [Google Scholar]
- VanTeeffelen J.W., and Segal S.S.. 2006. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 290:H119–H127. 10.1152/ajpheart.00197.2005 [DOI] [PubMed] [Google Scholar]
- Wang H., Wu C., Wan S., Zhang H., Zhou S., and Liu G.. 2013. Shikonin attenuates lung cancer cell adhesion to extracellular matrix and metastasis by inhibiting integrin β1 expression and the ERK1/2 signaling pathway. Toxicology. 308:104–112. 10.1016/j.tox.2013.03.015 [DOI] [PubMed] [Google Scholar]
- Williams B. 1998. Mechanical influences on vascular smooth muscle cell function. J. Hypertens. 16:1921–1929. 10.1097/00004872-199816121-00011 [DOI] [PubMed] [Google Scholar]
- Yano Y., Geibel J., and Sumpio B.E.. 1997. Cyclic strain induces reorganization of integrin alpha 5 beta 1 and alpha 2 beta 1 in human umbilical vein endothelial cells. J. Cell. Biochem. 64:505–513. [DOI] [PubMed] [Google Scholar]
- Yip K.P., and Marsh D.J.. 1997. An Arg-Gly-Asp peptide stimulates constriction in rat afferent arteriole. Am. J. Physiol. 273:F768–F776. [DOI] [PubMed] [Google Scholar]
- Ziegler T., Silacci P., Harrison V.J., and Hayoz D.. 1998. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension. 32:351–355. 10.1161/01.HYP.32.2.351 [DOI] [PubMed] [Google Scholar]