In the late 1970s, Dunlap and Fischbach (1978) reported that synaptic activity in sensory neurons is modulated by neurotransmitters, and they subsequently attributed this phenomenon to the inhibition of voltage-gated Ca2+ channels (VGCCs) (Dunlap and Fischbach, 1981). Their seminal studies laid the groundwork for three decades of research that revealed how G protein–coupled receptors (GPCRs) and G protein signaling play an essential role in the regulation of Ca2+ entry at presynaptic nerve endings and the fine-tuning of neurotransmitter release. Inhibition of VGCCs by GPCRs is crucial for regulating the excitability and responsiveness of nerve cells and neuronal networks under typical physiological conditions. This inhibition is also exploited for therapeutic purposes, as underscored by the treatment of neuropathic pain by µ-opioid agonists such as morphine. Numerous GPCRs have now been reported to inhibit the activity of neuronal VGCCs, with the majority targeting Cav2.1 and Cav2.2 channels.
The mechanisms by which GPCRs inhibit neuronal VGCCs are as complex as the multicomponent nature of GPCR signaling pathways (Zamponi and Currie, 2013; Proft and Weiss, 2015). A key step in GPCR modulation of Ca2+ channels involves the activation of heterotrimeric G proteins by exchange of GDP for GTP on the Gα subunit, resulting in the dissociation of GTP-bound Gα and the Gβγ dimer from the receptor. Although both Gα and the Gβγ dimer potently inhibit Ca2+ influx through VGCCs, their mode of action and the signaling pathways they use differ strikingly. Inhibition by Gβγ dimer relies on its direct binding to various structural elements of the Cav2.x subunit. This inhibition is typically fast (i.e., develops within tens of milliseconds); spatially delimited (i.e., it requires the activation of GPCRs located in the vicinity of the Ca2+ channel); and most frequently reported for Gαi/o-coupled receptors, although Gαs- and Gαq-coupled receptors can also initiate this pathway. Gβγ-mediated inhibition is less pronounced at depolarized membrane potentials, and can be experimentally relieved by short, highly depolarizing voltage steps, giving rise to the term “voltage-dependent” regulation. In contrast, Gα-mediated inhibition, which is less well characterized, typically involves one of many downstream diffusible messengers and signaling pathways involving phosphorylation and lipid metabolism. Gα-mediated inhibition is comparatively slow and develops within tens of seconds, and is in essence “voltage independent” (i.e., it is not relieved by membrane depolarization steps). An additional level of regulation arises from the direct biochemical association of the Ca2+ channel with the GPCR to not only control the trafficking of the channel to the cell surface but also give rise to an agonist-mediated cointernalization of the channel–receptor complex, thus regulating channel density in the plasma membrane (Zamponi, 2015).
Association of channels with receptors can also support a tonic, voltage-dependent inhibition of the channel in the absence of receptor agonist, which is believed to arise from constitutive receptor activity and its associated activation of the Gβγ pathway. Increasing evidence supports a vital role of agonist-independent constitutive activity of GPCRs in the control of neuronal network activity, and to date, more than 60 GPCRs have been reported to exhibit constitutive activity (Meye et al., 2014). A new study by the group of Jesica Raingo, reported in this issue (López Soto et al.), sheds light on a previously unidentified regulatory pathway of neuronal VGCCs that is mediated by the growth hormone secretagogue receptor type 1a (GHSR1a). GHSR1a is an unusual GPCR in that, although it is broadly expressed in the central nervous system, its natural agonist ghrelin is generally undetectable other than in trace amounts in the hypothalamus. However, GHSR1a exhibits among the highest constitutive activities of members of the GPCR family, and agonist-independent constitutive activity of GHSR1a has been proposed to modulate neuronal activity, especially in the hypothalamus, where it controls hunger by modulating the hypothalamo–pituitary–adrenal axis (Wren et al., 2000). The implication of GHSR1a in appetite and energy metabolism is best exemplified by the occurrence of human mutations that cause a loss of GHSR1a constitutive activity, leading to short stature caused by growth hormone deficiency (Pantel et al., 2006). However, the signaling pathways and downstream effectors by which agonist-independent constitutive activity of GHSR1a regulates neuronal networks had remained incompletely understood.
López Soto et al. (2015) used a combination of electrophysiological recordings combined with genetic and pharmacological manipulations of GHSR1a activity/expression to test the hypothesis that GHSR1a may control neuronal activity by modulating presynaptic VGCCs and neurotransmitter release. The authors showed that Cav2.1 and Cav2.2 channels expressed in HEK cells are sensitive to modulation by GHSR1a. Notably, they demonstrated that ghrelin induces a fast, voltage-dependent, and agonist-dependent inhibition of the Ca2+ currents that is mediated by the direct inhibition of the channel by G protein βγ dimer and involves Gαq signaling. In addition, they demonstrated that constitutive activity of GHSR1a inhibits both Cav2.1 and Cav2.2 channels via an agonist-independent mechanism that depends on the expression level of the receptor, requires Gαi/o proteins, and is associated with a reduced Ca2+ channel density at the cell surface. Agonist-independent inhibition was not reproduced with a GHSR1a mutant that lacks constitutive activity, indicating that this regulatory pathway is not mediated by the receptor itself but requires activation of downstream signaling molecules.
To further analyze whether GHSR1a modulates VGCCs in native cells, López Soto et al. (2015) performed electrophysiological recording on primary hypothalamic neurons from transgenic GHSR-eGFP mice. Consistent with their initial observation, the application of ghrelin inhibited Cav2.1 and Cav2.2 channels in neurons expressing GHSR. In addition, GHSR-positive neurons displayed decreased Ca2+ current density, indicating that GHSR1a-induced agonist-independent inhibition occurs in a physiologically relevant setting. To confirm that ghrelin-independent inhibition is mediated by constitutive activity of GHSR, the authors transfected hypothalamic neurons with a GHSR1a mutant lacking constitutive activity, and found that Ca2+ currents were increased compared with neurons expressing the wild-type receptor. Finally, López Soto et al. assessed whether GHSR-mediated inhibition of Ca2+ channels affects neurotransmitter release in hypothalamic GABAergic neurons. This is particularly relevant, as it has been reported that γ-amino butyric acid (GABA) release is essential for regulating food intake (Wu et al., 2009), and that GHSR1a activity decreases inhibitory input of hypothalamic networks (Cabral et al., 2012). To make such studies possible, the authors used hypothalamic tissue explants of the arcuate nucleus (ARC) from fasted mice in which expression of GHSR1a is increased. Consistent with the hypothesis that increased GHSR1a expression inhibits presynaptic VGCCs and thus neurotransmitter release, these authors found that GABA release induced by high potassium depolarization is decreased in explants from fasted mice compared with explants from ad libitum–fed mice. Altered GABA release was further documented by recording of inhibitory postsynaptic currents (IPSCs) in hypothalamic neurons. The amplitude of IPSCs recorded from cultures expressing GHSR1a was significantly reduced compared with those in cultures expressing the mutant GHSR1. This effect was fully attributed to the alteration of Ca2+-dependent presynaptic GABA release.
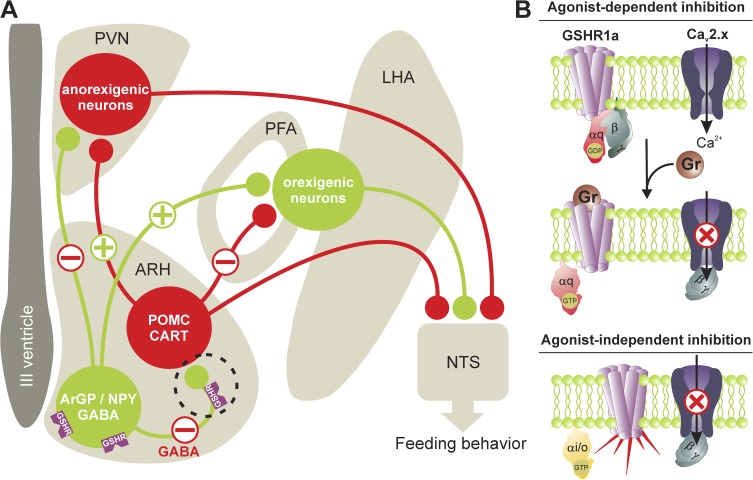
The novel and important findings of López Soto et al. (2015) provide compelling evidence for a role of GHSR1a in the control of the hypothalamic network, especially in the modulation of GABAergic activity in the ARC nucleus. Ghrelin-mediated hunger is believed to rely on the modulation of neuronal circuits within the ARC nucleus, where two different subpopulations of neurons are involved. The pro-opiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART) neurons are involved in the generation of an anorexigenic signal. In contrast, agouti-related peptide (AgRP)/neuropeptide Y (NPY)/GABA neurons are involved in the generation of an orexigenic signal. Both POMC/CART and AgRP/NPY/GABA neurons project to anorexigenic neurons in the paraventricular nucleus (PVN), and to orexigenic neurons located in the lateral (LHA) and perifornical (PFA) regions of the posterior hypothalamus, which in turn project to the nucleus of the solitary tract (NTS) to modulate food intake (Fig. 1). Ghrelin is believed to bind to and stimulate AgRP/NPY/GABA neurons expressing GHSR1a, which has two main effects: (1) inhibition of POMC/CART neuron activity through the inhibitory GABAergic input from activated AgRP/NPY/GABA neurons (which in turn suppresses the anorexigenic tone in the PVN and relieves the inhibitory input of orexigenic neurons from POMC/CART neurons), and (2) a direct stimulation of the orexigenic pathway by activated AgRP/NPY/GABA neurons. As a consequence, an orexigenic signal is sent to the NTS, which triggers feeding behavior.
Figure 1.
Ghrelin receptor signaling in the hypothalamus. (A) Hypothalamic circuits involved in ghrelin-mediated feeding behavior. Two distinct neuronal populations in the ARC represented by ArGP/NPY/GABA neurons and POMC/CART neurons are considered first-order sensory neurons in the control of food intake. They project to anorexigenic neurons in the PVN and orexigenic neurons in the PFA and LHA, which in turn project to the NTS to control feeding behavior. Upon stimulation of ArGP/NPY/GABA neuron activity by ghrelin, POMC/CART neurons are inhibited through GABAergic input from activated ArGP/NPY/GABA neurons, which in turn suppress the anorexigenic tone in the PVN. This inhibition may be balanced by the inhibition of Cav2.1 and Cav2.2 channels on the ArGP/NPY/GABA neurons by presynaptic GHSR1a (dotted circle). This in turn inhibits GABA release and suppresses inhibitory GABAergic input of POMC/CART neurons. The orexigenic and anorexigenic pathways are outlined in green (plus symbols) and red (minus symbols), respectively. (B) GHSR1a-mediated inhibition of presynaptic Cav2.1 and Cav2.2 channels occurs via two distinct mechanisms: (1) an agonist-dependent inhibition that involves the activation of Gαq-coupled receptors and a direct channel inhibition by Gβγ dimer; and (2) an agonist-independent inhibition mediated by constitutive GHSR1a activity and Gαi/o signaling that not only supports Gβγ-mediated inhibition but also decreases channel density in the plasma membrane, possibly by stimulating the internalization of channel–receptor complexes or by suppressing channel trafficking to the cell surface.
The exact downstream intracellular mechanisms by which ghrelin stimulates AgRP/NPY/GABA neurons are not fully understood, but it was proposed that the binding of ghrelin to GHSR1a induces an increase of intracellular Ca2+ concentration mediated by a PKA-dependent potentiation of Cav2.2 channels, and increased neurotransmitter release (Kohno et al., 2003). In contrast, the findings of López Soto et al. (2015) support an inhibition of neuronal Ca2+ channels and GABA release in an agonist-dependent and -independent manner. A physiological consequence therefore is a suppression of the inhibitory GABAergic input of POMC/CART neurons, which in turn is expected to stimulate the anorexigenic pathway. Thus, this contrasts with the established role of ghrelin in stimulating appetite. The work of López Soto et al. highlights the complexities of the ghrelin system in the control of appetite, and may help us understand the apparent discrepancy between results showing reduced weight in diet-induced obese mice treated with GHSR1a antagonists, whereas other studies reported a gain weight (Patterson et al., 2011).
In addition to providing essential information on the regulation of the hypothalamic network by the ghrelin–GHSR1a system, López Soto et al. also shed light on a new GPCR signaling pathway through which GPCRs control the density of presynaptic Ca2+ channels at the cell surface, thus providing for an additional level of control of synaptic activity. Control of channel surface density by GPCRs was reported previously for Cav2.2 channels complexed with nociceptin (ORL1) and dopamine (D1 and D2) receptors (Zamponi, 2015). This regulation relies on the direct interaction between the channel and the receptor, leading to channel–receptor co-trafficking to the plasma membrane and agonist-dependent cointernalization (Altier et al., 2006). An association of GHSR1a with D2 receptors has been reported in thalamic neurons, which in turn modulated dopamine signaling and appetite (Kern et al., 2012). Hence, it is possible that a similar association with neuronal Ca2+ channels exists and contributes to the control of channel surface expression by GHSR1a. However, the observation that a GHSR1a lacking constitutive activity failed to modulate channel surface density suggests that G protein signaling also plays a crucial role in this control. There is evidence in the literature that heterotrimeric G proteins, especially Gαi signaling, contribute to the control of membrane trafficking and regulate exocytosis from intracellular organelles (Stow et al., 1991; Aridor et al., 1993). In addition, G proteins of the Gαi family have been reported to control the trafficking of aquaporin 2 channels in kidney epithelial cells (Valenti et al., 1998), and also the trafficking and distribution of connexin 43 hemichannels in Novikoff hepatoma cells (Lampe et al., 2001). In this last study, the inhibition of G proteins with pertussis toxin resembled the effects of the trafficking inhibitor brefeldin A, supporting a key role of Gαi signaling in the transport of ion channels to the plasma membrane. All of these aspects of G protein signaling in intracellular trafficking are certainly of direct relevance for the observation of López Soto et al. (2015) on the role of GHSR1a in controlling of Ca2+ channel plasma membrane density. Further investigation is warranted to identify the precise molecular details of this signaling mechanism.
Overall, the finding of López Soto et al. provide novel insights into the physiology of the hypothalamus, and establish the ghrelin-GHSR1a signaling pathway as a key regulator of neuronal Ca2+ channels and synaptic activity in the control of the hypothalamic networks.
Acknowledgments
Work in the Weiss laboratory is supported by the Czech Science Foundation (grant 15-13556S), the Czech Ministry of Education Youth and Sports (grant 7AMB15FR015), and the Institute of Organic Chemistry and Biochemistry. Work in the Zamponi laboratory is supported by a Canada research Chair and grants from the Canadian Institutes for Health Research and the Natural Sciences and Engineering Research Council.
The authors declare no competing financial interests.
Elizabeth M. Adler served as editor.
References
- Altier C., Khosravani H., Evans R.M., Hameed S., Peloquin J.B., Vartian B.A., Chen L., Beedle A.M., Ferguson S.S., Mezghrani A., et al. 2006. ORL1 receptor-mediated internalization of N-type calcium channels. Nat. Neurosci. 9:31–40. 10.1038/nn1605 [DOI] [PubMed] [Google Scholar]
- Aridor M., Rajmilevich G., Beaven M.A., and Sagi-Eisenberg R.. 1993. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 262:1569–1572. 10.1126/science.7504324 [DOI] [PubMed] [Google Scholar]
- Cabral A., Suescun O., Zigman J.M., and Perello M.. 2012. Ghrelin indirectly activates hypophysiotropic CRF neurons in rodents. PLoS ONE. 7:e31462 10.1371/journal.pone.0031462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., and Fischbach G.D.. 1978. Neurotransmitters decrease the calcium component of sensory neurone action potentials. Nature. 276:837–839. 10.1038/276837a0 [DOI] [PubMed] [Google Scholar]
- Dunlap K., and Fischbach G.D.. 1981. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J. Physiol. 317:519–535. 10.1113/jphysiol.1981.sp013841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A., Albarran-Zeckler R., Walsh H.E., and Smith R.G.. 2012. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 73:317–332. 10.1016/j.neuron.2011.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno D., Gao H.Z., Muroya S., Kikuyama S., and Yada T.. 2003. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 52:948–956. 10.2337/diabetes.52.4.948 [DOI] [PubMed] [Google Scholar]
- Lampe P.D., Qiu Q., Meyer R.A., TenBroek E.M., Walseth T.F., Starich T.A., Grunenwald H.L., and Johnson R.G.. 2001. Gap junction assembly: PTX-sensitive G proteins regulate the distribution of connexin43 within cells. Am. J. Physiol. Cell Physiol. 281:C1211–C1222. [DOI] [PubMed] [Google Scholar]
- López Soto E.J., Agosti F., Cabral A., Mustafa E.R., Martínez Damonte V., Gandini M.A., Rodríguez S., Castrogiovanni D., Felix R., Perelló M., and Raingo J.. 2015. Constitutive and ghrelin-dependent GHSR1a activation impairs CaV2.1 and CaV2.2 currents in hypothalamic neurons. J. Gen. Physiol. 146:205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye F.J., Ramakers G.M., and Adan R.A.. 2014. The vital role of constitutive GPCR activity in the mesolimbic dopamine system. Transl. Psychiatr. 4:e361 10.1038/tp.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel J., Legendre M., Cabrol S., Hilal L., Hajaji Y., Morisset S., Nivot S., Vie-Luton M.P., Grouselle D., de Kerdanet M., et al. 2006. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J. Clin. Invest. 116:760–768. 10.1172/JCI25303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Bloom S.R., and Gardiner J.V.. 2011. Ghrelin and appetite control in humans—Potential application in the treatment of obesity. Peptides. 32:2290–2294. 10.1016/j.peptides.2011.07.021 [DOI] [PubMed] [Google Scholar]
- Proft J., and Weiss N.. 2015. G protein regulation of neuronal calcium channels: Back to the future. Mol. Pharmacol. 87:890–906. 10.1124/mol.114.096008 [DOI] [PubMed] [Google Scholar]
- Stow J.L., de Almeida J.B., Narula N., Holtzman E.J., Ercolani L., and Ausiello D.A.. 1991. A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J. Cell Biol. 114:1113–1124. 10.1083/jcb.114.6.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti G., Procino G., Liebenhoff U., Frigeri A., Benedetti P.A., Ahnert-Hilger G., Nürnberg B., Svelto M., and Rosenthal W.. 1998. A heterotrimeric G protein of the Gi family is required for cAMP-triggered trafficking of aquaporin 2 in kidney epithelial cells. J. Biol. Chem. 273:22627–22634. 10.1074/jbc.273.35.22627 [DOI] [PubMed] [Google Scholar]
- Wren A.M., Small C.J., Ward H.L., Murphy K.G., Dakin C.L., Taheri S., Kennedy A.R., Roberts G.H., Morgan D.G., Ghatei M.A., and Bloom S.R.. 2000. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 141:4325–4328. 10.1210/endo.141.11.7873 [DOI] [PubMed] [Google Scholar]
- Wu Q., Boyle M.P., and Palmiter R.D.. 2009. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 137:1225–1234. 10.1016/j.cell.2009.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G.W. 2015. Calcium channel signaling complexes with receptors and channels. Curr. Mol. Pharmacol. In press. [DOI] [PubMed] [Google Scholar]
- Zamponi G.W., and Currie K.P.. 2013. Regulation of CaV2 calcium channels by G protein coupled receptors. Biochim. Biophys. Acta. 1828:1629–1643. 10.1016/j.bbamem.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]