Abstract
Myocardial infarction (MI) is the leading cause of death worldwide. Notch1 signaling plays a critical role in cardiac development, in survival, cardiogenic lineage commitment, differentiation of cardiac stem/progenitor cells, and in regenerative responses following myocardial injury. The objective of this study was the evaluation of the therapeutic effect of delivering the Notch ligand-containing hydrogels in a rat model of MI. Self-assembling peptide (SAP) hydrogels were functionalized with a peptide mimic of the Notch1 ligand Jagged1 (RJ). In rats subjected to experimental MI, delivery of RJ-containing hydrogel to the infarcted heart resulted in improvement in cardiac function back to sham-operated levels. A significant decrease in fibrosis and an increase in the endothelial vessel area and Ki67 expression were also observed in rats treated with the RJ hydrogels compared to untreated rats or rats treated with unmodified or scrambled peptide hydrogels. This study demonstrates the functional benefit of Notch1-activating peptide delivered in SAP hydrogels for cardiac repair.
Introduction
Myocardial infarction (MI) is a leading cause of death worldwide.1 Occlusion of the coronary artery leads to MI characterized by local ischemia, myocyte apoptosis, fibrotic scar, and irreversible tissue damage, which progresses to heart failure.2 The infarct size and extent of remodeling are determinants of prognosis following acute MI. Current treatments such as pharmacological intervention with ACE inhibitors and β-blockers, myocardial reperfusion, left ventricular assistive device implantation, and bypass graft surgery salvage the injured heart acutely following MI. However, the continued loss of contractile myocardium as a consequence of MI leads to end-stage heart failure.
While recent progress in early clinical trials using stem cell therapy shows promise, heart transplantation is the most viable treatment option for patients with end-stage heart failure.2 However, limited availability of donor hearts and side effects of transplant-associated immunosuppression in patients with comorbidities have created the need for treatment alternatives. Early infarct expansion leading to increased regional stress has been identified as a key factor that accelerates cardiac remodeling with poor prognosis.3 Use of ventricular restraints that limit cardiac expansion and provide mechanical support in large animal models of MI has shown limited global remodeling.4 However, the invasive surgical procedure required has limited clinical application.
To circumvent this, our group and others have investigated the use of injectable cell-free biomaterials to support cardiac function following MI by serving as tissue bulking agents and growth factor delivery vehicles.5–10 Hydrogels are a class of biomaterials composed of cross-linked hydrophilic polymers of natural or synthetic origin characterized by high water content that can provide support to the damaged myocardium following MI. A tissue-compatible hydrogel is characterized by the ability to promote specific ligand–receptor interactions, host cell migration, minimal host inflammatory or foreign body response, controlled biofactor release, and tunable degradation kinetics. Hydrogels with diverse physical properties have been used in a variety of cardiac applications ranging from controlled delivery of drugs and small molecules to growth factors and stem cells.11
For use in the infarct environment, hydrogels with chemical and mechanical properties of native tissue are needed for successful cardiac regeneration.
Self-assembling peptides (SAPs) are a class of hydrogels composed of alternating hydrophilic and hydrophobic amino acids that self-assemble at physiologic pH and osmolarity to form a hydrogel. Two well-studied types of SAPs are RADA16-I (AcN-(RADARADA)2-CNH2) and RADA16-II (AcN-(RARADADA)2-CNH2).12 To maintain the viable myocardium lost after MI, cardioprotective cues have been delivered through SAP to the infarcted heart. For example, using the streptavidin–biotin conjugation system with SAP, sustained delivery of insulin-like growth factor (IGF)-1 to the infarcted rodent heart resulted in improved cardiomyocyte survival.6 Despite seeing improvements in transplanted cell therapy with IGF-1 tethered to growth factors, no improvement was seen due to the fact that IGF-1 as a soluble protein was locked in place. In addition, sustained platelet-derived growth factor (PDGF) delivery in SAP hydrogels has led to improved cardiac function and limited adverse remodeling after MI.13 Delivery of vascular endothelial growth factor (VEGF) in SAP hydrogels to the infarcted rat and pig heart following MI has promoted tissue repair and restored cardiac function.14 Dual delivery of PDGF and fibroblast growth factor in SAP hydrogels improved cardiac function of the infarcted rat heart compared to either growth factor alone.15 These studies demonstrate the use of SAPs as instructive biomaterials for cardiac applications.
Notch1 signaling is known to play a critical role in cardiac development and in the survival, cardiogenic lineage commitment, and differentiation of cardiac stem/progenitor cells.16–18 These varied critical roles played by Notch signaling have led to research on identifying ways to activate and control Notch signaling in vitro and in vivo. This has been achieved through soluble Notch ligands, overexpression of the active Notch intracellular domain in cells, ligand immobilization on tissue culture polystyrene surfaces, and development of biomaterials with immobilized notch ligands. While soluble Delta/Jagged ligands also allow for the ligand–receptor interaction, the lack of ligand-mediated receptor endocytosis may antagonize downstream signal activation.19 As overexpression of the receptors or ligands does not represent clinically translatable systems, ligand immobilization strategies have been attempted for controlled induction of Notch signaling.20
Endogenous progenitor cells and other resident cells are known to migrate to the infarct site due to the signals present in the infarct and in response to the presence of SAP.21–23 Moreover, following an infarction, an increase in levels of Notch activation along with increases in downstream targets and corresponding ligand expression has been observed.24 We have recently shown that tethering a Jagged peptide within SAP hydrogels improves transplanted cell therapy following ischemia–reperfusion (IR) injury.20 In this study, we examined SAP hydrogels with a peptide mimic of the Notch1 ligand Jagged1 (RJ) in a cell-free setting to evaluate the effect of hydrogel-mediated Notch activation in the endogenous healing response following MI.
Methods
Design of the hydrogel-ligand system
The SAP RADA16-II (H2N-RARADADARARADADA-OH) was synthesized with a seven glycine linker following the terminal alanine, followed by the active sequence of the Notch ligand Jagged-1 (H2N-CDDYYYGFGCNKFCRPR-OH)25 or with a scrambled peptide motif (H2NRCGPDCFDNYGRYKYCF-OH) to create the final peptides RJ or RS, respectively (New England Peptide). RS or RJ was combined with RADA-16-II in a ratio of 1:10 to ensure that the extra sequence did not interfere with a nanofiber assembly. A SAP hydrogel concentration of 2% w/v in 295 mM sucrose solution in the absence (2R) or presence of RS or RJ peptide (2RS or 2RJ) was used for all in vivo studies. Detailed synthesis and functionalization of hydrogels are provided by Boopathy et al.20
MI, hydrogel injection, and functional evaluation
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee at Emory University. MI was performed in female adult Sprague Dawley rats (Charles River Laboratories) in a randomized double-blinded manner. Briefly, the rats were anesthetized (1–3% isoflurane; Attane™), intubated, and heart exposed by separation of ribs. The left anterior descending coronary artery was ligated for 30 min. During reperfusion, hydrogels (2R, 2RS, or 2RJ, 40 μL volume, 31G Ultra-Fine short syringe; BD) were injected into the myocardium at three border zones. Cardiac function was evaluated 21 days after treatment by echocardiography (Acuson Sequoia 512 with a 14 MHz transducer) and invasive pressure–volume hemodynamics (Millar Instruments) to assess cardiac function. All functional evaluations were conducted and analyzed by investigators blinded to the animal treatment group. The rats were euthanized, and the hearts were excised for histological analysis. The hearts were fixed in 4% paraformaldehyde, dehydrated in ethanol, embedded in paraffin, and sectioned at 5 μm thickness.
Quantification of fibrosis
The paraffin-embedded heart tissue sections were dewaxed in Histo-Clear followed by a series of washes in ethanol and stained with the picrosirius red solution for 1 h (Sigma). The sections were washed in acidified water and ethanol and mounted with the resinous medium (Histomount). Images of the entire heart section were taken at 2.5× magnification on a bright field microscope (Olympus) and tiled together using Adobe Photoshop. To quantify picrosirius red-stained areas, the image was split into the red, green, blue stack. After performing white balance on the background, the threshold was set and maintained in all the comparisons. Fibrosis was quantified using ImageJ as the ratio of fibrotic tissue (stained red) to total tissue.
Quantification of endothelial vessel area
Paraffin-embedded heart sections were immunostained with isolectin-568 (Life Technologies) and DAPI. The infarcted region of the heart where the hydrogel could be detected was imaged at 20× magnification on a fluorescent microscope (Olympus). The average vessel area and number of vessels were quantified from five individual images per heart section using ImageJ.
Quantification of cardiomyocyte area
Paraffin-embedded heart sections were immunostained for wheat germ agglutinin (WGA-647; Life Tech) and nuclei (DAPI; Sigma). Infarcted region of the heart where the hydrogel could be detected was imaged at 40× magnification on a confocal microscope (Olympus FV1000). The average cardiomyocyte area was quantified from five individual images per heart section using ImageJ.
Immunohistochemistry for c-Kit and Ki67
Paraffin-embedded heart sections were immunostained for c-Kit (AF1356; R&D), Ki67 (Cat#16667; Abcam), and DAPI with Alexa Fluor anti-donkey 647 and Alexa Fluor anti-rabbit-568 secondary antibodies, respectively. The infarcted region of the heart was imaged at 40×magnification on a confocal microscope (Olympus FV1000). The number of c-Kit+ and Ki67+ cells was quantified from five individual images per heart section using ImageJ. Some sections were also double stained with isolectin to determine the lineage of proliferating cells.
Statistics
Data are represented as mean±SEM. All data were analyzed by one-way ANOVA followed by the indicated post-test (GraphPad Prism5). A p-value <0.05 was considered significant.
Results
Delivery of 2RJ hydrogel improves cardiac function
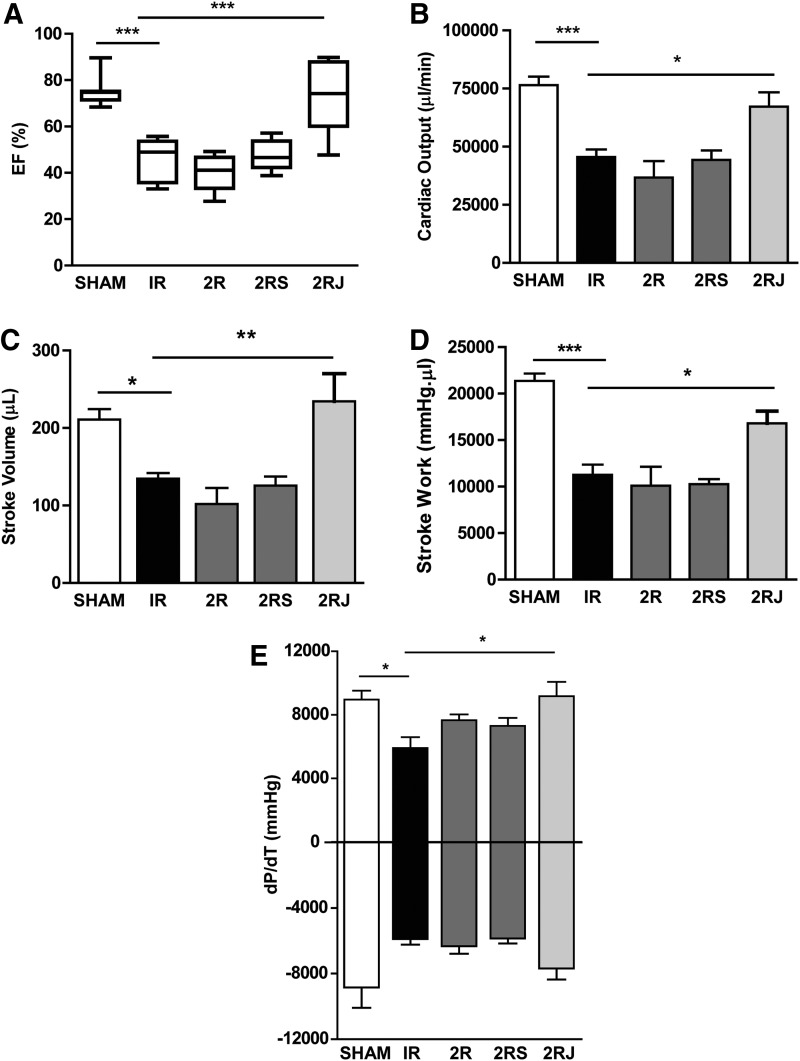
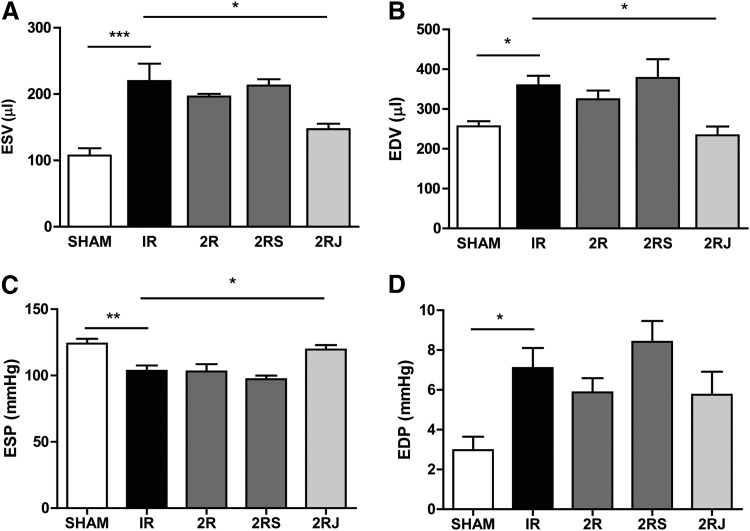
The functional consequences of implantation of 2R, 2RS, or 2RJ hydrogels were investigated in rats subjected to experimental MI. SAP hydrogels (2% w/v) containing empty, scrambled, or Jag1 peptide (2R, 2RS, or 2RJ) were injected intramyocardially (i.m.) in three border zones in adult rats following MI. Cardiac function was evaluated at 21 days by echocardiography and invasive hemodynamic measurements. IR injury for 30 min significantly decreased ejection fraction of the hearts when compared to sham-operated animals. Treatment with 2RJ hydrogels significantly improved ejection function comparable to levels in sham-operated animals as assessed by invasive hemodynamic analysis (Fig. 1A). No improvement in function was observed in animals treated with empty or scrambled (2R and 2RS) hydrogels indicating the importance of Notch1 activation in improving function following infarction. Among other parameters of ventricular function, the significant decrease in cardiac output, stroke volume, and stroke work following infarction was significantly improved to levels comparable to sham-operated rats on treatment with 2RJ hydrogels (Fig. 1B–D). Improvement was also observed in rats treated with 2RJ hydrogels for the cardiac contractility indicator +dP/dt (Fig. 1E). No effect of 2R and 2RS hydrogels was observed on any of the left ventricular parameters measured. As shown in Figure 2, a significant increase in end-systolic volume (ESV) and end-diastolic volume (EDV) was observed in untreated infarcted rats. This increase was normalized to sham levels upon delivery of 2RJ hydrogels (Fig. 2A, B). While no effect of hydrogel therapy was observed on levels of end-diastolic pressure, a significant improvement in end-systolic pressure was seen following 2RJ delivery (Fig. 2C, D). A comparison of heart rates between animals yielded no statistical differences between groups (sham=367±24 bpm, IR=352±39 bpm, 2R=388±53 bpm, 2RS=376±31 bpm, 2RJ=372±40 bpm).
FIG. 1.
Delivery of 2RJ hydrogel improves cardiac function following MI. Pressure–volume hemodynamic measurements of (A) ejection fraction (EF%), (B) cardiac output, (C) stroke volume, (D) stroke work, (E)±dP/dT; n≥6/group, *p<0.05, **p<0.01, ***p<0.001, one-way ANOVA followed by Tukey's post-test. MI, myocardial infarction; 2R, 2% RAD hydrogel; 2RS, 2% RAD hydrogel with scrambled peptide; 2RJ, 2% RAD hydrogel with Jagged peptide.
FIG. 2.
Delivery of 2RJ hydrogel improves left ventricular morphology following MI. Pressure–volume hemodynamic measurements of (A) ESV, (B) EDV, (C) ESP, and (D) EDP. *p<0.05, **p<0.01, n≥5/group. One-way ANOVA followed by Tukey's post-test. ESV, end-systolic volume; EDV, end-diastolic volume; ESP, end-systolic pressure; EDP, end-diastolic pressure.
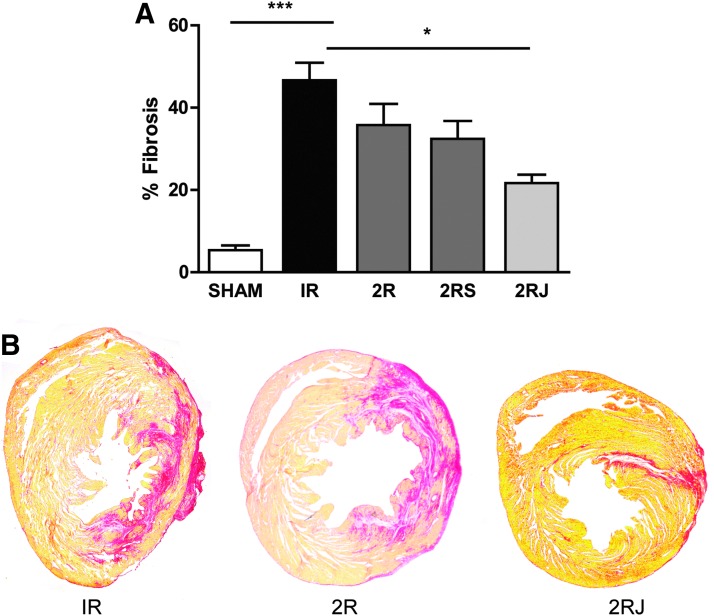
Delivery of 2RJ hydrogel reduces cardiac fibrosis
To evaluate a possible mechanism for the observed improvement in function, histological analysis of tissue sections was performed for collagen expression as a marker of fibrosis. Picrosirius red staining of heart sections showed that IR resulted in a significant increase in cardiac fibrosis. Only treatment with 2RJ hydrogel resulted in a significant decrease in fibrosis (p<0.01, n≥5, Fig. 3A). Representative images of heart sections stained with picrosirius red are shown in Figure 3B.
FIG. 3.
Delivery of 2RJ hydrogel decreases fibrosis. (A) Quantification of % fibrosis in rat hearts on day 21 shows a significant increase in fibrosis following 30 min of IR (black bar). Administration of 2RJ hydrogel attenuated this increase; ***p<0.001 versus sham, *p<0.05 versus IR, n≥5 per group. One-way ANOVA followed by Tukey's post-test. (B) Representative images of heart sections stained with picrosirius red and stitched together. IR, ischemia–reperfusion.
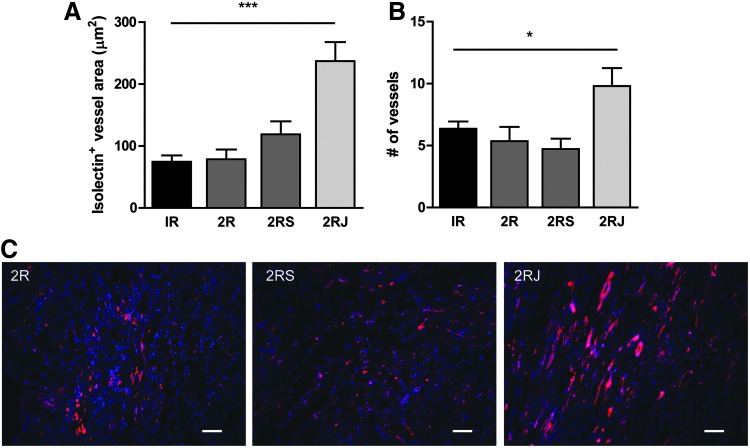
Delivery of 2RJ hydrogel enhances angiogenesis
To examine the effect of the 2RJ hydrogel on endothelialization at the infarct, paraffin-embedded heart sections were stained with isolectin. As shown in Figure 4A, a significant increase in isolectin+ vessel area was observed in hearts treated with 2RJ hydrogels compared to untreated, 2R, or 2RS hydrogel-treated hearts. In addition, the total number of isolectin+ vessels also increased significantly in 2RJ-treated hearts, whereas no effects were observed for 2R or 2RS-treated subjects (Fig. 4B). Representative images are shown in Figure 4C.
FIG. 4.
Delivery of 2RJ hydrogel increases endothelial vessel area. Quantification of endothelial vessel area (A) and number of vessels (B) in rat hearts on day 21 by isolectin staining shows a significant increase in both measurements in infarcted rats treated with 2RJ hydrogel. ***p<0.001 and *p<0.05 versus IR, n=4−7 per group. (C) Representative images of heart sections stained with isolectin (red) and DAPI (blue). Scale bar=50 μm.
Delivery of 2RJ hydrogel does not affect hypertrophy
Changes in the cardiomyocyte cross-sectional area were examined by immunostaining cell boundaries using wheat germ agglutinin. The increase in cardiomyocyte area observed in untreated infarcted hearts was not significantly decreased following treatment with hydrogels (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).
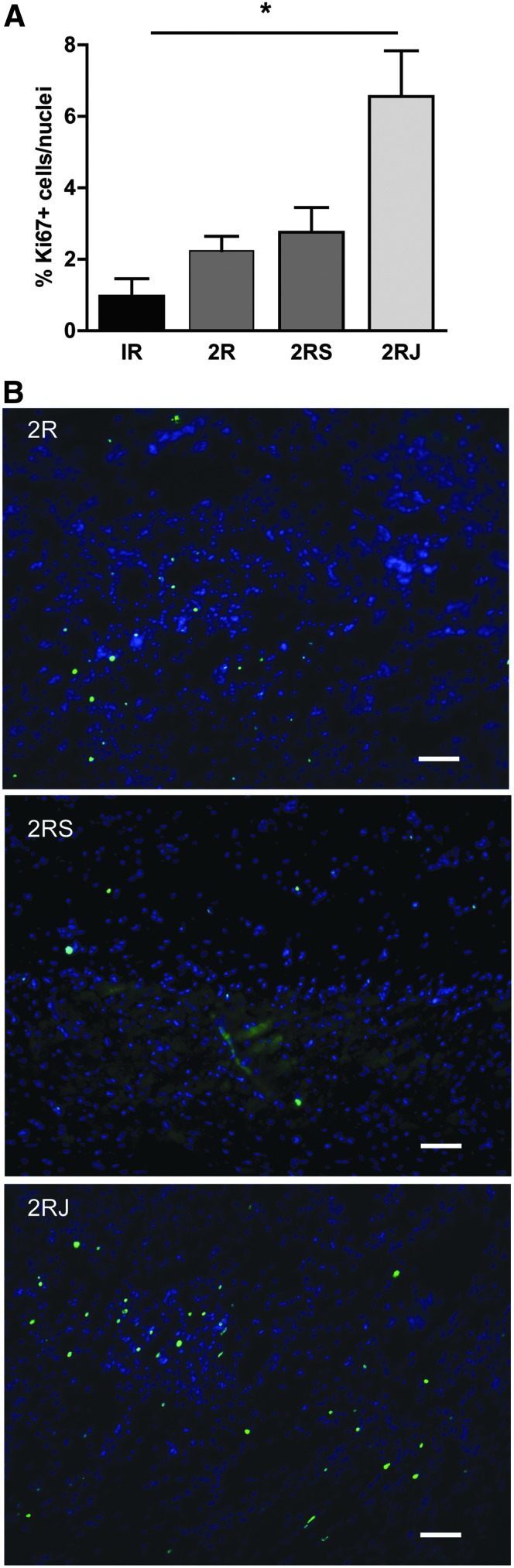
Delivery of 2RJ hydrogel induces cell proliferation and stem cell recruitment
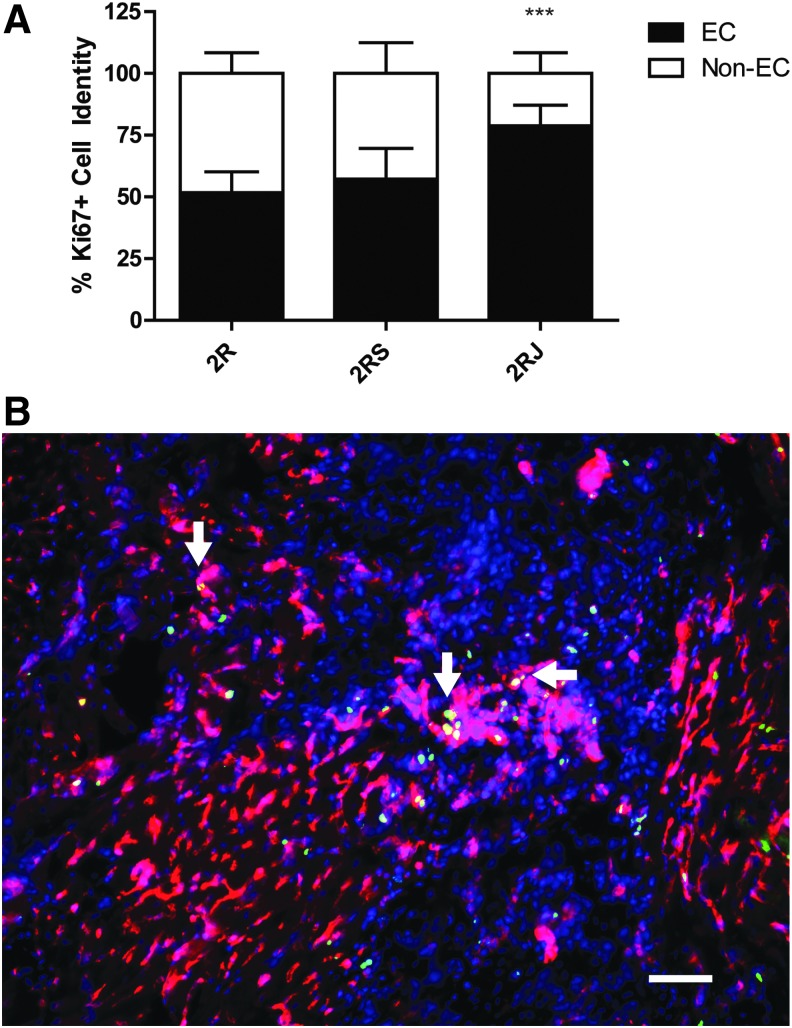
To determine if 2RJ hydrogel induced cell proliferation, sections were stained with Ki67. There was minimal staining in untreated injured hearts. As shown in the grouped data in Figure 5A and the representative images in Figure 5B, Ki67 staining was significantly upregulated in hearts treated with 2RJ hydrogel compared to low levels in 2R and 2RS hydrogel-treated hearts. To determine the source of these Ki67-positive cells, sections were counterstained with isolectin and double-positive cells were counted. As the data in Figure 6A and representative image in Figure 6B show, most of the Ki67-stained cells in 2RJ-treated rats were indeed endothelial cells.
FIG. 5.
Delivery of 2RJ hydrogel increases Ki67 expression. (A) Quantification of Ki67+ cells in rat hearts on day 21 shows an increase in Ki67+ cell number in infarcted rats treated with 2RJ hydrogel. *p<0.05, n=4 per group. (B) Representative images of Ki67 staining in the myocardium. Blue, DAPI; green, Ki67 nuclei. Scale bar=50 μm.
FIG. 6.
Ki67+ cells in RJ are mostly endothelial cells. (A) Quantification of double-positive Ki67 and isolectin cells in each group shows a significant change in cell lineage in 2RJ-treated rats. ***p<0.001 versus IR, n=4 per group. One-way ANOVA followed by Tukey's post-test. (B) Representative image from a 2RJ-treated rat heart showing isolectin (red), Ki67 (green), and DAPI (blue). Arrows denote areas of double staining. Scale bar=50 μm.
Discussion
Interest in acellular therapies is high due to relative ease of clinical translation, high reproducibility, and potential for off-the-shelf availability. Delivery of cell-free hydrogel therapy has resulted in improvement in cardiac function in animal models of MI through improved mechanical support and beneficial effects of delivered bioactive cargo.26 The established cardioprotective effects of Notch activation following infarction and the lack of inflammatory responses after myocardial delivery of SAPs containing VEGF in the porcine infarction model14 led us to investigate the role of SAP-mediated delivery of synthetic Notch1 ligand Jagged1 (2RJ) in attenuating cardiac dysfunction. The SAP hydrogel was also chosen to provide cues for Notch activation because (1) mechanical properties of the SAP can be tuned to match the host myocardium, (2) the peptide can be reproducibly synthesized chemically, (3) the ligand density can be regulated, and (4) acellular hydrogel systems are more clinically translatable. Previously, we demonstrated Jagged-1-presenting 2% SAP hydrogels to be the optimum concentration for promoting cardiogenic gene expression and regeneration, while 1% SAP hydrogels promoted vascular lineage. Furthermore, higher levels of Notch activation were observed in cells on 2RJ hydrogels than 1RJ.20 Based on these findings, and the mechanical properties of the higher density gels, we chose to use 2% hydrogels to study the effect of Notch activation using acellular hydrogels.
In the current study, hydrogels were injected immediately post-MI as reports suggest that effects of acellular hydrogels are most pronounced when injected immediately post-MI.26 As shown in Figure 1, ejection fraction recovered to sham-operated levels in rats treated with 2RJ hydrogels, whereas treatment with 2R or 2RS hydrogels had persistently decreased the ejection fraction comparable to untreated infarcted rats. Other metrics indicative of functional improvement were also preferentially improved in rats that received 2RJ hydrogels. Many acellular biomaterials improve cardiac function by providing mechanical support as tissue bulking agents. We have shown that the 2R, 2RS, and 2RJ hydrogels have the same Young's modulus (∼2 kPa) and swelling ratio (Supplementary Fig. S2 and Boopathy et al.20). However, the lack of functional improvement in 2R and 2RS hydrogel-treated rats shows that mechanical support of the infarcted heart does not explain the observed results.
Our results are in agreement with other studies that have examined the effect of Notch activation in the infarcted heart. Specifically, Notch activation in cardiac progenitor cells (CPCs) promoted cardiomyogenic differentiation of CPCs.16 Activation of Notch signaling in the border zone after infarction is known to be cardioprotective by promoting cardiomyocyte survival, cell cycle reentry, and improving cardiac function.24 Recent studies from our laboratory have shown the effect of RJ-mediated Notch activation on cardiogenic gene expression in CPCs in vitro with improvements in cardiac function following infarction in rats.20
Studies also demonstrate the inhibitory effect of Notch activation on cardiac fibroblast–myofibroblast transformation, which is critical for initiation of fibrosis.27 Moreover, Notch signaling has been shown to decrease cardiac fibrosis and promote CPC proliferation in a mouse model of cardiac pressure overload.28 These studies support the observed decrease in fibrosis following delivery of 2RJ hydrogels compared to untreated infarcted hearts. To clearly delineate the effects of 2RJ hydrogel, the effects on endogenous CPCs, myocytes, and endothelial vessel area were examined. As Notch activation has been shown to regulate the balance between fibrosis and regeneration, the extent of migration of endogenous CPCs into the infarcted region was investigated. We were unable to detect c-kit+ CPCs in untreated animals or rats treated with 2R and 2RS. While we did detect c-kit in 2RJ-treated rats, the levels were not high and this was not consistent among the animals (data not shown). Thus, we conclude that the main effects are likely due to improved vascularization.
Untreated infarction results in an initial compensatory increase in cardiomyocyte area and decrease in functional endothelial vessels. While treatment with 2RJ hydrogel did not affect the cardiomyocyte area, a significant increase in endothelial cell number and vessel area was observed indicative of 2RJ-mediated Notch activation in augmenting angiogenesis and possibly vasculogenesis. Indeed, several studies demonstrate a critical role for Notch signaling in regulating vessel maturation, and this may be the primary mechanism of action of the 2RJ hydrogel.29–31 We also observed a significant increase in cycling cells, specifically endothelial cells. Notch activation is long-known to have a potent effect on proliferation of endothelial cells32,33; however, we are unable to conclude whether the main effect is on proliferation of existing endothelial cells or of newly recruited stem cells differentiated to endothelial cells. In addition, we were unable to identify the nonendothelial cycling cells. Our data would suggest that they were not of smooth muscle or cardiac origin and likely conclude that they were proliferating fibroblasts or inflammatory cells. We also acknowledge limitations in the model being one of acute cardiac injury in rats. Toward this end, detailed analysis of the mechanism of action of the 2RJ hydrogels on the different cell types in the heart, the long-term effects of 2RJ hydrogel delivery, and possible paracrine effects should be investigated in the future. Functional studies should also be done in a large animal model of MI to determine scale up and translation of the hydrogel therapy. Despite this, as Notch-activating signals need to be membrane bound as opposed to soluble, we believe this tunable system provides the potential to have this critical mediator exert beneficial effects postinfarction. Given that previous studies using tethered IGF-1 did not show cell-free effects,6 the importance of choosing the ideal signal suitable for the targeted healing process is demonstrated in this report.
Conclusions
Delivery of a peptide mimic of the Notch1 ligand Jagged1, RJ, in a 2% SAP hydrogel to the infarcted rat heart resulted in improvements in cardiac function and contractility coupled with decreased fibrosis, increased endothelial vessel area, and ki67 expression. Intramyocardial injection of 2RJ hydrogels could be an effective acellular therapy for myocardial repair with the possibility for clinical translation.
Supplementary Material
Acknowledgments
This publication has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000043C to M.E.D. This work was also supported by an American Heart Association Predoctoral fellowship 11PRE7840078 to A.V.B.
Disclosure Statement
No competing financial interests exist.
References
- 1.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J., et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129, e28, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anversa P. Myocyte apoptosis and heart failure. Eur Heart J 19, 359, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Weisman H.F., and Healy B. Myocardial infarct expansion, infarct extension, and reinfarction: pathophysiologic concepts. Prog Cardiovasc Dis 30, 73, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Enomoto Y., Gorman J.H., 3rd, Moainie S.L., Jackson B.M., Parish L.M., Plappert T., et al. Early ventricular restraint after myocardial infarction: extent of the wrap determines the outcome of remodeling. Ann Thorac Surg 79, 881, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Christman K.L., Fok H.H., Sievers R.E., Fang Q., and Lee R.J. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng 10, 403, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Davis M.E., Hsieh P.C., Takahashi T., Song Q., Zhang S., Kamm R.D., et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A 103, 8155, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang X.J., Wang T., Li X.Y., Wu D.Q., Zheng Z.B., Zhang J.F., et al. Injection of a novel synthetic hydrogel preserves left ventricle function after myocardial infarction. J Biomed Mater Res A 90, 472–7, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Landa N., Miller L., Feinberg M.S., Holbova R., Shachar M., Freeman I., et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 117, 1388, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Salimath A.S., Phelps E.A., Boopathy A.V., Che P.L., Brown M., Garcia A.J., et al. Dual delivery of hepatocyte and vascular endothelial growth factors via a protease-degradable hydrogel improves cardiac function in rats. PLoS One 7, e50980, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singelyn J.M., DeQuach J.A., Seif-Naraghi S.B., Littlefield R.B., Schup-Magoffin P.J., and Christman K.L. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 30, 5409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science 336, 1124, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Hauser C.A., and Zhang S. Designer self-assembling peptide nanofiber biological materials. Chem Soc Rev 39, 2780, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Hsieh P.C., Davis M.E., Gannon J., MacGillivray C., and Lee R.T. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest 116, 237, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y.D., Luo C.Y., Hu Y.N., Yeh M.L., Hsueh Y.C., Chang M.Y., et al. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Sci Transl Med 4, 146ra09, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Kim J.H., Jung Y., Kim S.H., Sun K., Choi J., Kim H.C., et al. The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self-assembling peptides. Biomaterials 32, 6080, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Boni A., Urbanek K., Nascimbene A., Hosoda T., Zheng H., Delucchi F., et al. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A 105, 15529, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Bray S.J. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7, 678, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Niessen K., and Karsan A. Notch signaling in cardiac development. Circ Res 102, 1169, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Meloty-Kapella L., Shergill B., Kuon J., Botvinick E., and Weinmaster G. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev Cell 22, 1299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boopathy A.V., Che P.L., Somasuntharam I., Fiore V.F., Cabigas E.B., Ban K., et al. The modulation of cardiac progenitor cell function by hydrogel-dependent Notch1 activation. Biomaterials 35, 8103, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis M.E., Motion J.P., Narmoneva D.A., Takahashi T., Hakuno D., Kamm R.D., et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 111, 442, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang S.X., and Phillips W.D. Migration of resident cardiac stem cells in myocardial infarction. Anat Rec (Hoboken) 296, 184, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Liu J., Wang Y., Du W., and Yu B. Sca-1-positive cardiac stem cell migration in a cardiac infarction model. Inflammation 36, 738, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Gude N.A., Emmanuel G., Wu W., Cottage C.T., Fischer K., Quijada P., et al. Activation of Notch-mediated protective signaling in the myocardium. Circ Res 102, 1025, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickoloff B.J., Qin J.Z., Chaturvedi V., Denning M.F., Bonish B., and Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ 9, 842, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Tous E., Purcell B., Ifkovits J.L., and Burdick J.A. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res 4, 528, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Fan Y.H., Dong H., Pan Q., Cao Y.J., Li H., and Wang H.C. Notch signaling may negatively regulate neonatal rat cardiac fibroblast-myofibroblast transformation. Physiol Res 60, 739, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Nemir M., Metrich M., Plaisance I., Lepore M., Cruchet S., Berthonneche C., et al. The Notch pathway controls fibrotic and regenerative repair in the adult heart. Eur Heart J 35, 2174, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limbourg F.P., Takeshita K., Radtke F., Bronson R.T., Chin M.T., and Liao J.K. Essential role of endothelial Notch1 in angiogenesis. Circulation 111, 1826, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheppke L., Murphy E.A., Zarpellon A., Hofmann J.J., Merkulova A., Shields D.J., et al. Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood 119, 2149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Akker N.M., Caolo V., Wisse L.J., Peters P.P., Poelmann R.E., Carmeliet P., et al. Developmental coronary maturation is disturbed by aberrant cardiac vascular endothelial growth factor expression and Notch signalling. Cardiovasc Res 78, 366, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Roca C., and Adams R.H. Regulation of vascular morphogenesis by Notch signaling. Genes Dev 21, 2511, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Sainson R.C., and Harris A.L. Regulation of angiogenesis by homotypic and heterotypic notch signalling in endothelial cells and pericytes: from basic research to potential therapies. Angiogenesis 11, 41, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.