Abstract
Background:
The comparative data in the literature regarding rates of reoperation, revision ligament surgery, and contralateral surgery following anterior cruciate ligament reconstruction (ACLR) are variable and are often derived from studies with multiple surgeons, multiple centers, different surgical techniques, and a wide variety of graft choices.
Purpose:
To describe and analyze a single surgeon’s experience with ACLR using bone–patellar tendon–bone (BPTB) as the primary graft choice over a 25-year period.
Study Design:
Retrospective case series.
Methods:
All patients who underwent ACLR from 1986 to 2012 were identified from a prospectively maintained database. Traditional follow-up was only for patients who sought subsequent surgery with the index surgeon or presented with contralateral ACL injury. Covariates of interest included age, sex, time, and graft selection. Outcomes of interest included reoperation rates after primary/revision ACLR, rate of revision ACLR, success of meniscal repair with concomitant ACLR, and the proportion of patients undergoing contralateral surgery.
Results:
A total of 1981 patients (mean age, 29 years; 49% male) were identified. Of patients undergoing primary ACLR (n = 1809), 74% had BPTB autograft and 26% had a central third BPTB allograft. The mean age of patients undergoing autograft and allograft ACLR was 26 and 36 years, respectively (P < .05). Allograft tissue usage increased over time (P < .05). The rate of personal ACLR revision surgery was 1.7% (n = 30) for primary cases and 3.5% (n = 6) for revision cases. There were no significant differences in revision rates between primary autograft (1.6%) and allograft (2.0%) ACLR. With allograft use, the method of sterilization did not affect revision rates. The overall reoperation rate following primary ACLR was 10%; the 5-year reoperation rate was 7.7%. The reoperation rate was lower for primary cases reconstructed with allograft versus autograft (5% vs 12%) (P < .0001). Among primary ACLR cases, 332 patients (18%) underwent concomitant meniscal repair; 14% required revision meniscal surgery. The rate of contralateral ACLR was 6%.
Conclusion:
This information is useful for patients in the informed consent process, for perioperative decision making regarding graft choice, and for identifying patients who are at risk for injuring the uninvolved knee. The observed results in this series also emphasize that allograft ACLR can produce sustainable results with low complication rates in appropriately selected patients.
Keywords: anterior cruciate ligament, revision rate, reoperation rate, allograft, contralateral
The frequency of anterior cruciate ligament reconstruction (ACLR) performed in the United States has been estimated to range between 60,000 and 175,000 cases per year.8 Over a 10-year period, there has been a 21.5% and 68% increase in the number of ACLRs performed in New York State and in the United States, respectively.8 Despite the common nature of the procedure, controversy remains regarding the natural history of ACL injuries, surgical technique, graft choice, and long-term outcomes.10,11
While the importance of patient-based outcome measures cannot be overstated, there has been a recent renewed interest in monitoring hard endpoints or surgeon-based outcome measures such as the rate of reoperation and revision ligament surgery following ACLR, as well as rates of contralateral injury and surgery.1,5,7–9,14 A meta-analysis of six level 1 and 2 studies demonstrated recently that the pooled rate of ipsilateral graft rupture was 5.8% and the pooled contralateral injury rate was 11.8% at a minimum 5-year follow-up.16 In another systematic review of 11 randomized trials, Lewis et al5 demonstrated that the rate of graft failure following single-bundle ACLR was 4%, while the overall complication rate was 6%. Hettrich et al4 demonstrated an overall reoperation rate of 24.8% and a revision ACLR rate of 6.4% at 6-year follow-up in the Multicenter Orthopaedic Outcomes Network (MOON) cohort.
The aforementioned surgeon-based outcome measures can be assessed through a variety of methods ranging from single/multicenter retrospective or prospective case series (eg, MOON), administrative databases, and through registries. The latter can be formed to capture a cohort of patients from an international, national, regional, or local level, such as a hospital.1 In this manner, registries can be used to audit practice patterns and variations in practice between surgeons or different geographic locations. Furthermore, patients can be followed prospectively to assess for patient-reported and surgeon-based outcomes that are determined a priori.1 In contrast, administrative databases use procedural, billing, or diagnostic codes to often identify patients in a retrospective fashion. The outcomes of interest are restricted to the information that is collected in the database, and patient-reported outcomes are generally not available.14 Nonetheless, administrative databases allow access to large numbers of patients that can increase the power of subgroup comparisons.10,14 One of the disadvantages of multicenter cohort studies, administrative databases, and registries is that for surgical procedures such as ACLR, there can be large variation in surgical indications, surgical techniques, and graft choice. Furthermore, there are usually a large number of surgeons whose patients are enrolled, which introduces another confounding variable.
To decrease the potential for variability with respect to the above independent variables, the single-center, single-provider prospective or retrospective case series is one solution that remains of value. When appropriate selection criteria are employed and cases are adequately defined without significant attrition bias, the utility of such study designs allows for an analysis of pertinent patient- and surgeon-based outcomes.
Using a prospectively maintained database, the objective of the current study was to describe and analyze a single surgeon’s experience with primary and revision single-bundle ACLR over a 25-year period. Over this time period, the surgical technique remained relatively consistent, with bone–patellar tendon–bone (BPTB) as the primary graft choice. The specific objectives of this study were to determine the (1) demographics of the patient cohort at our institution, (2) patterns of graft use for ACLR, (3) rate of reoperation after ACLR, (4) rate of revision ACLR, and (5) the rate of contralateral ACLR.
Materials and Methods
This is a retrospective analysis of a prospectively maintained database of patients undergoing ACLR. The database was initially constructed at the commencement of the senior author’s (B.R.B.) surgical practice in September 1986 and has been maintained prospectively by the senior author. Data points that have been consistently observed and entered include age, sex, dates of all surgical procedures, side of surgery, concomitant procedures, revision procedures, acute and delayed complications (eg, arthrofibrosis, infection, hardware removal) with associated surgical procedures, and graft choice.
In this study, all patients who underwent a primary or revision ACLR from September 1986 to March 2012 were identified. Traditional follow-up was only for patients who sought subsequent surgery with the index surgeon or presented with contralateral ACL injury. Covariates of interest included age, sex, time, and graft selection for ACLR. Outcomes of interest included trends in graft selection and use over time, reoperation rates for primary and revision ACLR, the rate of revision and re-revision ACLR, success of meniscal repair in the setting of ACLR, and proportion of patients undergoing contralateral surgery.
Surgical Technique
Between 1986 and October 1991, a 2-incision, arthroscopically assisted single-bundle ACLR was performed, and since October 1991, the preferred single-bundle, transtibial technique has been used. The preferred technique of the senior surgeon (B.R.B.) is a single-bundle ACLR with BPTB auto- or allograft. An accessory inferolateral portal is utilized through the patellar tendon to facilitate creation of the tibial tunnel, and metal interference screws are utilized for fixation on both the femoral and tibial aspects. An accelerated postoperative rehabilitation program with full range of motion and full weightbearing was employed in 1990; whereas prior to this, weightbearing was delayed until 6 weeks postoperatively along with terminal extension.
Statistical Analysis
Data were extracted from the database and analyzed by an independent analyst. Descriptive statistics were used to describe the cohort undergoing primary and revision ACLR. The Student t test and analysis of variance (ANOVA) were used for continuous data and to compare outcomes over 5-year incremental time periods, respectively. The chi-square test was used for categorical data (sex, graft choice, rate of reoperation, rate of revision ACLR). An alpha level of .05 was determined to be of statistical significance.
Results
Between September 1986 and March 2012, the senior surgeon in this study (B.R.B.) performed 1981 ACLRs. Of these, 1809 were primary reconstructions, whereas 172 cases involved revision ligament reconstruction.
Patient Demographics
Among patients treated with a primary ACLR, the mean age was 28.6 years (range, 11-65 years; standard deviation [SD], 10.8 years); 59% (n = 1074) of these patients were male, and 50% (n = 892) were completed on right knees. The mean age of patients undergoing revision ACLR was 30 years (range, 14-51 years; SD, 8.6 years); 58% (n = 100) of these patients were male, and 54% (n = 93) were completed on right knees. There were no statistically significant differences in age or sex distribution among patients treated with primary and revision surgery.
Follow-up
Follow-up was only for patients who sought subsequent surgery with the index surgeon or presented with contralateral ACL injury. The mean follow-up for all such patients who had primary ACLR was 11.0 years (SD, 7.31 years). Among the primary cases treated with autograft, the mean follow-up was 12.8 years (SD, 7.4 years), compared with primary cases reconstructed with allograft, in which the mean follow-up was 6.2 years (SD, 4.3 years). The mean follow-up period for patients undergoing revision ACLR was 7.4 years (SD, 5.2 years).
Contralateral Surgery
In total, 5.5% (n = 109) of patients underwent contralateral ACLR at a mean of 49 months after initial index ACLR (range, 0-241 months; SD, 53.2 months). The mean age at index and contralateral reconstruction was 25 years (range, 12-55 years; SD, 10.2 years) and 29 years (range, 15-62 years; SD, 11.4 years), respectively. Among patients who underwent bilateral ACLR, females were significantly younger at index ACLR (21 vs 28 years; P = .003) and at contralateral ACLR (24 vs 32 years; P = .0003). There was also a trend for decreased time to contralateral reconstruction in females (39 months) compared with males (55 months) (P = .10). The age distribution of patients undergoing bilateral ACLR is illustrated in Figure 1.
Figure 1.
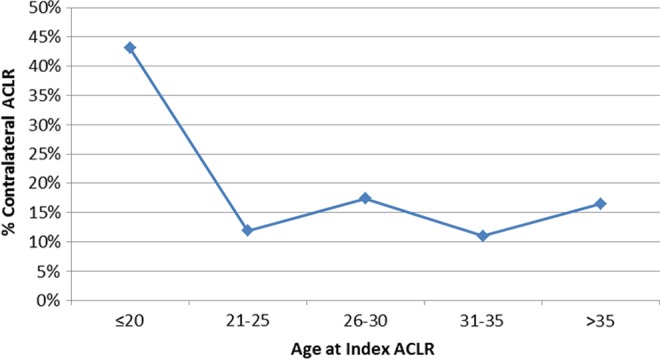
Age distribution of patients undergoing bilateral anterior cruciate ligament reconstruction (ACLR).
Patterns of Autograft and Allograft Use
Among patients treated with primary ACLR, 74% (n = 1332) were treated with an autograft, while 26% (n = 477) had a reconstruction with an allograft. In the autograft group, 1321 (99%) patients had a BPTB graft, compared with 11 patients reconstructed with quadrupled semitendinosis-gracilis hamstring grafts. In allograft ACLRs, there were 458 cases (96%) of central-third BPTB graft and 19 cases (4%) of hamstring allograft use; 116 patients had a nonirradiated allograft (prior to September 2003) and 361 patients had allografts that were processed and sterilized with low-dose (1.5 Mrad) irradiation (September 2003 and beyond). The mean age of patients undergoing autograft and allograft reconstruction was 26 years (range, 12-57 years; SD, 8.8 years) and 36 years (range, 11-65 years; SD, 11.5 years), respectively (P < .05). Allograft tissue use increased over time from 1% of all primary cases in 1986-1991 to 23% (1998-2003) and, most recently, to 46% (2009-2012) (P < .05). Trends for allograft use over time are illustrated in Figure 2. The use of allograft tissue also increased with patient age (P < .05) (Figure 3, A and B).
Figure 2.
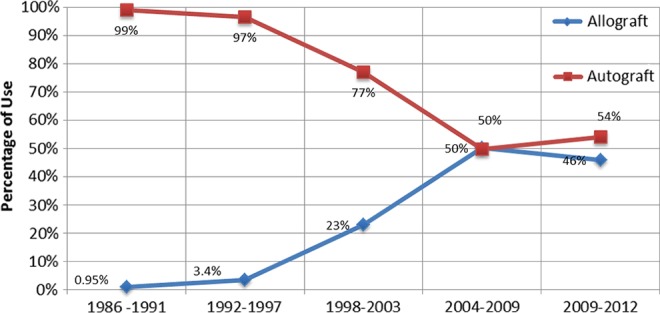
Changes in graft selection over time.
Figure 3.
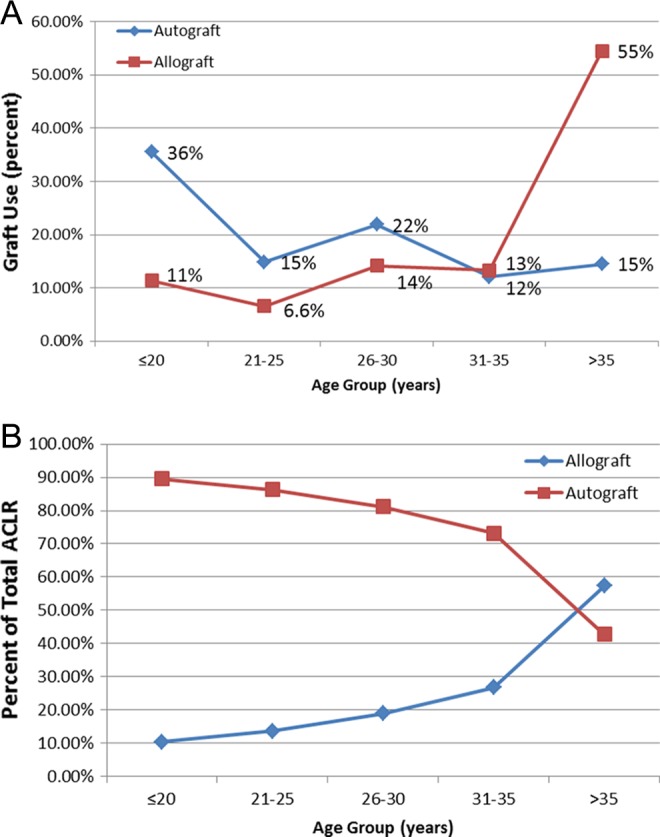
Graft selection based on patient age. (A) Distribution of total autograft and allograft use according to age. More than 50% of autograft cases are in patients <25 years, while more than 50% of allograft cases are in patients >35 years. (B) Percentage of total anterior cruciate ligament reconstructions (ACLRs) performed with autograft versus allograft based on patient age.
Among patients undergoing revision ACLR, 81% (n = 140) were treated with central-third BPTB allograft while 17% (n = 29) had a BPTB autograft; 1 patient had a quadriceps tendon autograft and 2 patients had a tendo-Achilles allograft.
Rate of Revision and Re-revision ACLR
The rate of personal ACLR revision surgery was 1.7% (n = 30) for all primary cases. The mean age at index and revision ligament reconstruction was 23 years (range, 13-41 years; SD, 8.6 years) and 28 years (range, 15-51 years; SD, 10.1 years), respectively. The mean time to revision ACLR was 57 months (range, 3-170 months; SD, 47.7 months). Revision rates remained stable over time between 1986 and 2012. There were no statistically significant differences in the personal revision rate between males and females or between autograft (1.5%, n = 20) and allograft (2.2%, n = 10) ACLR cases. Among patients undergoing allograft ACLR, there was also no statistically significant differences in revision rates among patients treated with nonirradiated patellar grafts (1.7%, n = 2 of 116) and low-dose–irradiated grafts (2.2%, n = 8 of 361). In the 30 failed primary cases in the current study, 17 patients had a BPTB autograft, 9 had a BPTB allograft, 2 had a hamstring autograft, and 1 patient had a hamstring allograft as the primary graft.
The personal re-revision rate of patients who had undergone a revision ACLR procedure was 3.5% (n = 6). All failed revision cases were BPTB allograft. The mean age of patients at the time of the index revision procedure was 27 years (range, 18-44 years), and the mean time to re-revision surgery was 37 months (range, 11-49 months). Fifty percent of all re-revision cases were male.
Rate of Reoperation
The overall nonrevision reoperation rate following primary or revision ACLR was 10.1% (n = 201) at a mean 40.2 months (range, 0.5-276 months; SD, 51.1 months) from the time of index ACLR. The rate of reoperation within 5 years of index ACLR reconstruction was 7.8%. The indications for reoperation in the current cohort of patients are illustrated in Figure 4. The most common reasons for repeat surgery included new meniscal tears (n = 65), failed meniscal repair (n = 50), arthrofibrosis (n = 32), hardware removal (n = 20), and superficial/deep infection (n = 9). The observed incidence of complications over the study period was 0.5% for infection, 1.0% for hardware removal, 1.5% for arthrofibrosis, and 3.3% for new meniscal tears.
Figure 4.
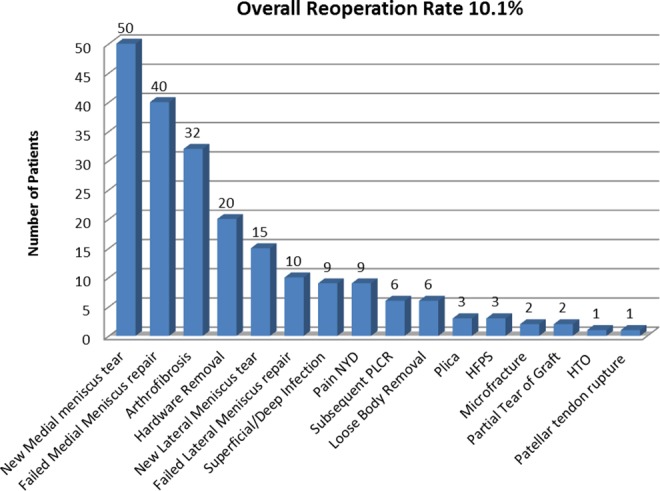
Reoperation following index primary or revision anterior cruciate ligament reconstruction (ACLR). PLCR, posterolateral corner reconstruction; HFPS, hypertrophic fat pad syndrome; HTO, high tibial osteotomy; NYD, not yet diagnosed.
There was a trend for lower reoperation rates in primary (9.8%, n = 177) compared with revision (14%, n = 24) cases (P = .08). A significant decrease in reoperation rates was observed over time, as illustrated in Figure 5 (P < .05). The reoperation rate was lower for primary cases reconstructed with allograft (5.0%, n = 24) compared with autograft (11.5%, n = 153) (P < .0001). The mean follow-up at the time of reoperation for allografts and autografts was 26.3 ± 27.3 and 41.9 ± 52.6 months, respectively (P = .16).
Figure 5.
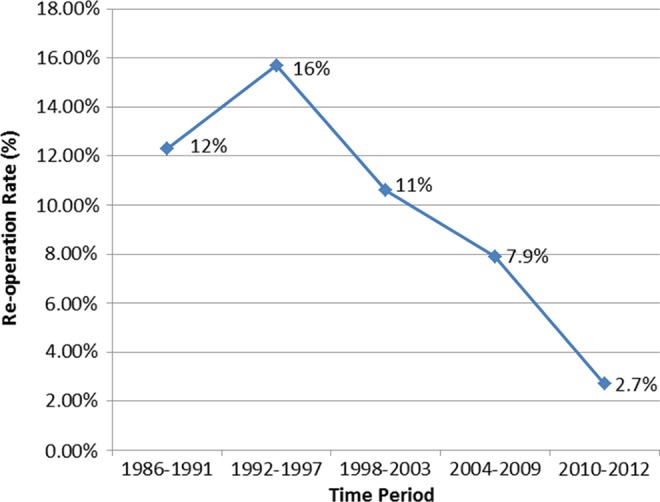
Reoperation rates over time for all patients.
Among primary ACLR cases, 332 patients (18%) underwent a concomitant meniscal repair; 14% (n = 46) of these patients required revision meniscal surgery at a mean 37 months (range, 4-116 months; SD, 32.2 months). The overall rate of revision meniscal surgery following meniscal repair at the time of ACLR was 15% (n = 41) in patients undergoing autograft ACLR and 7.7% (n = 5) in allograft cases (P < .0001).
Discussion
The current study utilizes a prospectively maintained administrative database, developed and maintained by the senior surgeon (B.R.B.), to describe the demographics and pertinent surgeon-based outcomes of a consecutive cohort of 1981 patients who have undergone primary or revision ACLR over a 25-year period. The value of the information derived from the current descriptive analysis relates to the long-term follow-up of patients from the practice of a single provider where the surgical techniques of choice (2-incision or 1-incision ACLR with BPTB) have remained consistent over time. The mean follow-up of patients who required further surgery in this series was 11 and 7 years for primary and revision ACLR, respectively, which provides a representative overview of the natural history following ACLR when performed by a single individual with little variation in surgical technique. Follow-up is not reported for patients who did not have repeat surgery by the index surgeon.
In the present series, the observed personal revision rates with primary and revision ACLR was 1.7% and 3.5%, respectively. Comparatively, there is a large amount of variability in the literature with respect to length of follow-up and revision rates. For example, 2 systematic reviews on primary ACLR demonstrated an objective failure rate of approximately 6% at variable follow-up periods.5,16 In 2 community-based registries in the United States and Norway, the rate of revision surgery was 0.9% and 1.6% at a follow-up of less than 3 years, respectively.9 Using an administrative database for a cohort of more than 34,000 patients from Ontario, Canada, Wasserstein et al14 demonstrated a 7.7% revision rate following primary ACLR at a mean of 4.2 years. In the national Danish registry, the revision rate after primary ACLR (n = 12,193 procedures) and re-revision rate (n = 1099 procedures) after revision ACLR was 4.1% and 5.4%, respectively, at 5 years.6 In a systematic review of outcomes following revision ACLR, Wright et al15 reported the pooled rate of objective failure to be 14% at a minimum 2-year follow-up. The observed personal revision rate for both primary and revision cases over the 25-year period in the current study is lower than the reported results in the literature. However, we would like to emphasize that a personal revision rate in a single practice is an underestimation of the true revision rate. The reasons for this include possible (1) loss to follow-up, (2) subsequent revision surgery by a different surgeon, and (3) the possibility that patients may have moved to a different geographic location due to the transient nature of young patients who tend to undergo ACLR.
There was also no difference in the rate of revision ACL surgery based on the use of autograft versus allograft tissue. The literature is replete with conflicting results with respect to failure rates following ACLR with different types of tissues. Foster et al3 systematically reviewed Oxford level 1 and 2 studies demonstrating graft failure rates of 4.7% and 8.2% for autograft and allograft tissue, respectively, and concluded there was no significant difference. In a systematic review of nonrandomized studies, Carey et al2 demonstrated that there was no difference in the clinical failure rate between autograft and allograft reconstructions. However, in a young military population, Pallis et al demonstrated a higher failure rate of allograft tissue compared to both BPTB and hamstring autografts (Pallis MP, Svoboda SJ, Cameron KL, Owens BD, Faegin JA. “Survival comparison of allograft and autograft ACL reconstruction at US military academy.” Presented at American Orthopaedic Society for Sports Medicine, 2011). We hypothesize that the observed similarities in objective failure and revision rates following autograft and allograft ACLR in the current study are related to differences in age between the 2 subgroups. The mean age of patients treated with autograft and allograft reconstruction was 26 and 36 years, respectively. It is likely that the older patients chosen for allograft reconstructions would place less in vivo forces on the ACL graft, which theoretically would translate into lower rates of clinical failure. Based on these findings, we believe that patient selection based on age (and presumably activity) is of paramount importance and has a direct influence on the potential longevity of a given reconstruction. As such, we believe it is important to tailor a given ACLR to the patient rather than have one solution that would be appropriate for all patients.
Among patients treated with allograft reconstruction, we noted no significant difference in the rate of revision ligament surgery according to graft processing and sterilization. In the senior surgeon’s practice, all allograft reconstructions prior to September 2003 were performed using nonirradiated grafts. Subsequent to September 2003, there was a transition to low-dose–irradiated grafts—specifically with 1.5 Mrad. In a randomized trial comparing outcomes of ACLR with irradiated BPTB allograft versus nonirradiated allograft and autograft, Sun et al13 demonstrated a significantly higher failure rate in the irradiated group (34.4%). Unlike our study, the authors sterilized grafts with 2.5 Mrad of irradiation prior to distribution. We believe that this higher dose of irradiation differentiates the results observed in this trial compared with those in our cohort. There were no cases of deep joint infection associated with allograft use in our series.
With respect to allograft use, we have noticed a gradual rise in the number of cases in which allograft reconstruction was performed. While only 1% of ACLRs were performed with allograft tissue from 1986 to 1991, there was an increase to 3%, 13%, 23%, 50%, and 46% over the ensuing 5-year increments of time. The increased use of allograft tissue over time is related to the empirical introduction of this graft type into the senior surgeon’s practice. That is, based on the early success of patients treated with allograft tissue in this series, allograft was subsequently offered to appropriately selected patients at increased rates over time. Furthermore, the mean age for patients treated with allograft was significantly higher than patients treated with autograft; more than 50% of allograft reconstructions were in patients older than 35 years, while more than 50% of autograft cases were performed in patients younger than 25 years. The observed findings emphasize the importance of patient selection when it comes to recommending graft choice.
The overall nonrevision reoperation rate following ACLR was approximately 10%, while the 5-year reoperation rate was 7.7%. Incidence rates of 0.5%, 1.0%, 1.5%, and 3.3% were observed for infection, hardware removal, arthrofibrosis, and new meniscal tears, respectively. A significant decrease in reoperation rates over time was observed. This may be explained by a learning curve that was experienced by the senior author early in his career, or the fact that patients who have had a more recent ACLR have not had the time to present with an indication requiring reoperation. Furthermore, patients with a primary autograft ACLR had a higher reoperation rate compared with patients reconstructed with allograft. Although this can be explained by the shorter observed follow-up period in patients undergoing allograft, the vast majority of reoperations occurred within 5 years of surgery in both groups. Another explanation for the increased reoperation in the autograft group may be the presumably greater activity levels in this younger cohort of patients. Our observed results, when compared with the literature, are lower than that reported for the MOON cohort by Hettrich et al,4 where an overall 25% reoperation rate was observed on the ipsilateral knee at 6-year follow-up. In the systematic review performed by Lewis et al,5 there was a 9.6% overall nonrevision reoperation rate at follow-up intervals ranging between 24 and 114 months across the included studies. With regard to the success of meniscal repair, an overall reoperation rate of 14% in the current series was noted. Furthermore, the rate of reoperation after failed meniscal repair was greater in patients treated with autograft (15%) versus allograft (8%), which may in turn reflect the younger age in the former group as well as an attempt to preserve as much meniscus as possible and the presumably the higher activity levels in younger patients. Our reoperation rate following meniscal repair is similar to that observed in the literature. In a systematic review of outcomes following meniscal repair, Paxton et al12 demonstrated a reoperation rate of 14% (148 of 1044 cases) when a repair was performed concomitantly with ACLR.
There are some limitations in the current study related to the use of an administrative single-surgeon database. First, the possibility of attrition bias cannot be ruled out. Based on the nature of the database, we were unable to ascertain how many patients may have moved to a different geographic area or may have been treated by another physician. This may result in an underestimation of revision ACLR rates. Furthermore, the data points entered are largely demographic, technique, and procedure related. Hence, we lack pertinent clinical information such as mechanism of injury, activity level, body mass index, and patient-reported outcome measures. Also, patient and surgeon factors unique to the tertiary care referral practice of the senior surgeon may not reflect the general population undergoing ACLR. Although these factors may impact the generalizability of the current findings, the data derived from the database of a single surgeon with a consistent technique over a 25-year period provide a high degree of internal validity with respect to the surgical procedures in the context of BPTB autograft and allograft cases. Finally, while the revision rates of ACLR performed with autograft and allograft are similar, the mean follow-up was less for the allograft group. It is possible that with longer mean follow-up, the revision rates for allograft ACLR may change.
Conclusion
The information derived from this study is useful for patients in the informed consent process, for perioperative decision making regarding graft choice, and for identifying patients who are at risk for injuring the uninvolved knee. The observed results in this series also emphasize that autograft and allograft ACLR using BPTB as the primary graft choice can produce sustainable results with low complication rates in appropriately selected patients. Future directions include establishing a registry at our institution with all sports surgeons and with our former fellows such that both surgeon- and patient-based outcome measures can be followed prospectively over time.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: B.R.B. has received educational grants to support the sports medicine fellowship from Smith & Nephew Endoscopy, Ossur, MioMed, Conmed Linvatec, Athletico, Arthrex, and Mitek and has received royalties from Slack Inc.
References
- 1. Ahn H, Court-Brown CM, McQueen MM, Schemitsch EH. The use of hospital registries in orthopaedic surgery. J Bone Joint Surg Am. 2009;91(suppl 3):68–72. [DOI] [PubMed] [Google Scholar]
- 2. Carey JL, Dunn WR, Dahm DL, Zeger SL, Spindler KP. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg Am. 2009;91:2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38:189–199. [DOI] [PubMed] [Google Scholar]
- 4. Hettrich CM, Dunn WR, Reinke EK, MOON Group, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate liagment reconstrucion: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41:1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewis PB, Parameswaran AD, Rue JP, Bach BR., Jr Systematic review of single-bundle anterior cruciate ligament reconstruction outcomes: a baseline assessment for consideration of double-bundle techniques. Am J Sports Med. 2008;36:2028–2036. [DOI] [PubMed] [Google Scholar]
- 6. Lind M, Mehnert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results fom the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40:1551–1557. [DOI] [PubMed] [Google Scholar]
- 7. Lind M, Menhert F, Pedersen AB. The first results from the Danish ACL reconstruction registry: epidemiologic and 2 year follow-up results from 5,818 knee ligament reconstructions. Knee Surg Sports Traumatol Arthrosc. 2009;17:117–124. [DOI] [PubMed] [Google Scholar]
- 8. Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91:2321–2328. [DOI] [PubMed] [Google Scholar]
- 9. Maletis GB, Granan LP, Inacio MC, Funahashi TT, Engebretsen L. Comparison of community-based ACL reconstruction registries in the U.S. and Norway. J Bone Joint Surg Am. 2011;93(suppl 3):31–36. [DOI] [PubMed] [Google Scholar]
- 10. Marx RG, Jones EC, Angel M, Wickiewicz TL, Warren RF. Beliefs and attitudes of members of the American Academy of Orthopaedic Surgeons regarding the treatment of anterior cruciate ligament injury. Arthroscopy. 2003;19:762–770. [DOI] [PubMed] [Google Scholar]
- 11. McRae SM, Chahal J, Leiter JR, Marx RG, Macdonald PB. Survey study of members of the Canadian Orthopaedic Association on the natural history and treatment of anterior cruciate ligament injury. Clin J Sport Med. 2011;21:249–258. [DOI] [PubMed] [Google Scholar]
- 12. Paxton ES, Stock MV, Brophy RH. Meniscal repair versus partial meniscectomy: a systematic review comparing reoperation rates and clinical outcomes. Arthroscopy. 2011;27:1275–1288. [DOI] [PubMed] [Google Scholar]
- 13. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17:464–474. [DOI] [PubMed] [Google Scholar]
- 14. Wasserstein D, Khoshbin A, Dwyer T, et al. Risk factors for recurrent anterior cruciate ligament reconstruction: a population study in Ontario, Canada, with 5-year follow-up [published online ahead of print July 15, 2013]. Am J Sports Med. doi:10.1177/0363546513493580. [DOI] [PubMed] [Google Scholar]
- 15. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wright RW, Magnussen RA, Dunn WR, Spindler KP. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: a systematic review. J Bone Joint Surg Am. 2011;93:1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]


