Abstract
Background:
Recalcitrant lateral epicondylitis (elbow extensor–origin tendinosis) is a common cause of elbow pain with many treatment options. In the present study, the medium-term results after open release and radiofrequency microtenotomy are reported.
Hypothesis:
Microtenotomy would provide long-term pain relief that was as good as the open release method.
Study Design:
Prospective, randomized trial.
Methods:
Twenty-four patients randomized to either open release or microtenotomy were assessed after 5 to 7 years. Clinical examination and dynamic infrared thermography (DIRT) of both elbows were performed preoperatively and at the medium-term follow-up. Magnetic resonance imaging (MRI) of both elbows was performed at the medium-term follow-up.
Results:
Significant pain reduction was found using a visual analog scale (VAS) at the medium-term follow-up in both groups compared with the preoperative assessment (P < .005). The Mayo Elbow Performance Score (MEPS) increased significantly in both groups (P < .01). The improvement in grip strength was not significant in either group. There was no significant difference between the groups in terms of VAS, strength, and the MEPS. On the DIRT examinations, there were significantly fewer hot spots at the medium-term follow-up than preoperatively (P = .0067, both study groups together). The MRI examinations revealed grade II changes in the operated elbow in 1 patient in each group at the medium-term follow-up, while all the other MRI examinations revealed a normal tendon.
Conclusion:
In this prospective, randomized trial with a medium-term follow-up, the results were similar after surgical release and microtenotomy in patients with recalcitrant lateral epicondylitis. The hypothesis was thus verified.
Keywords: tendinosis, epicondylitis, microtenotomy, infrared thermography
Recalcitrant lateral epicondylitis (elbow extensor–origin tendinosis) is a painful condition affecting the lateral part of the elbow. The incidence of the disease is about 3 per 1000 persons per year.36
This disease is common in the fourth decade of life, with a frequent consequence being absence from work for several weeks or months and even change of occupation. In the literature, local injury, aging, and overuse have been mentioned as causes of lateral epicondylitis.11,14,21,26,34
The diagnosis is mainly a clinical one. Tenderness on the lateral epicondyle and exacerbation of pain by resisted extension of the wrist with the elbow extended (Thomsen test) suggest affection of the extensor carpi radialis brevis (ECRB) and extensor carpi radialis longus (ECRL). Affection of the extensor digitorum communis (EDC) can be tested by resisted active extension of the middle finger. A number of conditions may be associated with pain laterally on the elbow, including posterior interosseous nerve entrapment syndrome, osteochondritis dissecans, varus instability, osteoarthritis, radial tunnel syndrome, posterior lateral rotatory instability, lateral plica syndrome, and cervical and shoulder pathology.
A routine radiographic examination (anteroposterior and lateral views) is important to rule out alternative lesions such as tumors and osteoarthritis. Radiographs may show enthesopathic exostosis, loose bodies, or evidence of past fracture. Calcification in the region of the lateral epicondyle has been reported in up to 7% of cases, a finding that does not alter the treatment strategy.34
Lateral epicondylitis is a degenerative condition associated with angiofibroplastic hyperplasia.21 However, many treatment approaches are based on the view that it is an inflammation. Most patients respond to conservative management, and only 5% to 10% of patients require surgery.2 There are more than 40 methods for treating lateral epicondylitis, but no particular one is recognized as being superior.8,11 Conservative treatment, such as rest, nonsteroidal anti-inflammatory drugs (NSAIDs), stretching, splinting, local injections of corticosteroid,14 and shock wave therapy12,24 have been reported with varying results. In several studies, the treatment of chronic elbow extensor–origin tendinosis with buffered platelet-rich plasma (PRP) reduced pain significantly.13,18,19
A percutaneous soft tissue release for treating lateral epicondylitis was reported by Lin et al15 with beneficial effects. Surgical procedures consisting of the release of the extensor tendon, epicondylar resection, and the excision and debridement of affected tissue are reported to render good to excellent results in up to 90% of patients.21,22
Tasto et al30 reported that radiofrequency (RF)-based microtenotomy could be employed as a safe and effective procedure for the treatment of chronic tendinosis. The treatment goal is to initiate healing by stimulating angiogenic responses. Bipolar RF-based microtenotomy is thought to induce healing by a controlled inflammatory response, followed by the stimulation of angiogenic healing in the tendon.10 In opposition to this, it has been reported that RF incites a healing response mediated by growth factors and cytokines,37 while Takahashi et al29 suggested that RF induces acute degeneration and/or ablation of sensory nerve fibers, which might explain the early pain relief after RF microtenotomy for tendinopathy.
The aim of this study was to make a medium-term evaluation of patients with recalcitrant lateral epicondylitis, comparing the results after open release and microtenotomy in a prospective, randomized trial. The hypothesis was that microtenotomy would provide good medium-term pain relief that was as good as that for the open release method.
Patients and Methods
Twenty-four patients (11 women, 13 men) with tendinosis in the lateral epicondyle of the elbow, who were randomized using closed envelopes to either open release or microtenotomy between 2006 and 2007, were assessed after 5 to 7 years. The study was approved by the Regional Ethics Committee at the University of Tromsø, Norway. The mean age was 49.2 years (range, 36-62 years) in the release group and 46.2 years (range, 30-64 years) in the microtenotomy group. The mean symptom duration in the release group was 28 months (range, 12-60 months; standard deviation [SD], 14.3 months), while it was 22 months (range, 12-50 months; SD, 9.7 months) in the microtenotomy group (not significant).
All patients had completed at least 1 year of conservative therapy before they were considered for surgical intervention. This included multiple injections with corticosteroids, oral medication with NSAIDs for several weeks without improvement, and physical therapy, which had been prescribed by the primary health care facility over a period of at least 3 months.
Eleven patients underwent surgery with extensor tendon release and repair, while 13 patients were operated on with microtenotomy using a Topaz Micro Debrider electrode (ArthroCare, Austin, Texas, USA).
Patients with cancer, severe organic diseases, seriously reduced general health status, or those with an unclear diagnosis with diffuse pain were excluded. All operations were performed by the first author (KM). Routine radiographic examinations of the affected elbow were performed preoperatively. All radiographic examinations were normal without exostoses, osteophytes, calcification, or other pathology.
Before the randomization procedure, the treating surgeon made the clinical assessment. The medium-term follow-up was performed by an independent orthopaedic surgeon.
Pain was evaluated using a visual analog scale (VAS), grip strength was assessed using a dynamometer (Yamar; Sammons Perston Rolyan, Mississauga, Ontario, Canada), and function was assessed using the Mayo Elbow Performance Score (MEPS) preoperatively and at the medium-term follow-up. At follow-up, 20 patients (7 in the release group, 13 in the microtenotomy group) were examined physically, and a telephone interview was carried out with the remaining 3 patients in the release group who were unable physically to attend the follow-up control because of relocation (Figure 1).
Figure 1.
Flowchart of the study.
Dynamic Infrared Thermography (DIRT)
Preoperatively, 18 of 24 patients (9 in the open release group, 9 in the microtenotomy group) underwent skin surface temperature measurement of the lateral aspect of both elbows using an infrared (IR) camera (FLIR ThermaCAM S65 HS; FLIR Systems, Boston, Massachusetts, USA). At the medium-term follow-up, 17 of 23 patients (6 in the open release group, 11 in the microtenotomy group) underwent the same examination (Figure 1). The technique is based on the relationship between dermal perfusion and the rate of change in skin temperature after transient thermal challenges.7,35 After a stabilization period of 10 minutes in a draught-free room kept at a constant temperature of 22°C, the elbow was subjected to mild convective cooling with a desktop fan for a period of 2 minutes.3,31 Thermal images were recorded for 3 minutes during the rewarming period. The IR images were subsequently processed using image-analyzing software (ThermaCAM Researcher Pro 2.8 SR-2, FLIR Systems). The images were classified by an independent clinical physiologist as showing or not showing an increased temperature (hot spot).
Magnetic Resonance Imaging (MRI)
Bilateral MRI scanning of the elbows was performed in 22 patients (10 in the open release group, 12 in the microtenotomy group) at the medium-term follow-up (Figure 1). The MRI examinations were evaluated by an independent radiologist with special interest in musculoskeletal pathology according to a 4-grade scale, as ascribed by Walton et al32 (Table 1).
Table 1.
Classification of Tendinosis Changes as Seen on Magnetic Resonance Imaginga
| Grade I: | Grade II: | Grade III: | Grade IV: |
|---|---|---|---|
| Normal tendon | Slight changes | Moderate changes | Severe changes |
| Homogenous low intensity | Mild focal increased tendon signal on proton density or fat-suppressed T2 images | Moderate focal increased signal not equal to that of fluid | Generalized increased signal in tendon |
aAccording to Walton et al.32
Surgical Technique
The extensor tendon release and repair technique was similar to that described by Nirschl and Pettrone,22 with slight modification. First, an approximately 3- to 4-cm incision was centered slightly distal to the lateral epicondyle, and the origin of the common extensor was exposed by dissection. Violation of the joint capsule was avoided. The interval between the ECRL and EDC was identified and widened by a small incision in line with the fibers, exposing the ECRB deeper and posterior to the ECRL. The proximal origin of the ECRB was released from its attachment. In some cases, the EDC was fibrillated and discolored, and it was released in a manner similar to the ECRB. The exposed fibrous-like granulation tissue was then removed with a curette or periosteal sleeve. The lateral epicondyle was decorticated. The tendon was repaired using a side-to-side suture technique without tension. The extensor aponeurosis was then closed before wound closure. A soft dressing was applied to the elbow. Pain permitting, postoperative mobilization was encouraged.
For the microtenotomy procedure, an incision approximately 3 cm in length was made over the affected epicondyle to expose the involved extensor tendon. The electrode, which was connected to a sterile isotonic saline flow system, was used for the microtenotomy. An RF apparatus provided the energy delivered through the electrode. The electrode was placed on the tendon perpendicular to its surface. The routine consisted of performing RF applications on the tendon in a grid-like pattern where each stimulated spot was placed 5 mm from the neighboring one. First, 2 to 3 light touches were made, followed by penetration of the tendon to a depth of 3 to 5 mm, depending on tendon thickness.30 The activation time for the electrode is fixed by the manufacturer at 0.5 seconds. The affected tendon usually requires 3 to 6 microablations.
All patients underwent this outpatient procedure under local anesthesia. One patient in the release group and 2 patients in the microtenotomy group required additional sedation.
Statistical Methods
The data are reported as mean values with standard deviations unless otherwise indicated. The comparisons between the groups were performed using the Mann-Whitney U test, and within-group comparisons were made using the Wilcoxon signed rank test. The comparison between dichotomous variables was made using the Fisher exact test. In the statistical analyses, the intention to treat principle was respected initially. Subsequent analyses were performed after excluding 1 patient in the microtenotomy group, who underwent a reoperation with open release, to further compare the study groups.
Results
Twenty-four patients were originally enrolled in this study, and 23 were contacted for the medium-term follow-up assessment. One patient in the open release group died during the follow-up period, and 1 patient in the microtenotomy group underwent reoperation with an open release.
The mean follow-up for the release group was 75.5 months (SD, 8.1 months), while it was 68.4 months (SD, 6.2 months) for the microtenotomy group (P = .02). The median length of the operation for the release group was 30 minutes (range, 22-40 minutes), whereas it was 18 minutes for the microtenotomy group (range, 10-27 minutes) (P < .001).
The preoperative mean VAS pain score in the release group was 6.4 (range, 4-8; SD, 1.5). The mean pain score at the medium-term follow-up was 1.3 (range, 0-5; SD, 1.7). The preoperative mean VAS pain score for the microtenotomy group was 7.1 (range, 5-10; SD, 1.6). The mean pain score at the medium-term follow-up was 1.4 (range, 0-5; SD, 2.3). A significant pain reduction was found in both groups compared with preoperative values (P < .005). No significant differences in terms of pain score were found between the study groups either preoperatively or at the medium-term follow-up.
The mean grip strength at the medium-term follow-up increased from 29.1 kg (range, 15-54 kg; SD, 12.9 kg) to 37.7 kg (range, 28-42 kg; SD, 6.1 kg) in the release group (not significant) and from 28.3 kg (range, 8-54 kg; SD, 16.9 kg) to 33.8 kg (range, 8-58 kg; SD, 13.1 kg) in the microtenotomy group (not significant). There was no significant difference in grip strength between the study groups preoperatively or at the medium-term follow-up. The median MEPS improved from 60 (range, 30-85) to 100 points (range, 70-100) (P < .01) in the release group and from 55 (range, 40-80) to 100 points (range, 65-100) in the microtenotomy group (P < .01). There was no significant difference in MEPS between study groups either preoperatively or at the medium-term follow-up (Tables 2 and 3).
Table 2.
MEPS Preoperatively and at the Medium-Term Follow-up in the Release Groupa
| Patient | MEPS Preoperative | MEPS 5-7 Years |
|---|---|---|
| 1 | 30 | 100 |
| 2 | 60 | 95 |
| 3 | 50 | Missing value |
| 4 | 80 | 100 |
| 5 | 85 | 100 |
| 6 | 80 | 95 |
| 7 | 50 | 100 |
| 8 | 60 | 100 |
| 9 | 54 | 100 |
| 10 | 50 | 100 |
| 11 | 70 | 70 |
| Median value | 60 | 100 |
aMEPS, Mayo Elbow Performance Score.
Table 3.
MEPS Preoperatively and at Medium-Term Follow-up in the Microtenotomy Groupa
| Patient | MEPS Preoperative | MEPS 5-7 Years |
|---|---|---|
| 1 | 40 | 100 |
| 2 | 40 | 100 |
| 3 | 45 | 100 |
| 4 | 65 | 100 |
| 5 | 60 | 100 |
| 6 | 55 | 100 |
| 7 | 40 | 85 |
| 8 | 65 | 100 |
| 9 | 50 | 65 |
| 10 | 65 | 100 |
| 11 | 70 | 100 |
| 12 | 80 | 100 |
| 13 | 45 | 100 |
| Median value | 55 | 100 |
aMEPS, Mayo Elbow Performance Score.
Reanalyzing the study groups after excluding the patient in the microtenotomy group who underwent reoperation with an open release revealed no significant differences between the study groups in terms of the VAS, MEPS, and grip strength at the medium-term follow-up. Furthermore, the improvements at the medium-term follow-up in terms of the VAS and MEPS were still significant in both study groups.
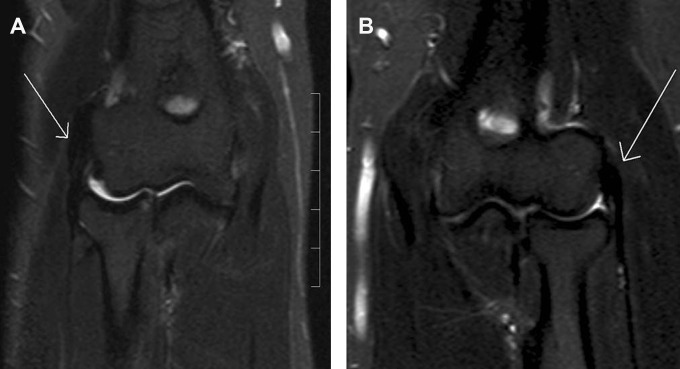
Ten patients in the release group and 12 patients in the microtenotomy group underwent MRI scanning of their elbows bilaterally at the medium-term follow-up. It was not possible to evaluate the MRI for 1 patient in the release group because of artifacts, and 1 patient in the microtenotomy group (the patient who underwent reoperation) was unable to undergo MRI because of claustrophobia. One patient in the release group and 1 in the microtenotomy group were classified as having grade II MRI changes in their index elbow. All the other patients undergoing MRI were classified as grade I on the index side. All patients undergoing MRI were classified as grade I on their contralateral elbow (Figure 2, A and B, Table 1).
Figure 2.
(A) T1 sequence from a magnetic resonance imaging scan of the right elbow in a 46-year-old man who underwent surgery for lateral elbow tendinosis with microtenotomy 5 years earlier, showing normal tendon, classified as grade I. (B) Left healthy elbow in the same patient on the same occasion, also classified as grade I.
Preoperatively, 14 of 18 (78%) patients who underwent DIRT (7 in the open release group, 7 in the microtenotomy group) displayed a hot spot. At the medium-term follow-up, 5 of 17 (29%) patients who underwent DIRT (1 in the open release group, 4 in the microtenotomy group) displayed a hot spot (Figure 3, A and B). There was a significant reduction in the number of patients with a hot spot in the entire study group and in the open release group (P = .0067, both groups together; P = .041, open release group; P = .092, microtenotomy group).
Figure 3.
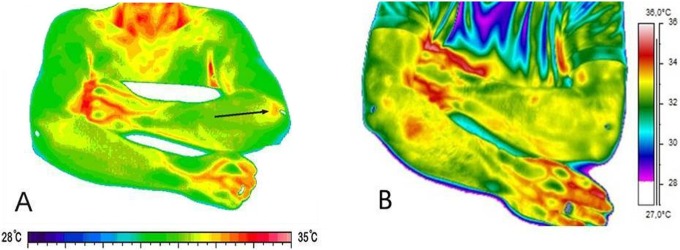
(A) Preoperative and (B) 5-year postoperative dynamic infrared thermographic images of a 48-year-old man who underwent microtenotomy of the left elbow. The hot spot on the preoperative image is not visible on the image from the medium-term follow-up.
There were no significant differences in terms of the number of hot spots between the study groups either preoperatively or at the medium-term follow-up.
Reanalyzing the study groups after excluding the patient in the microtenotomy group who underwent reoperation with an open release revealed no significant differences between the study groups in terms of the number of hot spots or the change over time.
No postoperative infections, neuroma formations, or other complications were registered in either group.
Discussion
In the present study, we compared the medium-term result of 2 methods for the surgical treatment of recalcitrant lateral epicondylitis: open tendon release and RF-based microtenotomy.
In the microtenotomy group, the VAS and the MEPS improved significantly at medium-term follow-up compared with preoperative findings. In the open release group, the VAS, MEPS, and number of hot spots as seen on DIRT showed significant improvements. However, the small number of patients in the study might have left it underpowered and therefore unable to demonstrate a significant difference between the 2 groups.
In the literature, different mechanisms have been suggested for the effect of the RF-based microtenotomy procedure, such as induced healing due to an angiogenic response in the tendon tissue.25,30 In an animal study, Harwood et al10 showed an increase in the expression of the angiogenic markers αv integrin subunit and vascular endothelial growth factor after RF-based microtenotomy. An early inflammatory response with new vessel formation after 28 days was found in an animal study using the same method.30 Another study documenting the quick degeneration or ablation of nerve fibers after RF-based microtenotomy may explain the rapid effect of this method.29 This theory is supported by Waseem et al,33 who discussed the existence of neurogenic inflammation and the presence of neuropeptides such as substance-P and calcitonin gene-related peptide (CGRP) in the tendon in lateral epicondylitis. The short-term effectiveness of the RF-based microtenotomy can be explained by instant neuroablation; however, the longer effectiveness may involve an angiogenic response and tendon regeneration.
In 2 recently published studies, Lin et al15 and Nazar et al20 argued that the percutaneous release of extensor origin on the humeral epicondylitis has a high rate of success without complications. Han et al9 discussed the possibility that corticosteroids have an inhibitory effect on cytokines and neuropeptides such as CGRP, and that the viability of the tenocytes is compromised. Furthermore, Maher16 found no evidence of the effectiveness of laser therapy for lateral epicondylitis.
In a recently published randomized study comparing PRP with glucocorticoid and saline, Krogh et al13 found no superior effect of PRP injections versus glucocorticoid or saline injections for the treatment of lateral epicondylitis. The results after corticosteroid injection and other injections for management of tendinopathy have been reported by Coombes et al.4
The RF microtenotomy procedure is based on a cold ablation and may induce less damage to the surrounding tissues during the operation, which makes the method less invasive and the surgical time shorter.
In a retrospective study, Szabo et al28 compared open arthroscopic and percutaneous release for lateral elbow extensor origin tendinosis. They found that all 3 methods were highly effective for the treatment of tendinosis, with no significant difference between them.28 The resection of the epicondyle and transfer of the anconeus muscle was found to be effective in a retrospective study by Almquist et al.1 Arthroscopy has been a promising procedure but it is technically demanding and can be associated with complications.2,26,27 Nirschl and Pettrone22 reported an 85% good to excellent outcome after the open release of extensor tendon, and Rubenthaler et al26 reported 88% effectiveness for the open technique and 93% for the arthroscopic technique.
In the present randomized controlled study, the VAS score, MEPS, grip strength, DIRT, and MRI were used to evaluate the effect of treatment. Comparing the present study with retrospective studies without preoperative data is somewhat problematic. The results in the present study appear to have a similar success rate compared with other reports.22,26,28
The mean grip strength at the medium-term follow-up had not improved significantly in either group. One explanation for this could be natural aging during the follow-up period.
Using DIRT is a new approach to evaluate extensor origin tendinosis. The DIRT technique is based on the relationship between dermal perfusion and the rate of change in skin temperature after transient thermal challenges.7,35 Since hypervascularity has been documented in tendonosis,5,30 and Pauling et al23 showed that DIRT is a reproducible method for assessment of digital vascular perfusion, it appears reasonable to use it for evaluation of hypervascularity associated with tendinosis.
Regardless of the surgical techniques used and their success rates, a number of complications may be associated with surgery. Postoperative problems may include restricted function, elbow instability, persistent muscle weakness, and painful neuroma formation of the posterior cutaneous nerve.6,21,30 In the present study, no such complications were found.
It is generally accepted that it is difficult to achieve complete pain relief in all patients with operative treatment.1,21,26,27 It has previously been reported that the advantages of microtenotomy are a rapid, significant improvement in the VAS just 3 weeks postoperatively and a significant improvement in strength 12 weeks after the operation.17
The weakness of the present study is its limited number of patients and the fact that no power analysis was performed for the primary variable. Furthermore, the differences in the follow-up in both groups may be considered a weakness. The strengths include its randomized design with a medium-term follow-up, the fact that the clinical follow-up evaluation was made by an independent observer, and that the patients were assessed using both MRI and DIRT.
Compared with the release procedure, the surgical time for microtenotomy is shorter and it is easy and safe to perform. Since the medium-term results are equal to those after surgical release, microtenotomy is now our preferred method for the surgical treatment of patients with recalcitrant lateral epicondylitis.
Conclusion
In this prospective, randomized trial with a medium-term follow-up, the results after surgical release and microtenotomy were similar in patients with recalcitrant lateral epicondylitis. The hypothesis was thus verified.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Almquist EE, Necking L, Bach AW. Epicondylar resection with anconeus muscle transfer for chronic lateral epicondylitis. J Hand Surg Am. 1998;23:723–731. [DOI] [PubMed] [Google Scholar]
- 2. Baker CL, Jr, Murphy KP, Gottlob CA, Curd DT. Arthroscopic classification and treatment of lateral epicondylitis: two-year clinical results. J Shoulder Elbow Surg. 2000;9:475–482. [DOI] [PubMed] [Google Scholar]
- 3. Binder A, Parr G, Thomas PP, Hazleman B. A clinical and thermographic study of lateral epicondylitis. Br J Rheumatol. 1983;22:77–81. [DOI] [PubMed] [Google Scholar]
- 4. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751–1767. [DOI] [PubMed] [Google Scholar]
- 5. Danielson P, Andersson G, Alfredson H, Forsgren S. Extensive expression of markers for acetylcholine synthesis and of M2 receptors in tenocytes in therapy-resistant chronic painful patellar tendon tendinosis—a pilot study. Life Sci. 2007;80:2235–2238. [DOI] [PubMed] [Google Scholar]
- 6. Dellon AL, Kim J, Ducic I. Painful neuroma of the posterior cutaneous nerve of the forearm after surgery for lateral humeral epicondylitis. J Hand Surg Am. 2004;29:387–390. [DOI] [PubMed] [Google Scholar]
- 7. Francis JE, Roggli R, Love TJ, Robinson CP. Thermography as a means of blood perfusion measurment. J Biomech Eng. 1979;101:246–249. [Google Scholar]
- 8. Goguin JP. Lateral epicondylitis. What is it really. Curr Orthop. 2003;17:386–389. [Google Scholar]
- 9. Han SH, An HJ, Song JY, et al. Effects of corticosteroid on the expressions of neuropeptide and cytokine mRNA and on tenocyte viability in lateral epicondylitis. J Inflamm (Lond). 2012;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harwood F, Bowden K, Ball S, Tasto JP, Amiel D. Structural and angiogenic response to bipolar radiofrequency treatment of normal rabbit Achilles tendon: a potential application for the treatment of tendinosis. Trans Orthop Res Soc. 2003:28:819. [Google Scholar]
- 11. Hong QN, Durand MJ, Loisel P. Treatment of lateral epicondylitis: where is the evidence? Joint Bone Spine. 2004;71:369–373. [DOI] [PubMed] [Google Scholar]
- 12. Hsu RW, Hsu WH, Tai CL, Lee KF. Effect of shock-wave therapy on patellar tendinopathy in a rabbit model. J Orthop Res. 2004;22:221–227. [DOI] [PubMed] [Google Scholar]
- 13. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625–635. [DOI] [PubMed] [Google Scholar]
- 14. Lewis M, Hay EM, Paterson SM, Croft P. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Results from a randomized controlled trial. Clin J Pain. 2005;21:330–334. [DOI] [PubMed] [Google Scholar]
- 15. Lin MT, Chou LW, Chen HS, Kao MJ. Percutaneous soft tissue release for treating chronic recurrent myofascial pain associated with lateral epicondylitis: 6 case studies. Evid Based Complement Alternat Med. 2012;2012:142941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maher S. Low-level laser therapy and lateral epicondylitis. Phys Ther. 2006;86:1161–1167. [PubMed] [Google Scholar]
- 17. Meknas K, Odden-Miland A, Mercer JB, Castillejo M, Johansen O. Radiofrequency microtenotomy: a promising method for treatment of recalcitrant lateral epicondylitis. Am J Sports Med. 2008;36:1960–1965. [DOI] [PubMed] [Google Scholar]
- 18. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–1778. [DOI] [PubMed] [Google Scholar]
- 19. Mishra AK, Skrepnik NV, Edwards SG, et al. Platelet-rich plasma significantly improves clinical outcomes in patients with chronic tennis elbow: a double-blind, prospective, multicenter, controlled trial of 230 patients [published online 3 Jul 2013]. Am J Sports Med. [DOI] [PubMed] [Google Scholar]
- 20. Nazar M, Lipscombe S, Morapudi S, et al. Percutaneous tennis elbow release under local anaesthesia. Open Orthop J. 2012;6:129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nirschl RP, Ashman ES. Elbow tendinopathy: tennis elbow. Clin Sports Med. 2003;22:813–836. [DOI] [PubMed] [Google Scholar]
- 22. Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am. 1979;61:832–839. [PubMed] [Google Scholar]
- 23. Pauling JD, Shipley JA, Raper S, et al. Comparison of infrared thermography and laser speckle contrast imaging for the dynamic assessment of digital microvascular function. Microvasc Res. 2012;83:162–167. [DOI] [PubMed] [Google Scholar]
- 24. Pettrone FA, McCall BR. Extracorporeal shock wave therapy without local anesthesia for chronic lateral epicondylitis. J Bone Joint Surg Am. 2005;87:1297–1304. [DOI] [PubMed] [Google Scholar]
- 25. Placzek R, Drescher W, Deuretzbacher G, Hempfing A, Meiss AL. Treatment of chronic radial epicondylitis with botulinum toxin A. A double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg Am. 2007;89:255–260. [DOI] [PubMed] [Google Scholar]
- 26. Rubenthaler F, Wiese M, Senge A, Keller L, Wittenberg RH. Long-term follow-up of open and endoscopic Hohmann procedures for lateral epicondylitis. Arthroscopy. 2005;21:684–690. [DOI] [PubMed] [Google Scholar]
- 27. Smith AM, Castle JA, Ruch DS. Arthroscopic resection of the common extensor origin: anatomic considerations. J Shoulder Elbow Surg. 2003;12:375–379. [DOI] [PubMed] [Google Scholar]
- 28. Szabo SJ, Savoie FH, 3rd, Field LD, Ramsey JR, Hosemann CD. Tendinosis of the extensor carpi radialis brevis: an evaluation of three methods of operative treatment. J Shoulder Elbow Surg. 2006;15:721–727. [DOI] [PubMed] [Google Scholar]
- 29. Takahashi N, Tasto JP, Ritter M, et al. Pain relief through an antinociceptive effect after radiofrequency application. Am J Sports Med. 2007;35:805–810. [DOI] [PubMed] [Google Scholar]
- 30. Tasto JP, Cummings J, Medlock V, Hardesty R, Amiel D. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy. 2005;21:851–860.16012499 [Google Scholar]
- 31. Thomas D, Siahamis G, Marion M, Boyle C. Computerised infrared thermography and isotopic bone scanning in tennis elbow. Ann Rheum Dis. 1992;51:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walton MJ, Mackie K, Fallon M, et al. The reliability and validity of magnetic resonance imaging in the assessment of chronic lateral epicondylitis. J Hand Surg Am. 2011;36:475–479. [DOI] [PubMed] [Google Scholar]
- 33. Waseem M, Nuhmani S, Ram CS, Sachin Y. Lateral epicondylitis: a review of the literature. J Back Musculoskelet Rehabil. 2012;25:131–142. [DOI] [PubMed] [Google Scholar]
- 34. Whaley AL, Baker CL. Lateral epicondylitis. Clin Sports Med. 2004;23:677–691. [DOI] [PubMed] [Google Scholar]
- 35. Wilson SB, Spence VA. Dynamic thermographic imaging method for quantifying dermal perfusion: potential and limitations. Med Biol Eng Comput. 1989;27:496–501. [DOI] [PubMed] [Google Scholar]
- 36. Wolf JM, Mountcastle S, Burks R, Sturdivant RX, Owens BD. Epidemiology of lateral and medial epicondylitis in a military population. Mil Med. 2010;175:336–339. [DOI] [PubMed] [Google Scholar]
- 37. Yeap EJ, Chong KW, Yeo W, Rikhraj IS. Radiofrequency coblation for chronic foot and ankle tendinosis. J Orthop Surg (Hong Kong). 2009;17:325–330. [DOI] [PubMed] [Google Scholar]