Abstract
Background:
Postoperatively, signal changes of the reconstructed anterior cruciate ligament (ACL) graft on magnetic resonance imaging (MRI) images commonly occurs, which may be a cause for concern. The signal intensity changes are usually expressed by signal/noise quotient (SNQ) value, representing graft maturity. To date, little is known about the factors influencing the SNQ value of the reconstructed ACL graft.
Purpose:
To evaluate ACL graft SNQ value and associated factors after ACL reconstruction.
Study Design:
Case series; Level of evidence, 4.
Methods:
Male patients who underwent ACL reconstruction using autograft or allograft tendon from September 2004 to September 2011 were randomly invited to take part in this investigation, including functional scores, physical examination, and MRI scan. The femoral side graft was fixed with Endobutton CL or Rigidfix pins, and the tibial side graft was fixed with a bio-intrafix. SNQ values of each graft were measured on MRI to represent graft maturity. Sagittal ACL angle, ACL–Blumensaat line angle, and medial and lateral posterior tibial slope (PTS) were measured using MRI 3-dimensional dual-echo steady-state images. Potential risk factors, including age, body mass index, postoperative time, Tegner activity scale (TAS), sagittal ACL angle, ACL–Blumensaat line angle, medial PTS, lateral PTS, and primary graft diameter, were tested for their association with the graft SNQ value by multivariate stepwise regression analysis.
Results:
A total of 104 male subjects (mean follow-up, 30.7 months) were examined, including 62 allograft and 42 autograft reconstructions. There was a significant association between graft SNQ and postoperative time (r = −0.431, P < .001), TAS (r = 0.295, P = .002), and ACL–Blumensaat line angle (r = −0.304, P = .002). Univariate regression analysis showed that TAS (β = 6.15, P < .001) positively correlated, postoperative time (β = −0.26, P < .001) negatively correlated, and ACL–Blumensaat line angle (β = −0.40, P = .038) negatively correlated with graft SNQ. Multivariate stepwise regression analysis showed that TAS, postoperative time, ACL–Blumensaat line angle, and age were significant independent factors associated with graft SNQ.
Conclusion:
The graft SNQ value had a significant positive correlation with physical activity level and a significant negative correlation with postoperative time in this study. Males with a shorter postoperative time and a higher physical activity level had higher graft signal intensity postoperatively.
Keywords: MRI, ACL, autograft, allograft, signal intensity, graft maturity
In the management of the anterior cruciate ligament (ACL) injury, reconstruction with autograft or allograft tendons has been a widely accepted procedure used to restore joint stability and improve knee function.12,14 The increased number of ACL reconstructions has led to the need for better postoperative evaluation of the reconstructed graft. As a noninvasive tool, magnetic resonance imaging (MRI) has been used to monitor graft status after implantation.1,25,26 Postoperatively, high signal intensity of the reconstructed graft on MRI images is commonly observed, which may be cause for concern.16,19,34
A previous investigation reported that quantitative analysis of graft strength was possible by measuring graft signal intensity on MRI.5 As reported, graft signal intensity has a significant negative linear correlation with the material strength of the ACL graft.2,11,37 High signal intensity on MRI indicates a decrease of mechanical properties of the reconstructed graft. Based on MRI images, the signal/noise quotient (SNQ) represents graft maturity22: A high graft signal intensity represents high SNQ value, which indicates inferior graft maturity.
In addition, an increasing number of studies have investigated the failure rate of allograft and autograft ACL reconstructions.4,20,28 Potential risk factors influencing the incidence of graft failure have been identified through clinical investigation, including age, sex, body weight, postoperative time, graft type, irradiation of allografts, graft placement, posterior tibial slope (PTS), and physical activity level.21,29,30,35 It has been reported that higher activity level can cause an increased failure rate of ACL reconstructions.3,36 However, little is known about the correlation of these factors with the maturity of the reconstructed ACL graft. Understanding the factors that influence graft maturity is critical in the prevention and management of graft failure.
The purpose of this study, therefore, was to identify factors that influenced graft maturity after anatomic ACL reconstruction based on 3.0-T MRI. We hypothesized that physical activity level would be significantly correlated to increased graft maturity after anatomic ACL reconstruction.
Methods
Male patients with unilateral ACL reconstruction from September 2004 to September 2011 were invited to participate in this investigation. Females were excluded because of different hormonal level, which might greatly influence graft maturity. One senior surgeon (S. Chen) performed all the operations using arthroscopic single-bundle ACL reconstruction techniques as described by Li et al.22 For the autograft reconstruction, the semitendinosus and gracilis tendons were harvested and prepared as a 4-strand double-looped hamstring autograft with a minimum of 12 cm in length. In the allograft group, a fresh-frozen tibialis anterior allograft tendon (Osteolink Biomaterial Co) was thawed in sterile physiologic fluid at room temperature and prepared as a 4-strand double-looped graft similar to the autograft. Subsequently, the tibial and femoral holes were made using a transtibial technique. After graft passage, the femoral side graft was fixed with an Endobutton CL (Smith & Nephew) or Rigidfix pins (Mitek Inc), and on the tibial side, the graft was fixed with a bio-intrafix (Mitek Inc). The inclusion criterion were (1) a unilateral ACL reconstruction, (2) no history of reinjury of the reconstructed knee, (3) time from the injury to surgery ranging from 3 months to 1 year, and (4) minimum follow-up time of 1 year. Exclusion criteria were (1) knee instability, (2) osteoarthritis, (3) ACL reconstruction combined with ligament reconstruction, and (4) severe synovial reaction at the time of surgery. The study was approved by the ethics committee of our hospital.
Clinical Evaluation
Patients were asked to return for examination and MRI at a minimum of 1 year after surgery. Clinical examination, including subjective, functional, and physical examinations, was performed on the same day as the MRI. Subjective functional examinations consisted of International Knee Documentation Committee (IKDC) score and Tegner activity scale (TAS).10 Physical examinations consisted of the anterior drawer test and Lachman test.
MRI Scan and Image Analysis
After resting for 1 hour, the knees were imaged in a relaxed extended position with a 3.0-T MRI scanner (MAGNETOM Verio, A Tim system; Siemens). Sagittal oblique fat-saturated proton density images were obtained as follows: repetition time, 3000 ms; echo time, 28 ms; flip angle, 160°; matrix, 320 × 272; field of view, 15 × 15 cm; slice thickness, 3 mm; scan time, 2 minutes 41 seconds. Three-dimensional dual-echo steady-state (3D-DESS) imaging: repetition time, 14.1 ms; echo time, 5 ms; flip angle, 25 degree; matrix, 256 × 238; field of view, 15 × 15 cm; slice thickness, 0.6 mm; scan time, 5 minutes 58 seconds. Image data were transferred into Siemens Software Packages workstation (NUMARIS/4, SyngoMR B17; Siemens) for calculation.
The SNQ of each graft site (femoral-adjacent, middle, and tibial-adjacent) was estimated using the following equation: SNQ = (signal of ACL graft − signal of quadriceps tendon)/signal of background (Figure 1).22 The SNQ value of the graft was calculated by averaging the SNQ value of the 3 sites. In addition, the sagittal ACL angle and the ACL–Blumensaat line angle were measured. Measurements of the medial and lateral PTS using MRI 3D-DESS images were performed according to a previous method.23 All measurements were made by the same investigator, and repeated measurements were made on 2 days at least 1 week apart.
Figure 1.
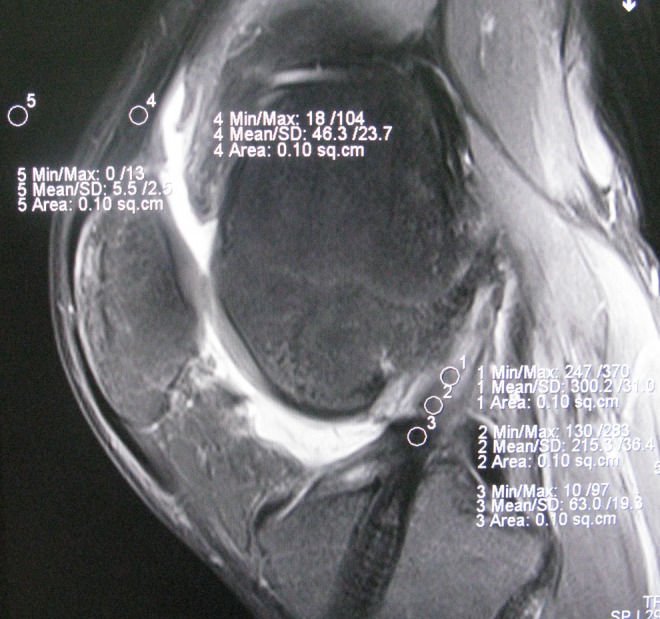
Sagittal magnetic resonance image of the knee shows the positions of the 5 regions of interest (area of the circle, 0.10 cm2), which included the (1) distal third, (2) middle third, (3) proximal third, (4) quadriceps tendon, and (5) background site (approximately 2 cm anterior to the quadriceps tendon).
Statistical Analysis
All analyses were carried out using SPSS 18.0 software (PASW Statistics v18.0; SPSS Inc), and the results were reported as mean and standard deviation for description. The intraclass correlation coefficient (ICC) was assessed by examining the intraobserver reliabilities. The coefficients were interpreted as poor if ICC < 0.4, marginal if 0.4 ≤ ICC ≤ 0.75, and as good if ICC > 0.75. Spearman correlation coefficients were calculated between graft SNQ value and potential risk factors, including age, body mass index (BMI), postoperative time, TAS, primary graft diameter, medial PTS, lateral PTS, sagittal ACL angle, and ACL–Blumensaat line angle. Multivariate stepwise regression analysis was performed to further assess the independent correlated factors of graft SNQ value. The level of significance was set as P < .05.
Results
A total of 104 male subjects using allograft or autograft tendons aged between 20 and 47 years (mean, 29.5 years) took part in this study, including 62 tibialis anterior tendon allograft and 42 hamstring tendon autograft reconstructions. The mean follow-up time was 30.7 months (range, 12-114 months). The demographic data of the participants are presented in Table 1, including age, BMI, operative side, primary graft diameter, graft type, follow-up time, and IKDC score.
TABLE 1.
Characteristics of Study Participantsa
| Variable | Total (N = 104) | Allograft (n = 62) | Autograft (n = 42) |
|---|---|---|---|
| Age, y | 29.5 ± 6.3 | 29.9 ± 6.9 | 29.0 ± 5.5 |
| Body mass index, kg/m2 | 24.3 ± 2.7 | 24.0 ± 2.8 | 24.7 ± 2.6 |
| Operative side, left/right | 40/64 | 25/37 | 15/27 |
| Graft diameter, n | |||
| 7 mm | 17 | 10 | 7 |
| 8 mm | 87 | 52 | 35 |
| Follow-up time, mo | 30.7 ± 15.5 | 28.1 ± 11.4 | 34.4 ± 19.7 |
| TAS | 3.4 ± 0.8 | 3.5 ± 0.8 | 3.3 ± 0.8 |
| IKDC score | 92.2 ± 5.8 | 92.1 ± 6.1 | 92.2 ± 5.8 |
| Sagittal ACL angle, deg | 64.3 ± 6.9 | 63.6 ± 7.4 | 65.3 ± 6.1 |
| ACL–Blumensaat line angle, deg | 17.5 ± 7.5 | 17.6 ± 7.3 | 17.5 ± 7.8 |
| Medial PTS | 6.7 ± 3.4 | 6.7 ± 3.4 | 6.6 ± 3.3 |
| Lateral PTS | 7.2 ± 3.3 | 7.1 ± 3.5 | 7.3 ± 3.0 |
| Femoral fixation type, n | |||
| Endobutton CL | 47 | 27 | 20 |
| Rigidfix pins | 57 | 35 | 22 |
aValues are reported as mean ± SD unless otherwise indicated. ACL, anterior cruciate ligament; IKDC, International Knee Documentation Committee; PTS, posterior tibial slope; TAS, Tegner activity scale.
The ICC index of intraobserver reliabilities was 0.85 for the SNQ value of the graft. Possible associations between potential risk factors and SNQ value of the graft were explored. There was no significant association between graft SNQ value and age, BMI, sagittal ACL angle, medial PTS, lateral PTS, or primary graft diameter. Graft SNQ had a significant association with postoperative time (r = −0.431, P < .001), TAS (r = 0.295, P = .002), and ACL–Blumensaat line angle (r = −0.304, P = .002). The graft SNQ of the allograft tendon group was significantly higher than that of the autograft tendon group (14.4 ± 13.8 vs 9.2 ± 9.9, respectively; P = .038). For the allograft, graft SNQ had a significant association with postoperative time (r = −0.467, P < .001) and TAS (r = 0.385, P < .001). For the autograft, graft SNQ had a significant association with TAS (r = 0.392, P = .01) and ACL–Blumensaat line angle (r = −0.322, P = .038). Scatter plots of the graft SNQ according to the postoperative time, the TAS, and the ACL–Blumensaat line angle are shown in Figures 2 to 4.
Figure 2.
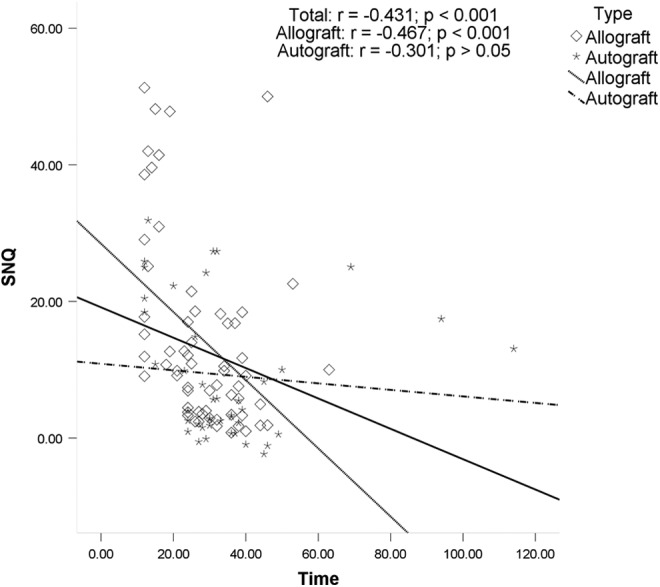
Correlation between postoperative time and the mean signal/noise quotient (SNQ) value. There was a significant negative association between the postoperative time and graft SNQ value in the total group and the allograft group.
Figure 3.
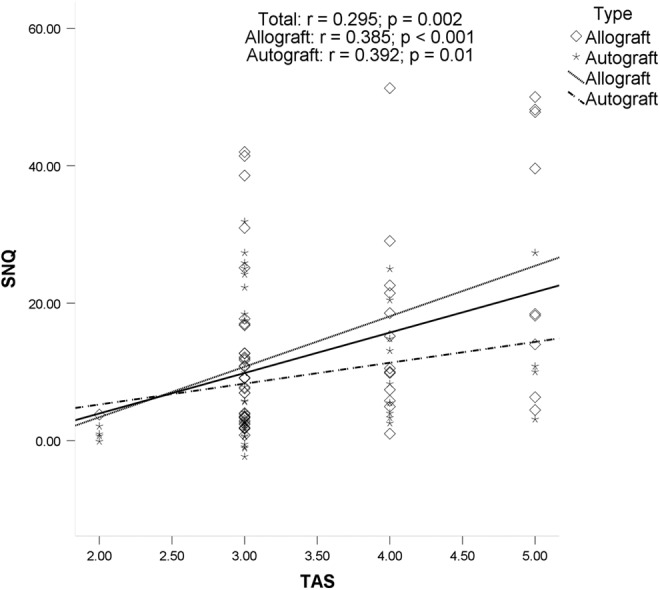
Correlation between the Tegner activity scale (TAS) and the mean signal/noise quotient (SNQ) value. There was a significant positive association between the TAS and graft SNQ value in the total group, the allograft group, and the autograft group.
Figure 4.
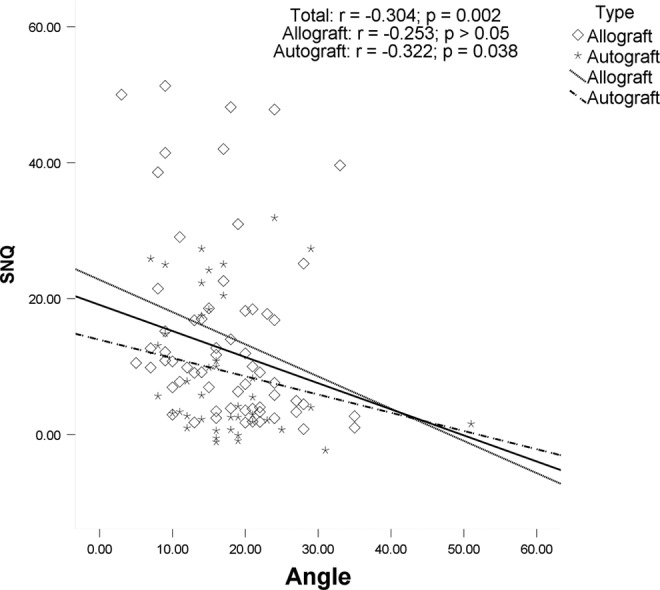
Correlation between the anterior cruciate ligament–Blumensaat line angle and the mean signal/noise quotient (SNQ) value. There was a significant negative association between the angle and graft SNQ value in the total group and the autograft group.
Univariate regression analysis (Table 2) showed that postoperative time (β = −0.26, P < .001), TAS (β = 6.15, P < .001), and ACL–Blumensaat line angle (β = −0.40, P = .038) correlated with graft SNQ value for the total 104 grafts. For the allograft, postoperative time (β = −0.51, P < .001) and TAS (β = 8.10, P < .001) correlated with graft SNQ value. Multivariate stepwise regression analysis (Table 3) showed that TAS, postoperative time, ACL–Blumensaat line angle, and age were significant independent correlated factors of graft SNQ value (β = 6.36 [TAS], −0.25 [postoperative time], −0.39 [ACL–Blumensaat line angle], 0.34 [age]; P < .05). For the allograft, high TAS and short postoperative time were significant independent predictors of high graft SNQ value (β = −0.55 [postoperative time], 8.03 [TAS]; P < .001).
TABLE 2.
Univariate Regression Analysisa
| Variable | Total (N = 104) | Allograft (n = 62) | Autograft (n = 42) | |||
|---|---|---|---|---|---|---|
| β | P Value | β | P Value | β | P Value | |
| Age | 0.39 | .024 | 0.41 | .050 | −0.20 | .551 |
| BMI | −0.39 | .327 | −0.29 | .568 | −0.15 | .822 |
| Postoperative time | −0.26 | <.001 | −0.51 | <.001 | −0.07 | .468 |
| TAS | 6.15 | <.001 | 8.10 | <.001 | 2.21 | .303 |
| Sagittal ACL angle | 0.07 | .731 | −0.10 | .695 | 0.39 | .396 |
| ACL–Blumensaat line angle | −0.40 | .038 | −0.28 | .290 | −0.54 | .119 |
| Medial PTS | 0.01 | .986 | 0.51 | .353 | −0.42 | .549 |
| Lateral PTS | 0.57 | .183 | −0.08 | .884 | 0.98 | .186 |
| Graft diameter | −34.67 | .252 | −55.18 | .181 | 0.92 | .983 |
aUnivariate analysis with age, BMI, postoperative time, TAS score, sagittal ACL angle, ACL–Blumensaat line angle, medial PTS, and lateral PTS. ACL, anterior cruciate ligament; BMI, body mass index; PTS, posterior tibial slope; TAS, Tegner activity scale.
TABLE 3.
Multivariate Stepwise Regression Analysis of SNQ Valuesa
| Variable | Regression Coefficient (95% CI) | SE | Standard Regression Coefficient | P Value |
|---|---|---|---|---|
| Total (N = 104) | ||||
| TAS | 6.36 (3.62 to 9.10) | 1.38 | 0.39 | <.001 |
| Follow-up time | −0.25 (−0.39 to −0.11) | 0.07 | −0.31 | <.001 |
| ACL–Blumensaat line angle | −0.39 (−0.68 to −0.11) | 0.14 | −0.23 | .007 |
| Age | 0.34 (0.01 to 0.68) | 0.17 | 0.17 | .046 |
| Allograft (n = 62) | ||||
| Time | −0.55 (−0.80 to −0.29) | 0.13 | −0.45 | <.001 |
| TAS | 8.03 (4.28 to 11.79) | 1.88 | 0.45 | <.001 |
aVariables of the original model included the following: age, BMI, postoperative time, TAS, primary graft diameter, medial PTS, lateral PTS, sagittal ACL angle, and ACL–Blumensaat line angle. ACL, anterior cruciate ligament; BMI, body mass index; PTS, posterior tibial slope; SNQ, signal/noise quotient; TAS, Tegner activity scale.
Discussion
The present study investigated associations between SNQ value and age, BMI, postoperative time, TAS, sagittal ACL angle, ACL–Blumensaat line angle, medial PTS, lateral PTS, and graft diameter in 104 patients. The most significant finding of the present study was that the SNQ value of the reconstructed graft was significantly positively associated with the TAS, which represents physical activity level. Furthermore, graft SNQ had a significant negative association with postoperative time and ACL–Blumensaat line angle.
In this study, graft SNQ was found to have a significant association with postoperative time. Autograft or allograft tendons undergo a healing process after implantation in the knee joint that includes initial avascular necrosis, revascularization, cell repopulation and resynovialization, and finally, remodeling.9,32 The tendon grafts undergo a series of changes, including initial avascular necrosis, revascularization, cellular repopulation and resynovialization, and finally, remodeling. The amount of revascularization tissue influences the MRI signal intensity of the graft, particularly during the first 2 years postoperatively.27 The MRI signal intensity of the graft varies considerably depending on the time interval since surgery.13 Muramatsu et al25 reported that graft SNQ decreased gradually after 1 year postoperatively. More recently, Miyawaki et al24 reported that graft signal intensity has a correlation with postoperative time. Furthermore, there was a significant association between graft SNQ and postoperative time for the autografts in this study. The reason might be due to the fact that the mean follow-up time of the autograft group was 34.4 months in the study. The grafts have no further changes at longer periods after surgery (48-120 months).38 No significant association was seen between graft signal intensity and postoperative time, with a mean follow-up time of 52 to 144 months.31
In this study, a high SNQ value was significantly positively associated with a high activity level. As mentioned before, patients with a higher activity level may have an increased rate of ACL graft failure.3,36 Stratum-specific odds ratios showed a multiplicative interaction between the higher activity level after ACL reconstruction and allograft use, greatly increasing the odds for ACL graft failure.6 Previously, Barrett et al4 found that active patients reconstructed with allografts were 2.6 to 4.2 times more likely to fail compared with less active patients.
In addition, younger patients tend to have higher activity levels, as demonstrated by higher TAS scores. Younger male soccer players are the most likely to return to sports after ACL reconstruction.8,17 Younger patients participated in more strenuous activities both before and after surgery than older patients.33 It was possible that the SNQ value might be associated with age. In this study, there was no association of SNQ values with age, regardless of the graft. This might be explained by the fact that the participants were all older than 22 years. Previously, it was found that patients younger than 20 years are at significantly increased risk for reconstructed graft rupture.36 Interestingly, in the present study, age was an independently correlated factor with graft SNQ value in multivariate stepwise regression analysis. It was presumed that older patients might have an inferior graft maturity.
Furthermore, there was a tendency for the ACL–Blumensaat line angle to be negatively associated with graft SNQ. A small ACL–Blumensaat line angle may cause graft impingement. As reported,18 the high signal intensity of the ACL graft on MRI was found to be caused by graft impingement. ACL implant failure is often caused by bone impingement in knee extension. The location of the drill tunnels is the decisive determinant of graft impingement.15 To prevent impingement and graft reinjury, ACL reconstruction should include anatomic graft placement.7
Admittedly, there were limitations to this study. First, we only included men in this study. Our findings cannot be generalized to women as the associations we found may be sex specific. Future in-depth studies in this field would provide valuable information regarding female patients. Second, there was a lack of baseline value to study the longitudinal change of the graft maturity. It was difficult to accurately measure the graft signal at the time of implantation due to edema or effusion. Furthermore, graft size for these patients is extremely small, with 15% of patients having a 7-mm graft and no patients with a graft >8 mm in our study. Finally, 2 types of fixation (Endobutton CL and Rigidfix cross pins) were employed in the femoral tunnel. The femoral fixation was with the Endobutton CL (n = 20 in the autograft group and n = 27 in the allograft group) or Rigidfix pins (n = 22 in the autograft group and n = 35 in the allograft group). It was thought that the femoral fixation might have minimal effect on the intra-articular site of the graft.
Conclusion
Collectively, the Tegner activity score, postoperative time, and ACL–Blumensaat line angle were significant independent correlated factors of graft signal intensity. Graft SNQ value has a significant positive correlation with physical activity level and a significant negative correlation with postoperative time from 12 to 114 months postoperatively. Patients who were further out from surgery or had lower activity levels had more mature appearing grafts.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This project was subsidized by the 973 Project (No. 2009CB930000) from the Ministry of Science and Technology of China and the Nano project of Shanghai Municipal Science and Technology Commission (1052nm03701).
References
- 1. Ahn JH, Lee SH, Choi SH, Lim TK. Magnetic resonance imaging evaluation of anterior cruciate ligament reconstruction using quadrupled hamstring tendon autografts: comparison of remnant bundle preservation and standard technique. Am J Sports Med. 2010;38:1768–1777. [DOI] [PubMed] [Google Scholar]
- 2. Anderson K, Seneviratne AM, Izawa K, Atkinson BL, Potter HG, Rodeo SA. Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med. 2001;29:689–698. [DOI] [PubMed] [Google Scholar]
- 3. Barrett AM, Craft JA, Replogle WH, Hydrick JM, Barrett GR. Anterior cruciate ligament graft failure: a comparison of graft type based on age and Tegner activity level. Am J Sports Med. 2011;39:2194–2198. [DOI] [PubMed] [Google Scholar]
- 4. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: Tegner activity level and failure rate. Arthroscopy. 2010;26:1593–1601. [DOI] [PubMed] [Google Scholar]
- 5. Biercevicz AM, Miranda DL, Machan JT, Murray MM, Fleming BC. In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am J Sports Med. 2013;41:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borchers JR, Pedroza A, Kaeding C. Activity level and graft type as risk factors for anterior cruciate ligament graft failure: a case-control study. Am J Sports Med. 2009;37:2362–2367. [DOI] [PubMed] [Google Scholar]
- 7. Bowers AL, Bedi A, Lipman JD, et al. Comparison of anterior cruciate ligament tunnel position and graft obliquity with transtibial and anteromedial portal femoral tunnel reaming techniques using high-resolution magnetic resonance imaging. Arthroscopy. 2011;27:1511–1522. [DOI] [PubMed] [Google Scholar]
- 8. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40:2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Claes S, Verdonk P, Forsyth R, Bellemans J. The “ligamentization” process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. Am J Sports Med. 2011;39:2476–2483. [DOI] [PubMed] [Google Scholar]
- 10. Faltstrom A, Hagglund M, Kvist J. Patient-reported knee function, quality of life, and activity level after bilateral anterior cruciate ligament Injuries. Am J Sports Med. 2013;41:2805–2813. [DOI] [PubMed] [Google Scholar]
- 11. Fleming BC, Vajapeyam S, Connolly SA, Magarian EM, Murray MM. The use of magnetic resonance imaging to predict ACL graft structural properties. J Biomech. 2011;44:2843–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38:189–199. [DOI] [PubMed] [Google Scholar]
- 13. Gohil S, Annear PO, Breidahl W. Anterior cruciate ligament reconstruction using autologous double hamstrings: a comparison of standard versus minimal debridement techniques using MRI to assess revascularisation. A randomised prospective study with a one-year follow-up. J Bone Joint Surg Br. 2007;89:1165–1171. [DOI] [PubMed] [Google Scholar]
- 14. Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hui C, Salmon LJ, Kok A, Maeno S, Linklater J, Pinczewski LA. Fifteen-year outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft for “isolated” anterior cruciate ligament tear. Am J Sports Med. 2011;39:89–98. [DOI] [PubMed] [Google Scholar]
- 16. Iriuchishima T, Shirakura K, Horaguchi T, Morimoto Y, Fu FH. Full knee extension magnetic resonance imaging for the evaluation of intercondylar roof impingement after anatomical double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19 (suppl 1):S22–S28. [DOI] [PubMed] [Google Scholar]
- 17. Kamien PM, Hydrick JM, Replogle WH, Go LT, Barrett GR. Age, graft size, and Tegner activity level as predictors of failure in anterior cruciate ligament reconstruction with hamstring autograft. Am J Sports Med. 2013;41:1808–1812. [DOI] [PubMed] [Google Scholar]
- 18. Kanamiya T, Hara M, Naito M. Magnetic resonance evaluation of remodeling process in patellar tendon graft. Clin Orthop Relat Res. 2004;(419):202–206. [DOI] [PubMed] [Google Scholar]
- 19. Kiekara T, Jarvela T, Huhtala H, Paakkala A. MRI of double-bundle ACL reconstruction: evaluation of graft findings. Skeletal Radiol. 2012;41:835–842. [DOI] [PubMed] [Google Scholar]
- 20. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41:2439–2448. [DOI] [PubMed] [Google Scholar]
- 21. Leys T, Salmon L, Waller A, Linklater J, Pinczewski L. Clinical results and risk factors for reinjury 15 years after anterior cruciate ligament reconstruction: a prospective study of hamstring and patellar tendon grafts. Am J Sports Med. 2012;40:595–605. [DOI] [PubMed] [Google Scholar]
- 22. Li H, Tao H, Cho S, Chen S, Yao Z. Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: a comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am J Sports Med. 2012;40:1519–1526. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Hong L, Feng H, et al. Posterior tibial slope influences static anterior tibial translation in anterior cruciate ligament reconstruction: a minimum 2-year follow-up study. Am J Sports Med. 2014;42:927–933. [DOI] [PubMed] [Google Scholar]
- 24. Miyawaki M, Hensler D, Illingworth KD, Irrgang JJ, Fu FH. Signal intensity on magnetic resonance imaging after allograft double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22:1002–1008. [DOI] [PubMed] [Google Scholar]
- 25. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24:1038–1044. [DOI] [PubMed] [Google Scholar]
- 26. Ntoulia A, Papadopoulou F, Ristanis S, Argyropoulou M, Georgoulis AD. Revascularization process of the bone–patellar tendon–bone autograft evaluated by contrast-enhanced magnetic resonance imaging 6 and 12 months after anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39:1478–1486. [DOI] [PubMed] [Google Scholar]
- 27. Ntoulia A, Papadopoulou F, Zampeli F, Ristanis S, Argyropoulou M, Georgoulis A. Evaluation with contrast-enhanced magnetic resonance imaging of the anterior cruciate ligament graft during its healing process: a two-year prospective study. Skeletal Radiol. 2013;42:541–552. [DOI] [PubMed] [Google Scholar]
- 28. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40:1242–1246. [DOI] [PubMed] [Google Scholar]
- 29. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38:1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saupe N, White LM, Chiavaras MM, et al. Anterior cruciate ligament reconstruction grafts: MR imaging features at long-term follow-up—correlation with functional and clinical evaluation. Radiology. 2008;249:581–590. [DOI] [PubMed] [Google Scholar]
- 32. Scheffler SU, Unterhauser FN, Weiler A. Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16:834–842. [DOI] [PubMed] [Google Scholar]
- 33. Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37:246–251. [DOI] [PubMed] [Google Scholar]
- 34. Tanaka Y, Yonetani Y, Shiozaki Y, et al. MRI analysis of single-, double-, and triple-bundle anterior cruciate ligament grafts. Knee Surg Sports Traumatol Arthrosc. 2014;22:1541–1548. [DOI] [PubMed] [Google Scholar]
- 35. van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH. Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med. 2012;40:800–807. [DOI] [PubMed] [Google Scholar]
- 36. Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42:641–647. [DOI] [PubMed] [Google Scholar]
- 37. Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med. 2001;29:751–761. [DOI] [PubMed] [Google Scholar]
- 38. Zaffagnini S, De Pasquale V, Marchesini Reggiani L, et al. Electron microscopy of the remodelling process in hamstring tendon used as ACL graft. Knee Surg Sports Traumatol Arthrosc. 2010;18:1052–1058. [DOI] [PubMed] [Google Scholar]


