Abstract
Background:
Treatment decision making for chondral defects in the knee is multifactorial. Articular cartilage pathology, malalignment, and meniscal deficiency must all be addressed to optimize surgical outcomes.
Purpose:
To determine whether significant clinical improvements in validated clinical outcome scores are observed at minimum 2-year follow-up after articular cartilage repair of focal articular cartilage defects of the lateral compartment of the knee with or without concurrent distal femoral osteotomy and lateral meniscus transplant.
Study Design:
Case series; Level of evidence, 4.
Methods:
Symptomatic adults who underwent surgical treatment (microfracture, autologous chondrocyte implantation [ACI], osteochondral autograft or allograft) of full-thickness lateral compartment chondral defects of the knee with or without a postmeniscectomy compartment or valgus malalignment by a single surgeon with minimum 2-year follow-up were analyzed. Validated patient-reported and surgeon-measured outcomes were collected pre- and postsurgery. Pre- and postoperative outcomes were compared via Student t tests.
Results:
Thirty-five subjects (mean age, 29.6 ± 10.5 years) were analyzed. Patients had been symptomatic for 2.51 ± 3.52 years prior to surgery and had undergone 2.11 ± 1.18 surgeries prior to study enrollment, with a mean duration of follow-up of 3.65 ± 1.71 years. The mean defect size was 4.42 ± 2.06 cm2. Surgeries included ACI (n = 18), osteochondral allograft (n = 14), osteochondral autograft (n = 2), and microfracture (n = 1). There were 18 subjects who underwent concomitant surgery (14 lateral meniscus transplant, 3 distal femoral osteotomy, and 1 combined). Statistically significant (P < .05) and clinically meaningful improvements were observed at final follow-up in Lysholm, subjective International Knee Documentation Committee (IKDS), Knee Injury and Osteoarthritis Outcome Score (KOOS) subscales, Short Form–12 (SF-12) scores, and patient satisfaction. At follow-up, patients undergoing isolated articular cartilage surgery had a significantly higher KOOS quality of life subscore than did those undergoing articular cartilage surgery and lateral meniscus transplant (P = .039). Otherwise, there were no significant postoperative differences between the isolated and combined surgery groups in any outcome score. Five patients underwent 6 reoperations (1 revision osteochondral allograft, 5 chondroplasties). No patient was converted to knee arthroplasty.
Conclusion:
In patients with lateral compartment focal chondral defects with or without lateral meniscal deficiency and valgus malalignment, surgical cartilage repair and correction of concomitant pathology can significantly improve clinical outcomes at 2-year follow-up with no significant differences between isolated and combined surgery and a low rate of complications and reoperations.
Keywords: knee, articular cartilage repair, meniscus transplantation, distal femoral osteotomy, lateral compartment
Full-thickness chondral and osteochondral defects are common sources of knee pain.8 Tibiofemoral (femoral condyles and tibial plateaus) lesions are more common than patellofemoral (patella and trochlea) lesions.8,20,28 Patients with symptomatic full-thickness chondral defects that have failed nonoperative treatment may undergo a variety of cartilage repair or restoration options. If surgical management is chosen, all comorbidities must be addressed. If the ipsilateral compartment is meniscal deficient, then meniscal allograft transplantation is a viable option. If the mechanical axis of the lower extremity preferentially loads or overloads the affected compartment, then an unloading osteotomy is recommended. Ligamentous insufficiency warrants reconstruction.
Outcomes of surgical management have generally demonstrated better results for patients with tibiofemoral defects versus patellofemoral.5,15 Within the tibiofemoral compartment, there is significant biomechanical evidence demonstrating differences between the medial and lateral compartments of the knee.11,12 However, the clinical outcomes are less well characterized.3 The shape of the lateral compartment has been theorized to create a more difficult, less congruent environment to permit cartilage repair.1,3,12,23 Additionally, with the relatively uncommon prevalence of lateral compartment chondral pathology, clinical outcomes of surgical treatment of isolated lateral compartment chondral defects are also reported less frequently. In addition, outcomes of distal femoral osteotomy for valgus malalignment of the lower extremity are not as commonly reported as high tibial osteotomy for varus malalignment.
Thus, the purpose of this investigation was to report the clinical outcomes at minimum 2-year follow-up after treatment of focal articular cartilage defects of the lateral compartment of the knee with or without concurrent distal femoral osteotomy and lateral meniscus transplant. The authors hypothesized that there would be statistically significant and clinically meaningful improvements in validated patient-reported clinical outcome scores (International Knee Documentation Committee [IKDC], Knee Injury and Osteoarthritis Outcome Score [KOOS], Lysholm) at minimum 2-year follow-up. The authors also hypothesized that there would be no significant difference in clinical outcomes between patients undergoing (1) isolated articular cartilage repair without malalignment or meniscal deficiency, (2) articular cartilage repair with distal femoral osteotomy for valgus malalignment without meniscal deficiency, (3) articular cartilage repair with lateral meniscus transplant for lateral meniscus deficiency without malalignment, and (4) articular cartilage repair with lateral meniscus transplant and distal femoral osteotomy for lateral meniscal deficiency and valgus malalignment, respectively. These hypotheses translate to equivalent outcomes between subjects undergoing isolated lateral compartment articular cartilage surgery and subjects undergoing additional concomitant surgeries (lateral meniscus transplant and/or distal femoral osteotomy).
Materials and Methods
Over a 10-year enrollment period (September 2000 to March 2010), patients undergoing surgical treatment of lateral compartment articular cartilage pathology by a single surgeon were identified. Institutional review board approval was obtained. All patients were informed that their information would be published, and patient consent for study participation was obtained. Patients who underwent distal femoral osteotomy for valgus malalignment and/or lateral meniscus transplant for lateral meniscal deficiency were also eligible for analysis. Data were prospectively collected and retrospectively analyzed. Additionally, in patients without recent follow-up, both mail and telephone surveys were utilized for follow-up.
Inclusion criteria were any symptomatic adult (>18 years of age) subject with a full-thickness (International Cartilage Repair Society [ICRS] grade III or IV) chondral defect of the lateral femoral condyle or lateral tibial plateau treated with cartilage repair or restoration techniques (microfracture, autologous chondrocyte implantation [ACI], osteochondral autograft, or osteochondral allograft). Patients were deemed symptomatic if the location of their pain corresponded to the appropriate compartment. Patients with a meniscectomized lateral compartment and/or valgus malalignment were eligible for inclusion. Asymptomatic patients with a known meniscectomized state, chondral pathology, and/or malalignment did not undergo this surgical treatment. Those with uncorrected cruciate and/or collateral deficiency and significant patellofemoral arthrosis or instability were excluded. Furthermore, patients with medial compartment disease, varus malalignment, and/or medial meniscal deficiency were excluded. For this study, the minimum follow-up length was 2 years duration from the date of surgery. Thirty-five subjects met the inclusion criteria and were definitively analyzed.
Prior to index surgery, patients had been symptomatic for 2.51 ± 3.52 years. Surgeries included 24 prior partial lateral meniscectomies (and 1 partial medial meniscectomy), 22 chondroplasties of a femoral condyle chondral defect, 18 prior microfractures, 8 prior loose body removals, 4 prior anterior cruciate ligament reconstructions, 3 prior arthroscopic reductions and internal fixation of an osteochondritis dissecans lesion, 2 lateral meniscal repairs, 1 synthetic plug grafting of an osteochondritis dissecans lesion, and 1 prior arthroscopic removal of hardware. All isolated and combined surgeries were performed at the same setting and simultaneously, not sequentially, staged.
Surgical Technique
If lateral meniscus transplantation was performed, it was the first technique in the procedure because of the significant varus stress required for graft passage, placement, and suture repair. If ACI was performed, it was performed last to avoid disruption of the type I to III collagen membrane or periosteal patch covering the implanted cells. Microfracture and osteochondral autograft or allograft was performed at any point during the surgery. Distal femoral osteotomy was always performed after meniscal transplant to avoid disruption of the osteotomy and internal fixation.
Varus-producing opening wedge distal femoral osteotomy (Figure 1B) was performed for correction of valgus deformity (Figure 1A). Internal fixation was achieved using a low-profile titanium locking plate with 4.5-mm diameter, fully threaded cortical screws proximally and 6.5-mm diameter, fully threaded cancellous screws distally (Femoral Opening Wedge Osteotomy Plate; Arthrex). The opened wedge was packed with local bone graft, demineralized bone matrix (StimuBlast DBM; Arthrex), allograft bone chips, beta–tricalcium phosphate wedges (OSferion [osteoconductive bone graft substitute]; Arthrex), and platelet-rich plasma. Degree of correction was determined preoperatively to unload the lateral compartment to 62% tibial width from the most lateral edge of the lateral compartment (62% width chosen based on valgus-producing high tibial osteotomy for varus malalignment correction literature).9 This corresponds approximately to the medial tibial spine as the desired correction point.
Figure 1.
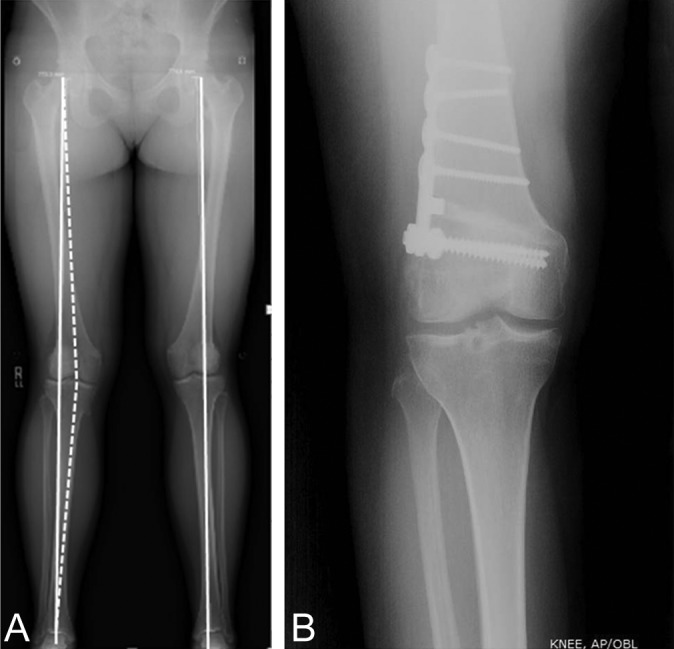
(A) Mechanical axis anteroposterior (AP) standing radiograph with valgus deformity of the right knee. The mechanical axis (from the center of the femoral head to the center of the talus) passes through the middle of the lateral compartment. The desired correction point for the mechanical axis is to 62% the distance from the most lateral aspect of the lateral compartment (approximately the medial tibial spine). (B) Healed postdistal femoral osteotomy AP standing knee radiograph with lateral distal femoral plate and screw construct.
Lateral meniscal transplantation was performed using the bridge-in-slot technique from 2005 to present. Prior to 2005, lateral meniscal transplant was performed via the keyhole technique. Grafts are preserved fresh-frozen. Grafts were placed into a slot 8 mm in width and 10 mm in depth and fixed with a 7 mm–diameter BioComposite Interference Screw (70% poly-l/d-lactic acid [PLDLA], 30% biphasic calcium phosphate; Arthrex). Once seated, the meniscus was repaired inside-out with No. 2-0 high-strength nonabsorbable sutures (Figure 2).
Figure 2.
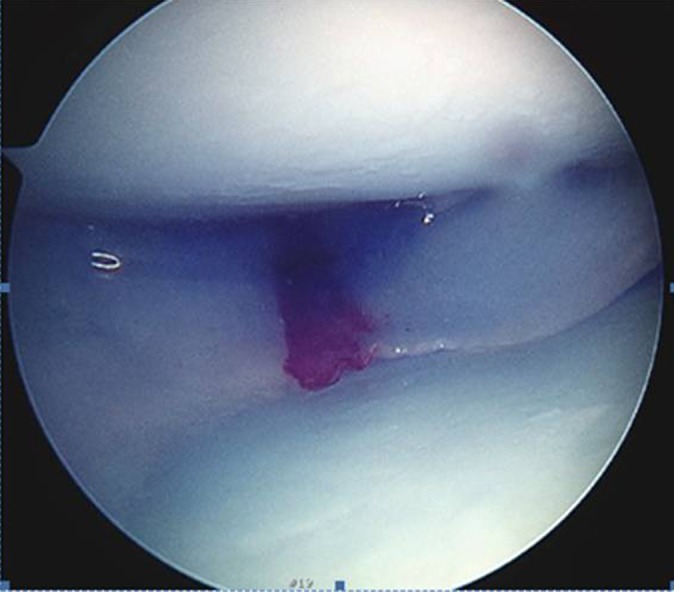
Arthroscopic photograph of lateral meniscal transplant in the right knee. Viewing portal is the anterolateral portal with the knee under a varus stress to open the lateral compartment.
Selection of articular cartilage technique was based on a patient-, limb-, and defect-specific based algorithm.6 This algorithm is multifactorial. However, marrow-stimulation techniques (eg, microfracture) are first-line treatments for smaller lesions (<2-4 cm2) and modest physical demands. In high-demand patients or those that have failed marrow stimulation, osteochondral autograft may be performed in lesions smaller than 2 to 4 cm2. Lesions larger than 2 to 4 cm2 are more often treated with osteochondral allograft or ACI. Shallow lesions, especially in the patellofemoral joint, are more amenable to ACI. Deeper lesions with subchondral bone loss are more amenable to osteochondral allograft. Microfracture was performed using the arthroscopic technique described by Steadman et al.25 Osteochondral autograft was performed using a donor site from the lesser weightbearing medial or lateral trochlea, with press-fit grafting of the plugs flush to the recipient site.14 Osteochondral allograft was performed with fresh dowel allograft (graft age, 14-28 days).10 If secure press-fit fixation was unable to be achieved, a centrally placed bioabsorbable screw (Bio-Compression screw, poly-l-lactic acid [PLLA]; Arthrex) was added for security. ACI was performed via a 2-stage arthroscopic procedure (first stage biopsy, second stage arthrotomy and cell implantation) using a type I to III collagen membrane cover (BioGide; Geistlich Pharma) with suture and fibrin glue fixation.13
Postoperative Rehabilitation
After surgery, patients were placed in a cryotherapy compression cooling device and hinged knee brace. Nonweightbearing precautions were employed for the first 6 postoperative weeks, in addition to 6 hours daily continuous passive motion (CPM). Formal physical therapy was commenced on suture removal approximately 10 days following surgery. Weightbearing was initiated at 6 weeks postoperatively; return to most activities of daily living at 3 months, with cutting and twisting at 4 months (if meniscal transplant performed to permit meniscal healing); return to impact and/or ballistic activities at 6 to 8 months (microfracture and osteochondral autograft), 8 months (osteochondral allograft integration), or 12 months (ACI); and return to activities without restrictions at 12 months.
Clinical outcomes assessed following surgery include the following questionnaires: Short Form–12 (SF-12), IKDC subjective form, KOOS, and Lysholm knee scores. These outcomes were assessed preoperatively, at 6 weeks, and 3, 6, 12, and 24 months after surgery. Additionally, at the time of final follow-up, the patients were contacted via telephone and outcome questionnaires were mailed to the subjects, their results recorded, and sent back for analysis. Descriptive statistics were calculated. Continuous data were reported as mean ± standard deviation, and categorical data reported as frequency. Shapiro-Wilk tests for normality were performed on the outcome score data sets of IKDC, KOOS subscales, Lysholm, and SF-12 physical component summary (PCS) and mental component summary (MCS). These all demonstrated normality. Thus, pre- versus postoperative outcome score comparisons were made using Student t tests. Statistical significance was defined as P value <.05. Clinically meaningful differences in these outcome scores were based on the minimal clinically important difference (MCID) for IKDC subjective score (11.5 in the setting of knee injury), the minimal detectable change (MDC) for KOOS subscales (range, 5-12 in the setting of knee injury), and MDC for Lysholm score (8.9 in the setting of ACL reconstruction).4,7 All statistical analyses were performed using PASW Statistics Student Version 18 (SPSS Inc).
Results
Thirty-five subjects (mean age, 29.6 ± 10.5 years; range, 18-46 years) met inclusion criteria and were analyzed (Table 1). Mean duration of clinical follow-up was 3.65 ± 1.71 years (range, 2-8.5 years). Nearly all defects (97%) were on the lateral femoral condyle. Seventeen (49%) subjects underwent isolated articular cartilage surgery, while 18 underwent combined (with either meniscal transplant and/or distal femoral osteotomy) surgery. Knee extension was –0.05° ± 1.0° preoperatively and –0.07° ± 0.59° postoperatively (P = .995). Knee flexion was 130° ± 10.7° preoperatively and 130° ± 9.3° postoperatively (P = .988). There was no significant difference in range of motion pre- or postoperatively in subjects undergoing and not undergoing osteotomy (P > .05 for both). For all subjects not undergoing osteotomy, mechanical axis alignment was between 2° valgus and 2° varus. For the 4 subjects undergoing osteotomy, the mean correction was 7.5° ± 1.6°.
TABLE 1.
Patient and Surgical Demographics (N = 35 Patients)
| Sex, n | |
| Male | 18 |
| Female | 17 |
| Affected knee, n | |
| Right | 25 |
| Left | 10 |
| Age, y, mean ± SD | 29.6 ± 10.5 |
| Body mass index, kg/m2, mean ± SD | 23.9 ± 4.13 |
| Mass, kg | 74.3 ± 18.6 |
| Height, m | 1.75 ± 0.96 |
| Length of preoperative duration of symptoms, y, mean ± SD | 2.51 ± 3.52 |
| No. of prior surgeries, mean ± SD | 2.11 ± 1.18 |
| Patients with prior surgeries, n | |
| 1 prior | 12 |
| 2 prior | 13 |
| 3 prior | 7 |
| 4 prior | 1 |
| 5 prior | 1 |
| 6 prior | 1 |
| Length of clinical follow-up, y, mean ± SD | 3.65 ± 1.71 |
| Defect area, cm2, mean ± SD | 4.42 ± 2.06 |
| Etiology, n | |
| Chondral defect | 19 |
| Osteochondritis dissecans | 16 |
| Avascular necrosis | 0 |
| Location of articular cartilage defect, n | |
| Lateral femoral condyle | 34 |
| Lateral tibial plateau | 1 |
| Isolated articular cartilage repair, n | 17 |
| Microfracture | 0 |
| Autologous chondrocyte implantation | 8 |
| Osteochondral autograft | 0 |
| Osteochondral allograft | 9 |
| Articular cartilage surgery + lateral meniscus transplant, n | 14 |
| Microfracture | 1 |
| Autologous chondrocyte implantation | 8 |
| Osteochondral autograft | 1 |
| Osteochondral allograft | 4 |
| Articular cartilage surgery + distal femoral osteotomy, n | 3 |
| Microfracture | 0 |
| Autologous chondrocyte implantation | 2 |
| Osteochondral autograft | 0 |
| Osteochondral allograft | 1 |
| Lateral meniscus transplant + distal femoral osteotomy + osteochondral autograft, n | 1 |
Preoperatively, there was no significant difference in any outcome score between subjects undergoing isolated lateral compartment articular cartilage surgery versus articular cartilage surgery and concomitant lateral meniscus transplant. At final follow-up, there were statistically significant and clinically meaningful improvements in IKDC subjective, Lysholm, and all KOOS subscales (vs preoperative scores for all 35 subjects) (Table 2). There were also statistically significant improvements in both the physical and mental components of the SF-12. Tegner activity score at final follow-up was 7.34 ± 2.16. Subjects undergoing isolated articular cartilage surgery had significantly higher KOOS quality of life subscore than did subjects undergoing articular cartilage surgery and concomitant lateral meniscus transplant (69.9 ± 22.7 vs 50.9 ± 25.9, respectively; P = .039). There was no significant difference (P > .05) in lesion size between isolated articular cartilage repair and combined articular cartilage repair and lateral meniscus transplant. There was no significant difference (P > .05) in outcomes between subjects whose defect etiology was a chondral defect versus osteochondritis dissecans. Otherwise, there were no significant postoperative differences (P > .05) between the latter 2 groups in any outcome score.
TABLE 2.
Postoperative Clinical Outcomes, Reoperations, and Complications
| Preoperative | Final Follow-up | P Value | |
|---|---|---|---|
| IKDC subjective | 39.4 ± 16.7 | 72.7 ± 20.4 | <.001 |
| KOOS | |||
| Pain | 60.8 ± 19.8 | 83.8 ± 16.9 | <.001 |
| Symptoms | 61.2 ± 15.2 | 77.1 ± 20.1 | <.001 |
| Activities of daily living | 73.1 ± 22.3 | 92.2 ± 11.5 | <.001 |
| Sport | 16.8 ± 24.9 | 64.4 ± 26.3 | <.001 |
| Quality of life | 27.1 ± 22.1 | 61.6 ± 24.5 | <.001 |
| Lysholm | 47.5 ± 19.4 | 75.1 ± 18.6 | <.001 |
| SF-12 | |||
| Physical component | 40.6 ± 7.57 | 45.0 ± 7.04 | .016 |
| Mental component | 49.6 ± 12.8 | 55.6 ± 8.54 | .026 |
| Satisfaction (0-10) | 5.23 ± 2.12 | 8.05 ± 1.79 | <.01 |
| Tegner | * | 7.34 ± 2.16 | * |
aValues are expressed as mean ± SD. IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; SF-12, Short Form–12. An asterisk indicates that no preoperative Tegner score was available.
Complications and Reoperations
Following surgery, 1 subject who underwent combined distal femoral osteotomy, lateral meniscus transplant, and lateral femoral condyle osteochondral autograft had a superficial wound infection with less than 1 cm of incision dehiscence that responded well to oral antibiotics and local wound care (Table 3). Five subjects underwent 6 reoperations (14% reoperation rate). One subject with 2 reoperations had debridement of an osteochondral allograft and then a revision osteochondral allograft (2.9% revision rate). One subject who had undergone index osteochondral allograft and lateral meniscus transplant underwent a partial lateral meniscectomy and lateral release reoperation. The other 3 patients underwent 1 reoperation each (2 had an index femoral condyle osteochondral allograft and 1 had an index lateral tibial plateau microfracture). No subject was converted to total knee or unicompartmental knee arthroplasty.
TABLE 3.
Reoperations After Surgery
| Reoperations | 6 |
| Total knee arthroplasty | 0 |
| Revision osteochondral allograft | 1a |
| Chondroplasty | 5a |
| Partial lateral meniscectomy | 1b |
| Lateral release | 1b |
aOne subject had undergone prior osteochondral allograft, followed by 2 reoperations (the first for chondroplasty of osteochondral allograft and the second for revision osteochondral allograft).
bOne subject had undergone prior osteochondral allograft and lateral meniscus transplant followed by reoperation for partial lateral meniscectomy of the meniscus transplant and lateral release for lateral patellar tilt.
Discussion
This retrospective case series of 35 patients who underwent lateral compartment articular cartilage repair has demonstrated statistically significant and clinically meaningful improvements in validated patient-reported clinical outcome scores at short-term follow-up, confirming our hypotheses. There was no significant difference in outcomes between patients with isolated defects and those with comorbidities requiring concomitant distal femoral osteotomy and/or lateral meniscus transplant. There was a low rate of complications and reoperations. Furthermore, the revision rate was low, and no patient was converted to arthroplasty.
The management of articular cartilage disease is complex and multifactorial. Comorbidities (malalignment, meniscal deficiency, cruciate and/or collateral ligament insufficiency) are addressed simultaneously or in a staged fashion (osteotomy, meniscal preservation via repair/transplantation, ligament repair/reconstruction). Significant improvements in validated clinical outcomes have been demonstrated for both isolated femoral condyle lesions with or without concurrent procedures.15,16,18,19,22,26 No study exists that exclusively evaluates clinical outcomes for lateral compartment focal chondral defects. However, there is ample literature that assesses the mid- and long-term outcome of varus-producing distal femoral osteotomy for valgus gonarthrosis.2,21,24,27 Varus-producing distal femoral osteotomy for valgus gonarthrosis success ranges from 64% to 82% at 10 years after surgery and drops to 45% at 15 years after surgery. In comparison to subjects undergoing isolated valgus-producing high tibial osteotomy (HTO), HTO with articular cartilage surgery, and HTO with meniscal transplant, survival rates and clinical outcome improvements are similar.18 For patients with varus gonarthrosis, survival was 92%, 85%, and 77% at 5-, 10-, and 15-year follow-up, respectively.18 For patients with medial compartment focal defects and varus malalignment, survival was 98% and 84% at 5 and 10 years, respectively.18 For patients with medial compartment focal defects and medial meniscal deficiency, survival was 91% at 5 years.18 Although the current study lacks the follow-up exhibited in the latter study, future long-term investigations will permit valid comparisons between these 2 patient cohorts.
Two significant difficulties are encountered in comparison of subjects undergoing distal femoral osteotomy for valgus gonarthrosis and those undergoing lateral compartment articular cartilage surgery with or without concomitant osteotomy for focal chondral defects without osteoarthritis: (1) differences in subject populations (eg, age, activity level, return-to-sport goals, medial/patellofemoral compartment disease) and (2) the use of validated focal chondral defect (vs osteoarthritis) clinical outcome scores. Therefore, it is difficult to compare the 2 cohorts. Currently, one must use patient-reported clinical outcome scores with suitable psychometric properties (these include proper development, validity, reliability, and responsiveness) for each condition. Then, one must determine if statistically significant differences in clinical outcome scores are actually perceived by the patient as important. Thus, the improvements in the current investigation are clinically meaningful (IKDC, KOOS subscales, Lysholm).
Limitations
Limitations to this comparative case series include its retrospective design with selection and transfer bias. As the purpose of biological knee reconstruction with articular cartilage repair, with or without meniscus transplantation and unloading osteotomy, is to improve patient symptoms and function and to slow or prevent the progression of degenerative changes in the knee, the follow-up in this study does not represent the mid- or long-term results intended to investigate. In patients undergoing articular cartilage repair, it is difficult to create a blinded controlled study. In fact, it has not been studied in the literature. This is a recognized limitation in articular cartilage repair investigations (derived from a meta-analysis of the articular cartilage repair literature [194 studies; 11,787 subjects]).17 Randomized studies in the articular cartilage literature compare at least 2 separate groups (often microfracture vs a cell-based therapy such as ACI or osteochondral grafting).17 The ideal isolated focal chondral defect control group would be a patient with a neutrally aligned lower extremity, with stable ligaments, normal menisci, and no subchondral bone loss or edema that has a focal full-thickness articular cartilage defect either left alone or minimally debrided (chondroplasty) to stable borders with vertical walls. Although the exclusive group analyzed in this study (lateral compartment of knee) does improve internal validity at the expense of external validity/generalizability, it does not evaluate medial or patellofemoral lesions. Also, beta error is possible in that there were no differences detected between groups analyzed (isolated lateral defect articular cartilage treatment versus combined surgery with meniscus transplant and/or osteotomy). Additionally, although the KOOS sports and recreation subscore demonstrated significant improvements, the specific sports played were unknown, and an isolated postoperative Tegner activity score does not explicitly report the sports played and the subjects’ ability to return to sport. Future research should focus on high-quality, prospective enrollment with randomized design in an appropriately powered study cohort using like surgical techniques and blinded observers for objective (clinical, radiographic, histologic) and subjective evaluation with validated patient-reported clinical outcome scores at long-term follow-up.
Conclusion
In patients with lateral compartment focal chondral defects with or without lateral meniscal deficiency and valgus malalignment, surgical cartilage repair and correction of concomitant pathology can significantly improve clinical outcomes at 2-year follow-up with no significant differences between isolated and combined surgery and a low rate of complications and reoperations.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: F.M.M. receives research support from Zimmer. B.J.C. receives research support from Arthrex, Medipost, National Institutes of Health NIAMS and NICHD, and Zimmer; receives other financial support from Athletico, Ossur, Smith & Nephew, and Tornier; is a paid consultant for Arthrex, Carticept, Regentis, and Zimmer; holds stock/stock options in Carticept and Regentis; and receives royalties from Arthrex, DJO, Elsevier, Saunders/Mosby, and SLACK Inc. G.D.A. is a paid consultant for Cytonics and holds stock in Cytonics.
References
- 1. Alford J, Lewis P, Kang R, Cole B. Rapid progression of chondral disease in the lateral compartment of the knee following meniscectomy. Arthroscopy. 2005;21:1505–1509. [DOI] [PubMed] [Google Scholar]
- 2. Backstein D, Morag G, Hanna S, Safir O, Gross A. Long-term follow-up of distal femoral varus osteotomy of the knee. J Arthroplasty. 2007;22:2–6. [DOI] [PubMed] [Google Scholar]
- 3. Behery OA, Harris JD, Karnes JM, Siston RA, Flanigan DC. Factors influencing the outcome of autologous chondrocyte implantation: a systematic review. J Knee Surg. 2013;26:203–211. [DOI] [PubMed] [Google Scholar]
- 4. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37:890–897. [DOI] [PubMed] [Google Scholar]
- 5. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. [DOI] [PubMed] [Google Scholar]
- 6. Cole B, Pascual-Garrido C, Grumet R. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91:1778–1790. [PubMed] [Google Scholar]
- 7. Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken). 2011;63 (suppl 11):S208–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curl W, Krome J, Gordon E, Rushing J, Smith B, Poehling G. Cartilage injuries: a review of 31,516 knee srthroscopies. Arthroscopy. 1997;13:456–460. [DOI] [PubMed] [Google Scholar]
- 9. Dugdale TW, Noyes FR, Styer D. Preoperative planning for high tibial osteotomy. The effect of lateral tibiofemoral separation and tibiofemoral length. Clin Orthop Relat Res. 1992;(274):248–264. [PubMed] [Google Scholar]
- 10. Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907–914. [DOI] [PubMed] [Google Scholar]
- 11. Flanigan DC, Harris JD, Brockmeier PM, Lathrop RL, Siston RA. The effects of defect size, orientation, and location on subchondral bone contact in oval-shaped experimental articular cartilage defects in a bovine knee model. Knee Surg Sports Traumatol Arthrosc. 2014;22:174–180. [DOI] [PubMed] [Google Scholar]
- 12. Flanigan DC, Harris JD, Brockmeier PM, Siston RA. The effects of lesion size and location on subchondral bone contact in experimental knee articular cartilage defects in a bovine model. Arthroscopy. 2010;26:1655–1661. [DOI] [PubMed] [Google Scholar]
- 13. Gomoll AH, Probst C, Farr J, Cole BJ, Minas T. Use of a type I/III bilayer collagen membrane decreases reoperation rates for symptomatic hypertrophy after autologous chondrocyte implantation. Am J Sports Med. 2009;37(suppl 1):20S–23S. [DOI] [PubMed] [Google Scholar]
- 14. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(suppl 2):25–32. [DOI] [PubMed] [Google Scholar]
- 15. Harris J, Brophy R, Siston R, Flanigan D. Treatment of chondral defects in the athlete’s knee. Arthroscopy. 2010;26:841–852. [DOI] [PubMed] [Google Scholar]
- 16. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy. 2011;27:409–418. [DOI] [PubMed] [Google Scholar]
- 17. Harris JD, Erickson BJ, Abrams GD, et al. Methodologic quality of knee articular cartilage studies. Arthroscopy. 2013;29:1243.e5–1252.e5. [DOI] [PubMed] [Google Scholar]
- 18. Harris JD, McNeilan R, Siston R, Flanigan D. Survival and clinical outcome of isolated high tibial osteotomy and combined biological knee reconstruction. Knee. 2013;20:154–161. [DOI] [PubMed] [Google Scholar]
- 19. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92:2220–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730–734. [DOI] [PubMed] [Google Scholar]
- 21. Kosashvili Y, Safir O, Gross A, Morag G, Lakstein D, Backstein D. Distal femoral varus osteotomy for lateral osteoarthritis of the knee: a minimum ten-year follow-up. Int Orthop. 2009;34:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053–2063. [DOI] [PubMed] [Google Scholar]
- 23. Paletta G, Manning T, Snell E, Parker R, Bergfeld J. The effect of allograft meniscal replacement on intraarticular contact area and pressures in the human knee—a biomechanical study. Am J Sports Med. 1997;25:692–698. [DOI] [PubMed] [Google Scholar]
- 24. Saithna A, Kundra R, Modi CS, Getgood A, Spalding T. Distal femoral varus osteotomy for lateral compartment osteoarthritis in the valgus knee. A systematic review of the literature. Open Orthop J. 2012;6:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steadman J, Briggs K, Rodrigo J, Kocher M, Gill T, Rodkey W. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477–484. [DOI] [PubMed] [Google Scholar]
- 26. Stone KR, Freyer A, Turek T, Walgenbach AW, Wadhwa S, Crues J. Meniscal sizing based on gender, height, and weight. Arthroscopy. 2007;23:503–508. [DOI] [PubMed] [Google Scholar]
- 27. Wang JW, Hsu CC. Distal femoral varus osteotomy for osteoarthritis of the knee. J Bone Joint Surg Am. 2005;87:127–133. [DOI] [PubMed] [Google Scholar]
- 28. Widuchowski W, Lukasik P, Kwiatkowski G, et al. Isolated full thickness chondral injuries. Prevalence and outcome of treatment. A retrospective study of 5233 knee arthroscopies. Acta Chir Orthop Traumatol Cech. 2008;75:382–386. [PubMed] [Google Scholar]


