Abstract
Background:
Optimal treatment of superior labral anterior-posterior (SLAP) tears is controversial, in part because the dynamic role of the long head of the biceps muscle (LHBM) in the glenohumeral joint is unclear. The aim of this study was to determine dynamic LHBM behavior during shoulder activity by studying (1) the electromyographic activity of the LHBM during shoulder motion, (2) the effect of elbow immobilization on this activity, and (3) the effect of a load applied to the distal humerus on this activity.
Hypothesis:
The LHBM would not play a significant role in active glenohumeral range of motion.
Study Design:
Controlled laboratory study.
Methods:
Thirteen normal volunteers underwent surface electromyography (EMG) measurement of the LHBM, short head biceps muscle (SHBM), deltoid, infraspinatus, and brachioradialis during shoulder motion from the neutral position (0° of rotation, flexion, and abduction) to 45° of flexion, 90° of flexion, 45° of abduction, and 90° of abduction. These motions were repeated both with and without splint immobilization of the forearm and elbow at 100° of flexion and neutral rotation and with and without a 1-kg weight placed on the lateral distal humerus.
Results:
Mean EMG activity within the LHBM and the SHBM was low (≤11.6% ± 9.1%). LHBM activity was significant increased by flexion and abduction (P < .049 in all cases), while SHBM activity was not. EMG activity from the middle head of the deltoid was significantly increased by loading with the shoulder positioned away from the body (ie, in abduction or flexion). When compared with the unloaded state, the addition of a distal humeral load significantly increased LHBM activity in 45° of abduction (P = .028) and 90° of flexion (P = .033) despite forearm and elbow immobilization. The SHBM showed similar trends.
Conclusion:
In normal volunteers with forearm and elbow immobilization and application of a load to the distal humerus, LHBM EMG activity is increased by both glenohumeral flexion and abduction, suggesting that this muscle plays a dynamic role in glenohumeral motion with higher demand activities.
Clinical Relevance:
Biceps tenodesis may result in dynamic change within the glenohumeral joint with higher demand activities.
Keywords: labral tear, biceps tendon, long head of biceps tendon, superior labral anterior posterior (SLAP) tear, upper extremity immobilization, electromyography
The role of the long head of the biceps muscle (LHBM) and its proximal tendon (LHBT) within the glenohumeral joint remains unknown. Various authors have proposed that the LHBT may play a role in glenohumeral stability,5,6,10,12 humeral head depression,15 and overall glenohumeral kinematics.14 These studies suggest that efforts should be made to preserve the LHBT in cases of proximal biceps tendon, biceps anchor, and superior labral anterior-posterior (SLAP) pathology. However, other studies have revealed that the LHBT does not play a role in glenohumeral stability4,17 or overall glenohumeral kinematics.16 Clinically, patients experience no definable deficit in shoulder function after biceps tenodesis.7 This evidence suggests that preservation of the pathologic intra-articular portion of the tendon would only serve to leave a potential pain generator within the joint without improving joint kinetics.1
To achieve our general aim to determine the dynamic role of the LHBT in the glenohumeral joint, we performed an electromyographic (EMG) analysis of the LHBM in normal volunteers. Our specific aims were (1) to determine LHBM and short head of the biceps muscle (SHBM) activity during a variety of glenohumeral joint motions, (2) to determine whether forearm and elbow immobilization affects this activity, and (3) to determine whether application of a load to the distal humerus affects this activity.
Our hypothesis was that the LHBM would not demonstrate significant EMG activity during active glenohumeral range of motion. Specifically, we hypothesized that regardless of glenohumeral joint motion (aim 1), LHBM activity would be decreased by forearm and elbow immobilization (aim 2) both with and without load application (aim 3).
Methods
This study was approved by our institutional review board as study protocol No. 11090808. All participants signed informed consent forms and a HIPAA (Health Insurance Portability and Accountability Act) waiver. Our participants were a convenience sample of normal, healthy volunteers without shoulder pain or any history of shoulder pathology. Exclusion criteria included complaints of shoulder pain, prior shoulder injury, or prior shoulder surgery. Participant sex, age, weight, and height were also collected. No participants were aware of the hypothesis of the study. In all cases the dominant extremity was tested. All testing was performed in our human motion analysis laboratory. No pre hoc power analysis was possible as no data exist comparing the immobilized to the nonimmobilized state for this experimental model.
Data Collection
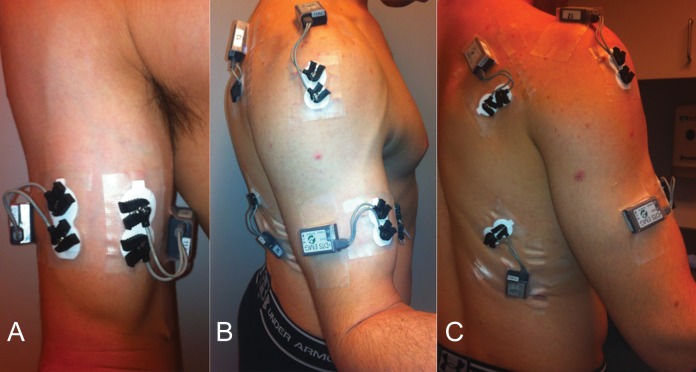
The surface EMG (sEMG) of the muscle activity from each subject was collected using a TeleMyo transmitter and receiver, model 2400T/2400R (Noraxon Inc, Scottsdale, Arizona, USA). Prior to electrode application, the skin was cleaned using antimicrobial wipes.13 Self-adhesive dual Ag/AgCl electrodes (Noraxon Inc) were placed on the palpable muscle bellies of the brachioradialis, LHBM, SHBM, middle head of the deltoid, and infraspinatus muscles in parallel of the muscle fibers at the midpoint of the muscle with the muscle held in midflexion to optimize the signal. Figure 1 shows electrode placement. For the LHBM and short head electrodes, if the bulk of the biceps muscle was split into thirds, the LHBM electrodes lay at the junction of the lateral and middle thirds and the short head electrodes lay at the junction of the middle and medial thirds with a minimum of 3 cm between the short and long head electrodes mediolaterally to avoid cross-talk, as previous described.2,3,14 As an internal check, a cadaveric dissection was performed to confirm that the long and short heads of the biceps had entirely separate muscular fibers until their attachment at the distal tendon. This dissection is shown in Figure 2; the separate heads of the muscle have anatomically distinct fascicles without cross-weaving, with the LHBM being lateral and the SHBM being medial up until they coalesce at the distal tendon. EMG signals were preamplified (500×) near the electrodes, with the band pass filtered between 10 and 500 Hz and sampled at a rate of 1500 Hz.
Figure 1.
This series of clinical photographs shows electrode placement. (A) Anterior view demonstrating electrode placement on the long and short heads of the biceps. (B) Lateral view demonstrating electrode location on the middle head of the deltoid. (C) Posterior view showing electrode placement on the infraspinatus. A latissimus dorsi electrode is also shown, although this electrode was not used for this particular study.
Figure 2.
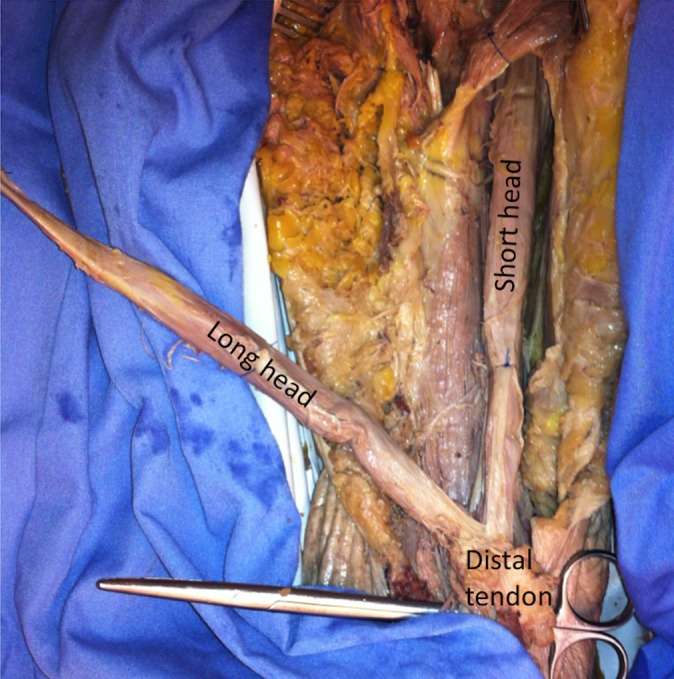
Cadaveric anatomical dissection showing that the long and short heads of the biceps exist as separate fascicles without cross-talk up to their coinsertion at the distal tendon and are thus candidates for separate electromyographic data collection.
Prior to measuring activity with the testing protocol, the maximal amount of muscle activity in each subject’s biceps brachii was determined to serve as an internal control. Three consecutive trials of 3- to 5-second maximal manual muscle testing (MMT) were performed. For both the long and short heads of the biceps brachii, the MMT involves maximal isometric elbow flexion force with the forearm in supination against a fixed flat surface and the elbow flexed at 90°. For the brachioradialis, the MMT is similar except that the forearm is held in neutral rotation. For the deltoid, the MMT involves a maximal shoulder abduction force with the humerus at 90° of abduction and neutral rotation. For the infraspinatus, the MMT involves a maximal external rotation force with the arm in adduction and neutral rotation. Each subject showed activity in the biceps with resisted flexion, and this activity was defined as 100% as follows: The 3- to 5-second interval with the highest sEMG activity was selected as the maximal MMT representing 100% biceps brachii muscle activity (100% MMT) and used to normalize both the SHBM and LHBM activity within each subject. Raw sEMG signals were rectified and smoothened using a root-mean-square algorithm with a window of 300 milliseconds prior to MMT normalization.
Testing Protocol
Once electrodes were placed, the subject was asked to move his or her shoulder to a series of shoulder positions while recording EMG activity with the elbow held at 100° of flexion, the forearm held in neutral rotation, and the wrist held in 20° of extension. These motions included the neutral position (ie, no abduction, elevation, or rotation for baseline) and then the motion from the neutral position to 45° of forward elevation, 90° of forward elevation, 45° of abduction, and 90° of abduction. The subject performed each of these motions in neutral rotation, maximal internal rotation, and maximal external rotation except for the baseline neutral position, which was only performed in neutral rotation. These motions were then repeated with a 1-kg weight applied at the lateral distal humerus.
A well-padded long-arm posterior mold and a “sugar-tong” plaster splint were then applied from the axilla to the metacarpal necks with the arm held in 20° of wrist extension, neutral forearm rotation, and 100° of elbow flexion. A standardized amount of padding plaster and an ACE wrap (Matrix Elastic Bandages; Medline, Mundelein, Illinois, USA) were used to construct each splint: each splint employed one 2-inch roll and three 4-inch rolls of padding (Kendall Webril 100% Cotton Undercast Padding; Covidien Inc, Mansfield, Massachusetts, USA), four rolls of 4-inch plaster (Specialist; Johnson & Johnson, New Brunswick, New Jersey, USA), and one 4- and one 6-inch ACE wrap. The motions were then repeated with the elbow and forearm immobilized. A 1-kg weight was then again applied to the lateral distal humerus with the splint in place, and the motions were repeated.
Statistical Analysis
All analyses were performed in SPSS 18 (IBM Inc, Armonk, New York, USA). After normalization to the MMT, mean EMG activity for each subject and each muscle was calculated for each motion both with and without immobilization and with and without load. Data were tested for normality using the Kolmogorov-Smirnov test, and parametric/nonparametric tests were used as appropriate based on data normality. To confirm accurate electrode placement, function of the data collection apparatus, and data preprocessing, both negative and positive control tests were performed on the data. As a negative control, nonimmobilized and immobilized brachioradialis activity was compared using the Mann-Whitney U test. As the brachioradialis only crosses the elbow joint, its activity should be extinguished by elbow immobilization. As positive controls, infraspinatus activity in external rotation and neutral rotation/internal rotation were compared using the Mann-Whitney U test, and deltoid activity in low and high abduction was compared using the Mann-Whitney U test. To answer our primary hypothesis, we compared LHBM EMG activity between the splinted and nonsplinted states both within the loaded and unloaded states and within each nonrotational activity using Wilcoxon signed-rank tests. We performed a similar analysis of SHBM EMG activity and deltoid EMG activity. We also compared LHBM EMG activity between the loaded and unloaded states within both the splinted and nonsplinted states and within each nonrotational activity using Wilcoxon signed-rank tests. A similar analysis of SHBM and deltoid EMG activity was performed. In addition, we compared LHBM to SHBM activity within each activity and each state to determine which head of the muscle was more active in the glenohumeral joint. We also compared LHBM, SHBM, and deltoid activity between the neutral position and each flexion and abduction motion to determine whether these motions increased activity within these muscles. Results are expressed as mean ± standard deviation.
Results
Thirteen subjects aged 26.2 ± 4.2 years, with a weight of 76.9 ± 15.0 kg and height of 177.8 ± 9.3 cm, participated in this study. There were 4 female and 9 male participants. No correlations were found between subject sex, height, or weight and LHBM/SHBM activity, although activity was found to positively correlate with age (Pearson r = 0.593 and 0.643, P = .033 and .018, respectively).
All positive controls were confirmatory: (1) The 90° of abduction motion had significantly more deltoid activation than the 45° of abduction motion (7.3% ± 4.2% vs 15.7% ± 7.8%; P < .001.) and (2) the external rotation motions had significantly more infraspinatus activity than the internal rotation motions (27.7% ± 27.1% vs 14.1% ± 15.2%; P < .001).
Our negative control, however, did not behave as expected. Splint application significantly increased brachioradialis activity (4.9% ± 2.7% vs 3.3% ± 2.2%; P < .001.) Overall activity was low for both the splinted and nonsplinted states.
With regard to aim 1, in the nonimmobilized and unloaded state, LHBM, deltoid, and SHBM activity were all highest with motion to 90° of abduction, with respective mean activities of 8.8% ± 4.5%, 11.4% ± 4.7%, and 3.8% ± 3.4%. Abduction and flexion both increased LHBM activity over the neutral position regardless of immobilization or loading (P < .049 in all cases) (Tables 1 and 2). With a few rare exceptions, neither abduction nor flexion increased SHBM activity (Tables 1 and 2). Both abduction and flexion increased deltoid activity (P < .024 in all cases) (Tables 1 and 2). The highest mean activities were observed after splinting and loading; for both LHBM and SHBM, these were observed at 90° of flexion at 11.6% ± 9.1% and 8.8% ± 7.7%, respectively, while for the deltoid, the highest mean activity was 18.4% ± 4.5% at 90° of abduction. LHBM activity was greater than SHBM activity in the nonsplinted state with flexion, abduction, unloading, and loading (P < .043 for all motions except for 90° of flexion after load application [P = .061]) (Table 3). In the splinted state, no differences were seen in LHBM and SHBM activity (P > .05 in all cases).
TABLE 1.
EMG Activity for the LHBM and SHBM Normalized to Maximal Manual Muscular Testing for Each Shoulder Positiona
| EMG Activity, % | ||||
|---|---|---|---|---|
| Nonloaded State | Loaded State | |||
| Shoulder Position/Muscle | Nonsplinted | Splinted | Nonsplinted | Splinted |
| Neutral | ||||
| LHBM | 4.2 ± 1.8 | 5.7 ± 3.8 | 5.3 ± 3.3 | 5.2 ± 3.4 |
| SHBM | 2.8 ± 2.2 | 5.2 ± 5.5 | 4.3 ± 4.3 | 5.0 ± 5.7 |
| Deltoid | 1.0 ± 0.9 | 1.1 ± 0.8 | 1.5 ± 1.4 | 1.3 ± 1.3 |
| 45° of abduction | ||||
| LHBM | 5.4 ± 2.7 | 7.8 ± 5.1 | 7.2 ± 4.0 | 9.4 ± 7.0 |
| SHBM | 2.9 ± 2.5 | 6.0 ± 5.6 | 4.2 ± 3.7 | 6.4 ± 4.6 |
| Deltoid | 5.1 ± 2.3 | 7.2 ± 4.0 | 7.2 ± 2.5 | 9.5 ± 6.1 |
| 90° of abduction | ||||
| LHBM | 8.8 ± 4.5 | 10.6 ± 7.4 | 11.5 ± 6.9 | 11.3 ± 8.0 |
| SHBM | 3.8 ± 3.4 | 6.8 ± 5.6 | 5.0 ± 4.1 | 6.4 ± 4.8 |
| Deltoid | 11.4 ± 4.7 | 16.7 ± 9.1 | 16.5 ± 7.0 | 18.4 ± 8.5 |
| 45° of flexion | ||||
| LHBM | 5.8 ± 2.6 | 7.4 ± 4.4 | 7.1 ± 3.4 | 8.0 ± 5.1 |
| SHBM | 3.5 ± 3.2 | 6.3 ±5.9 | 4.7 ± 3.8 | 6.7 ± 5.8 |
| Deltoid | 2.0 ± 1.3 | 2.4 ± 1.8 | 3.1 ± 2.3 | 5.3 ± 6.8 |
| 90° of flexion | ||||
| LHBM | 7.1 ± 4.0 | 9.4 ± 5.5 | 9.3 ± 5.2 | 11.6 ± 9.1 |
| SHBM | 3.7 ± 3.8 | 6.9 ± 6.1 | 5.7 ± 4.6 | 8.8 ± 7.7 |
| Deltoid | 4.5 ± 2.0 | 5.2 ± 3.0 | 6.6 ± 4.0 | 10.6 ± 12.2 |
aValues are expressed as mean ± standard deviation. EMG, electromyography; LHBM, long head of the biceps muscle; SHBM, short head of the biceps muscle.
TABLE 2.
P Values for Comparison of Baseline Activity in the Neutral Position With Activity for Each Muscle and Shoulder Positiona
| Nonloaded State | Loaded State | |||
|---|---|---|---|---|
| Baseline Activity vs Neutral Position | Nonsplinted | Splinted | Nonsplinted | Splinted |
| LHBM | ||||
| 45° of flexion | .001 | .002 | .001 | .003 |
| 90° of flexion | .002 | .005 | .001 | .005 |
| 45° of abduction | .003 | .049 | .012 | .005 |
| 90° of abduction | .001 | .005 | .003 | .003 |
| SHBM | ||||
| 45° of flexion | .071 | .002 | .532 | .020 |
| 90° of flexion | .150 | .020 | .126 | .008 |
| 45° of abduction | .850 | .099 | .926 | .123 |
| 90° of abduction | .062 | .031 | .299 | .088 |
| Middle head of the deltoid muscle | ||||
| 45° of flexion | .008 | .004 | .001 | .024 |
| 90° of flexion | .001 | .001 | .001 | .010 |
| 45° of abduction | .001 | .001 | .001 | .001 |
| 90° of abduction | .001 | .001 | .001 | .001 |
aBoldfaced values indicate statistical significance (P ≤ .05, paired Student t test). LHBM, long head of the biceps muscle; SHBM, short head of the biceps muscle.
TABLE 3.
P Values for Comparison of LHBM Activity With SHBM Activitya
| Shoulder Position | |||||
|---|---|---|---|---|---|
| Testing State, LHBM vs SHBM | Neutral | 45° of Abduction | 90° of Abduction | 45° of Flexion | 90° of Flexion |
| Nonsplinted, nonloaded | .077 | .017 | .002 | .017 | .017 |
| Splinted, nonloaded | .270 | .228 | .144 | .293 | .174 |
| Nonsplinted, loaded | .106 | .043 | .004 | .043 | .061 |
| Splinted, loaded | .555 | .293 | .130 | .317 | .369 |
aBoldfaced values indicate statistical significance (P ≤ .05, Mann-Whitney U test). LHBM, long head of the biceps muscle; SHBM, short head of the biceps muscle.
With regard to aim 2, immobilization of the elbow and forearm did not affect LHBM EMG activity regardless of activity or within loaded or unloaded states (Tables 1 and 4, Figure 3). Immobilization of the elbow and forearm increased SHBM EMG activity in all motions and combinations of load except for the neutral position with load (Tables 1 and 4, Figure 4). A similar trend was found for the middle head of the deltoid muscle in the plane of its action (eg, abduction) (Tables 1 and 4, Figure 5).
TABLE 4.
P Values for EMG Activity Between Splinted/Nonsplinted (Within Each Loading State) and Loaded/Nonloaded (Within Each Splinting State) Conditionsa
| Shoulder Position | |||||
|---|---|---|---|---|---|
| EMG Activity by Testing State | Neutral | 45° of Abduction | 90° of Abduction | 45° of Flexion | 90° of Flexion |
| LHBM | |||||
| Nonloaded, splinted vs not | .064 | .075 | .173 | .087 | .116 |
| Loaded, splinted vs not | .972 | .133 | .701 | .650 | .600 |
| Nonsplinted, loaded vs not | .046 | .002 | .006 | .016 | .003 |
| Splinted, loaded vs not | .196 | .028 | .463 | .463 | .033 |
| SHBM | |||||
| Nonloaded, Splinted vs not | .016 | .001 | .003 | .011 | .001 |
| Loaded, Splinted vs not | .807 | .004 | .019 | .033 | .028 |
| Nonsplinted, loaded vs not | .039 | .011 | .009 | .016 | .001 |
| Splinted, loaded vs not | .382 | .033 | .279 | .650 | .023 |
| Middle head of the deltoid muscle | |||||
| Nonloaded, splinted vs not | .046 | .011 | .002 | .055 | .345 |
| Loaded, splinted vs not | .101 | .133 | .173 | .173 | .075 |
| Nonsplinted, loaded vs not | .075 | .002 | .001 | .013 | .009 |
| Splinted, loaded vs not | .507 | .033 | .152 | .013 | .007 |
aBoldfaced values indicate statistical significance (P ≤ .05, related-samples Wilcoxon signed rank comparison). LHBM, long head of the biceps muscle; SHBM, short head of the biceps muscle.
Figure 3.
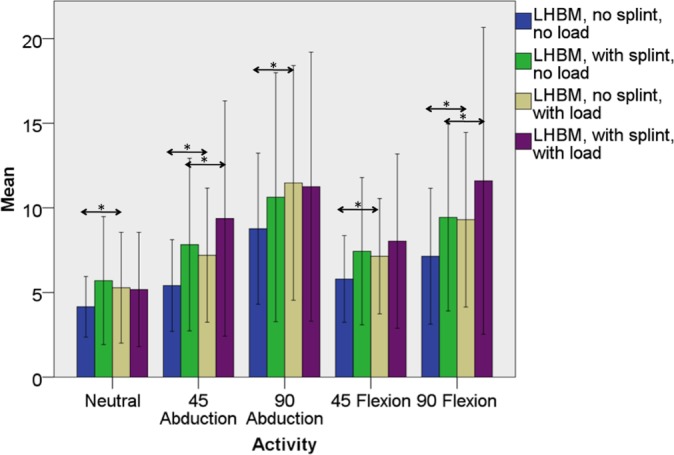
Mean maximal manual testing–normalized percent electromyographic (EMG) activity in the long head of the biceps muscle (LHBM) both with and without splint immobilization and both with and without elbow loading with the shoulder in the neutral position and with motion to 45° of abduction, 90° of abduction, 45° of forward flexion, and 90° of forward flexion. Significant differences between mean EMG activity in the splinted and nonsplinted (within the loaded and unloaded states) and loaded and unloaded (within the splinted and nonsplinted states) are denoted by asterisks. Error bars represent 1 standard deviation.
Figure 4.
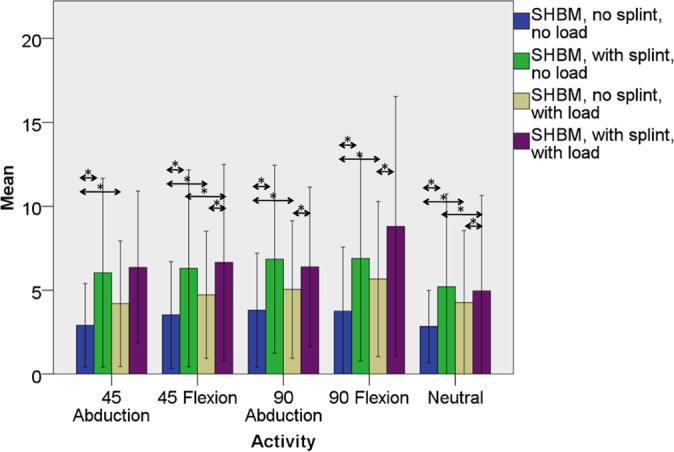
Mean maximal manual testing–normalized percent electromyographic (EMG) activity in the short head of the biceps muscle (SHBM) both with and without splint immobilization and both with and without elbow loading with the shoulder in the neutral position and with motion to 45° of abduction, 90° of abduction, 45° of forward flexion, and 90° of forward flexion. Significant differences between mean EMG activity in the splinted and nonsplinted (within the loaded and unloaded states) and loaded and unloaded (within the splinted and nonsplinted states) are denoted by asterisks. Error bars represent 1 standard deviation.
Figure 5.
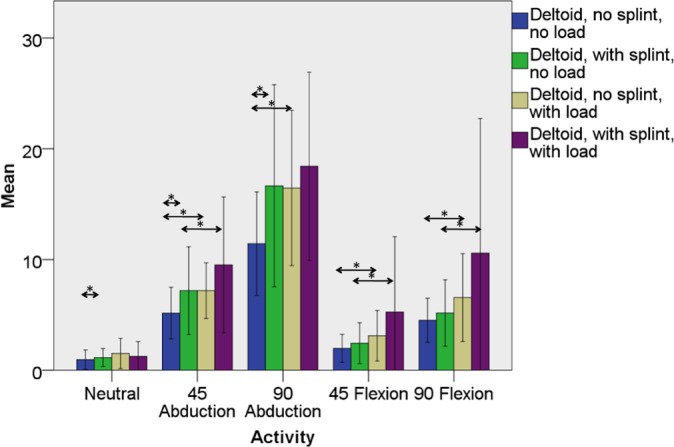
Mean maximal manual testing–normalized percent electromyographic (EMG) activity in the middle head of the deltoid muscle both with and without splint immobilization and both with and without elbow loading with the shoulder in the neutral position and with motion to 45° of abduction, 90° of abduction, 45° of forward flexion, and 90° of forward flexion. Significant differences between mean EMG activity in the splinted and nonsplinted (within the loaded and unloaded states) and loaded and unloaded (within the splinted and nonsplinted states) are denoted by asterisks. Error bars represent 1 standard deviation.
With regard to aim 3, loading increased LHBM EMG activity in the nonimmobilized state, regardless of motion (Table 1, Figure 3). When compared with the unloaded state, the addition of a distal humeral load increased LHBM activity, even in the immobilized state, in all planes of motion except the neutral position, with statistical significance in 45° of abduction (P = .028) and 90° of flexion (P = .033) (Table 1, Figure 3). The SHBM showed similar trends: loading increased SHBM activity in the nonimmobilized state in all cases but only increased activity in the splinted state with the arm held in 45° of abduction (P = .033) and 90° of flexion (P = .023) (Tables 1 and 4, Figure 4). EMG activity from the middle head of the deltoid was significantly increased by loading with the shoulder moving away from the body (eg, in abduction or flexion) regardless of elbow and forearm immobilization, except for 90° of abduction in the splinted state (Tables 1 and 4, Figure 5).
Discussion
The LHBT and its anchor on the superior labrum is a common source of pathology and is thus frequently managed with tenotomy or tenodesis without any known resultant clinical deficit on shoulder function.1,7,8,11 As a result, previous studies have called into question the dynamic role of the LHBM in the glenohumeral joint.4,7,16,17 However, other authors have demonstrated a static and dynamic role for the LHBT in glenohumeral motion and stability.5,6,9,12,14,15 These studies suggest that tenodesis may have subtle biomechanical consequences, and thus, the tendon should be preserved whenever possible. To address this issue, we performed an EMG analysis of LHBM and SHBM activity during a variety of shoulder motions both with and without elbow and forearm immobilization to neutralize LHBM activity as an elbow flexor and forearm supinator. Trends in LHBM activity were then compared with trends in SHBM and deltoid activity. Positive controls validated that our experimental model functioned as expected.
With respect to aim 1, our experimental model replicated expected physiologic activity for the LHBM, the SHBM, and the deltoid muscles. LHBM activity was increased by flexion and abduction while SHBM activity was not, suggesting that the LHBM is a flexor and stabilizer of the glenohumeral articulation, a not unexpected finding as the muscle passes anterior to the axis of the joint. After the application of a load, deltoid activity was highest in 90° of abduction, confirming the action of this muscle as an abductor.
With respect to aim 2, our hypothesis remains untested as our experimental model did not function as predicted by previous studies.14 Immobilization of the elbow and forearm did not affect LHBM EMG activity regardless of the plane of motion or load application. Immobilization of the elbow and forearm increased SHBM EMG activity in almost all planes of motion and combinations of load. A similar trend was found for the middle head of the deltoid muscle in the plane of its action (eg, abduction) (Figure 4). Because the deltoid does not cross the elbow and should not be affected by elbow and forearm immobilization, these results suggest that the weight of the plaster splint served to increase forces across the shoulder and thus led to a paradoxical increase in EMG activity in the deltoid with immobilization. A similar increase was also observed for our negative control, the brachioradialis, which may undergo increased isometric contraction due to the additional weight of the splint, although in both the splinted and nonsplinted states, brachioradialis activity was very low.
With respect to aim 3, we must reject our hypothesis and therefore must conclude that, with a load applied, the LHBM is active with glenohumeral motion despite forearm and elbow immobilization. Motion to 45° of abduction (P = .028) and 90° of flexion (P = .033) increased LHBM activity despite elbow and forearm immobilization with loading. This increase in activity with shoulder motion suggests that with higher demand activities, for example, loading with the arm held in abduction or flexion, the LHBM may be important for dynamic stabilization. Similarly, without forearm and elbow immobilization, LHBM activity was increased by application of a distal humeral load in almost all motions. The SHBM demonstrated a similar role, with loading increasing activity despite elbow and forearm immobilization with the arm held in 45° of abduction (P = .033) and 90° of flexion (P = .023). As a confirmation, these trends mirror those observed in the deltoid, which is known to play a critical role in glenohumeral abduction.
Several previous studies have been conducted to determine the electromyographic activity of the long head of the biceps in glenohumeral motion. Yamaguchi et al16 performed an EMG comparison of the LHBM function in normal patients and patients with rotator cuff tears during immobilization in an elbow brace. These authors described little to no EMG activity within the biceps with the arm taken through a variety of motions, leading the authors to conclude that that the LHBM does not play an active role in the glenohumeral joint.16 However, subsequent authors have called these findings into question as no load was applied, no control is available (ie, a nonimmobilized test phase), and the method of immobilization may be insufficient (a brace still allows trace flexion/extension and pronation/supination while a splint or cast provides superior immobilization).14 Indeed, a subsequent study performed using a similar protocol with an elbow brace for immobilization demonstrated high biceps activity in a variety of shoulder positions, calling these findings into question.14 The results from our splinted and nonimmobilized trial numerically mirror those of Yamaguchi et al16; however, the addition of a nonsplinted control group and a loaded group alters the interpretation of their results. Increased LHBM activity was seen with glenohumeral motion despite forearm and elbow immobilization once a load was applied. As confirmation, nonsplinted LHBM activity was significantly higher than nonsplinted SHBM activity, and LHBM activity was increased by flexion and abduction, both of which confirm LHBM activity in glenohumeral motion. With higher demand activities, the LHBM is active in the glenohumeral joint independent of the forearm and elbow. Yamaguchi et al16 insufficiently stressed the glenohumeral joint to show this activity and thus incorrectly concluded there was no activity.
The significance and function imparted by this activity remain unclear. While this activity may suggest an etiology for degenerative instability of the biceps anchor via repetitive traction stress, further research will be necessary to determine if the LHBM plays a role in glenohumeral stability. In addition, a dynamic role for the LHBM in glenohumeral motion does not suggest that the LHBT should be preserved in treatment of pathology of the proximal biceps tendon, biceps anchor, and superior labrum.
This study has several limitations. Our use of normal controls may not replicate the role of the LHBM in those patients under consideration for tenodesis. Ideally, these findings would be replicated in a population of patients with SLAP tears, bicipital tendonitis, and/or lesions of the biceps pulley. Surface EMG also has limitations, especially for muscles in close anatomic proximity such as the SHBM and LHBM. Ideally, this study would be replicated with fine-wire EMG. However, multiple previous studies have described the use of sEMG for the LHBM and SHBM,2,3,14 and multiple steps were taken in this study to separate these muscles including electrode spacing and an anatomical dissection to confirm anatomically distinct fascicles. In addition, the lack of a significant difference in several of our comparisons may be because of the relatively small number of subjects included in our study—in particular, the lack of a difference between the loaded and unloaded states for LHBM activity at 90° of abduction and 45°. A study with more subjects may better address this question. Given that significant differences were found in the primary comparisons in our study and the null hypothesis was rejected, the study is currently adequately powered, and type 2 error has not occurred.
Conclusion
In normal volunteers with forearm and elbow immobilization, application of a load to the distal humerus increases LHBM EMG activity in both the flexed and abducted position, suggesting that this muscle plays a dynamic role in glenohumeral motion with higher demand activities.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was funded by grants from the Arthroscopy Association of North America and KFx Medical Inc. B.J.C. receives royalties from Arthrex and DJ Orthopaedics; is a paid consultant for Arthrex, DJ Orthopaedics, Johnson & Johnson, Regentis, and Zimmer; has stock or stock options in Carticept and Regentis; receives research support from Johnson & Johnson, Medipost, and Zimmer; receives publication royalties from Elsevier, Lippincott, Smith & Nephew, and WB Saunders; serves on the boards of the American Academy of Orthopaedic Surgery, the American Journal of Orthopaedics, the American Journal of Sports Medicine, Cartilage, the Education Committee of the Arthroscopy Association of North America, Elsevier, the International Committee of the Arthroscopy Association of North America, the Journal of Bone and Joint Surgery, the Journal of Shoulder and Elbow Surgery, and the Arthroscopy Association of North America. A.A.R. receives royalties from Arthrex; serves on the speaker’s bureau for Arthrex; serves as a paid consultant for Arthrex; receives research support from Arthrex, DJO Surgical, Smith & Nephew, and Ossur; receives other financial support from Arthrex and DJO Surgical; receives publication royalties from Saunders/Mosby-Elsevier; serves on the boards of the Journal of Shoulder and Elbow Surgery, SLACK Inc, Orthopedics Today, Orthopedics, Sports Health, Techniques in Shoulder and Elbow Surgery, Operative Techniques in Sports Medicine, the Orthopaedic Journal of Sports Medicine, The American Orthopaedic Society for Sports Medicine, the American Shoulder and Elbow Surgeons, and the Arthroscopy Association of North America. N.N.V. receives royalties from Smith & Nephew; serves on the speaker’s bureau for Arthrosurface; serves as a paid consultant for Smith & Nephew and Arthrex; has stock or stock options in Omeros; receives research support from Arthrex, Smith & Nephew, Athletico, Conmed Linvatec, Miomed, Mitek, and Arthrosurface; receives publication royalties from Vindico Medical, Orthopedics Hyperguide, and Arthroscopy; serves on the boards for the Journal of Knee Surgery, Arthroscopy, SLACK Inc, and the Arthroscopy Association of North America Learning Center Committee.
References
- 1. Boileau P, Parratte S, Chuinard C, Roussanne Y, Shia D, Bicknell R. Arthroscopic treatment of isolated type II SLAP lesions: biceps tenodesis as an alternative to reinsertion. Am J Sports Med. 2009;37:929–936. [DOI] [PubMed] [Google Scholar]
- 2. Brown JM, Solomon C, Paton M. Further evidence of functional differentiation within biceps brachii. Electromyogr Clin Neurophysiol. 1993;33:301–309. [PubMed] [Google Scholar]
- 3. Dupont L, Gamet D, Pérot C. Motor unit recruitment and EMG power spectra during ramp contractions of a bifunctional muscle. J Electromyogr Kinesiol. 2000;10:217–224. [DOI] [PubMed] [Google Scholar]
- 4. Hawkes DH, Alizadehkhaiyat O, Fisher AC, Kemp GJ, Roebuck MM, Frostick SP. Normal shoulder muscular activation and co-ordination during a shoulder elevation task based on activities of daily living: an electromyographic study. J Orthop Res. 2011;30:53–60. [DOI] [PubMed] [Google Scholar]
- 5. McMahon PJ, Burkart A, Musahl V, Debski RE. Glenohumeral translations are increased after a type II superior labrum anterior-posterior lesion: a cadaveric study of severity of passive stabilizer injury. J Shoulder Elbow Surg. 2004;13:39–44. [DOI] [PubMed] [Google Scholar]
- 6. Morgan CD, Burkhart SS, Palmeri M, Gillespie M. Type II SLAP lesions: three subtypes and their relationships to superior instability and rotator cuff tears. Arthroscopy. 1998;14:553–565. [DOI] [PubMed] [Google Scholar]
- 7. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19:764–768. [DOI] [PubMed] [Google Scholar]
- 8. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18:645–656. [DOI] [PubMed] [Google Scholar]
- 9. Pagnani MJ, Deng XH, Warren RF, Torzilli PA, O’Brien SJ. Role of the long head of the biceps brachii in glenohumeral stability: a biomechanical study in cadavera. J Shoulder Elbow Surg. 1996;5:255–262. [DOI] [PubMed] [Google Scholar]
- 10. Pagnani MJ, Warren RF, Altchek DW, Wickiewicz TL, Anderson AF. Arthroscopic shoulder stabilization using transglenoid sutures: a four-year minimum followup. Am J Sports Med. 1996;24:459–467. [DOI] [PubMed] [Google Scholar]
- 11. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16:170–176. [DOI] [PubMed] [Google Scholar]
- 12. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22:121–130. [DOI] [PubMed] [Google Scholar]
- 13. Rojas IL, Provencher MT, Bhatia S, et al. Biceps activity during windmill softball pitching: injury implications and comparison with overhand throwing. Am J Sports Med. 2009;37:558–565. [DOI] [PubMed] [Google Scholar]
- 14. Sakurai G, Ozaki J, Tomita Y, Nishimoto K, Tamai S. Electromyographic analysis of shoulder joint function of the biceps brachii muscle during isometric contraction. Clin Orthop Relat Res. 1998;(354):123–131. [DOI] [PubMed] [Google Scholar]
- 15. Warner JJ, McMahon PJ. The role of the long head of the biceps brachii in superior stability of the glenohumeral joint. J Bone Joint Surg Am. 1995;77:366–372. [DOI] [PubMed] [Google Scholar]
- 16. Yamaguchi K, Riew KD, Galatz LM, Syme JA, Neviaser RJ. Biceps activity during shoulder motion: an electromyographic analysis. Clin Orthop Relat Res. 1997;(336):122–129. [DOI] [PubMed] [Google Scholar]
- 17. Youm T, Tibone JE, ElAttrache NS, McGarry MH, Lee TQ. Simulated type II superior labral anterior posterior lesions do not alter the path of glenohumeral articulation: a cadaveric biomechanical study. Am J Sports Med. 2008;36:767–774. [DOI] [PubMed] [Google Scholar]