Abstract
Background: Children with positive islet autoantibodies monitored prospectively avoid metabolic decompensation at type 1 diabetes (T1D) diagnosis. However, the effects of early diagnosis and treatment on preservation of insulin secretion and long-term metabolic control are unknown. We compared characteristics of children detected through research screening (Diabetes Autoimmunity Study in the Young [DAISY]) versus community controls at baseline and, in a subset, 6- and 12-month metabolic outcomes.
Materials and Methods: This was a case-control study comparing DAISY children with T1D to children diagnosed in the general community. All participants underwent mixed-meal tolerance testing; a subset wore a continuous glucose monitoring (CGM) device. Fasting and stimulated C-peptide levels, insulin dose-adjusted hemoglobin A1c (IDAA1c), and CGM variables were compared.
Results: Children (21 DAISY, 21 community) were enrolled and matched by age, time of diagnosis, and diabetes duration; 18 were enrolled within 2 months and 24 within 2.5 years on average from diagnosis. In the overall group and the subgroup of participants enrolled 2.5 years from diagnosis, there were no IDAA1c or C-peptide differences between DAISY versus community children. The subgroup of DAISY versus community children enrolled near diagnosis, however, had lower baseline hemoglobin A1c (6.5±1.4% vs. 9.2±2.9%; P=0.0007) and IDAA1c (7.4±2.1% vs. 11.2±3.5%; P=0.04) and higher stimulated C-peptide (2.5±0.5 vs. 1.6±0.2 ng/mL; P=0.02). In this subgroup, IDAA1c differences persisted at 6 months but not at 1 year. CGM analyses revealed lower minimum overnight glycemia in community children (72 vs. 119 mg/dL; P=0.01).
Conclusions: Favorable patterns of IDAA1c and C-peptide seen in research-screened versus community-diagnosed children with T1D within 2 months of diagnosis are no longer apparent 1 year from diagnosis.
Introduction
Type 1 diabetes (T1D) is a chronic autoimmune disease characterized by a preclinical period identifiable by the presence of diabetes-associated autoantibodies to insulin, 65-kDa glutamic acid decarboxylase (GAD), islet antigen-2 (IA-2), and zinc transporter isoform 8 (ZnT8).1 Prospective studies such as the Diabetes Autoimmunity Study in the Young (DAISY), BABYDIAB, the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study, The Environmental Determinants of Diabetes in the Young (TEDDY), and TrialNet have shown that positive autoantibodies identify children at a very high risk for the development of T1D,1–3 especially when two or more antibodies are present.4 Our group has previously demonstrated that children diagnosed through DAISY have a lower hemoglobin A1c (HbA1c) level at onset and through the first month of therapy, a lower insulin dose through the first year of therapy, and decreased hospitalization rates for diabetic ketoacidosis compared with patients diagnosed in the general community.5
C-peptide is a marker of the pancreas's native ability to produce insulin. In the Diabetes Prevention Trial-Type 1 (DPT-1), C-peptide levels were higher in those diagnosed by surveillance compared with those in the general community,6 although data are only available up to 3 months following diagnosis. The Diabetes Complications and Control Trial (DCCT) found that patients with sustained C-peptide production have lower HbA1c levels and lower rates of hypoglycemia, microalbuminuria, and retinopathy.7–9
Rates of C-peptide decline described in the literature are variable. Most data are derived from the control arms of intervention trials, and considerable heterogeneity among individual patients has been reported, with ranges from 0% to 58% decline in stimulated C-peptide levels during the first year after diagnosis.10 The duration of C-peptide persistence is variable as well, and one report described evidence of residual β-cell function up to 50 years after diagnosis.10 The Type 1 Diabetes TrialNet Study Group found that 93% of individuals have detectable C-peptide at least 2 years from diagnosis; however, the study subjects in that report had a mean HbA1c level of 6.5% (48 mmol/mol) on entry and 7.6% (60 mmol/mol) at 2 years and may not reflect the natural history of C-peptide change in the general T1D population.11 The DCCT found that half of the patients screened for the trial up to 5 years from diagnosis had detectable stimulated C-peptide levels.12 Whether or not early detection and treatment of diabetes through surveillance studies preserve β-cell function compared with individuals diagnosed in the general community is unknown.
This study was designed to follow a group of DAISY children diagnosed with T1D to test the hypothesis that earlier detection and treatment are associated with prolonged C-peptide production. We hypothesized that screening for diabetes and early treatment would result in higher C-peptide levels at diagnosis and slower C-peptide decline over time, which will be associated with better glycemic control and lower glycemic variability in the early years after diagnosis.
Materials and Methods
Study population and design
This is a case-control study where children diagnosed with T1D through the prospective DAISY study are compared with patients diagnosed with T1D from the general community and followed at the Barbara Davis Center for Childhood Diabetes in Aurora, CO. DAISY has been following 2,547 children at high risk for T1D, including 1,424 general population children with susceptibility human leukocyte antigen (HLA) genes for T1D and 1,123 children with a first-degree relative with T1D. The details of screening and follow-up in DAISY have been previously published.13
This substudy became available June 2010 to DAISY participants diagnosed after July 2006. DAISY cases were eligible if they had a DAISY follow-up visit within 1 year prior to diagnosis of diabetes. At the time of this analysis, 31 DAISY participants had been diagnosed with T1D since July 2006 and were eligible for this substudy. For those who agreed to participate, the first study visit occurred as soon as possible after 1 month from diagnosis, when autoantibody results are available and metabolic control is generally attained. Those recruited around diagnosis are followed at 6 months, 12 months, and then annually until C-peptide is undetectable. DAISY subjects within 4 years of T1D diagnosis when this study started in 2010 were also recruited as soon as possible and followed a similar visit schedule (i.e., 6 months after diagnosis [if applicable] and then annually). Fasting blood draws and stimulated C-peptide levels are obtained at each visit. All participants were provided the optional opportunity to wear a continuous glucose monitoring (CGM) device for 7 days.
Children diagnosed with T1D from the general community and seen at the Barbara Davis Center were informed of the study and identified as eligible based on matching with the DAISY cases on age (±1 year), time of diagnosis (±3 months), and duration of diabetes. Subjects diagnosed with T1D in the community were eligible if they had at least one positive islet autoantibody to insulin, GAD, IA-2, or ZnT8. They followed the same visit schedule as described for DAISY cases.
Diabetes was diagnosed according to American Diabetes Association criteria (i.e., with symptoms of diabetes and blood glucose level of ≥200 mg/dL, random plasma glucose level of ≥200 mg/dL at least twice, or abnormalities on oral glucose tolerance testing with fasting glucose level of ≥126 mg/dL and/or 2-h glucose level of ≥200 mg/dL at least twice). The presence or absence of diabetes symptoms at diagnosis (polyuria, polydipsia, nocturia, fatigue, weight loss, and/or increased appetite) was noted by chart review.
Preservation of C-peptide over time is the primary outcome. Secondary outcomes include changes in HbA1c, insulin dose, insulin dose-adjusted HbA1c (IDAA1c), number of blood glucose tests per day, and CGM variables. Participants are followed annually until C-peptide is undetectable. This article reports characteristics from all subjects as well as enrollment and 6- and 12-month follow-up data in the subgroup enrolled shortly after diagnosis.
Informed consent was obtained from the parents of each study subject. The Colorado Multiple Institutional Review Board approved all study protocols.
Study visits
Participants were instructed to fast for 8 h prior to the study visit. The mixed-meal tolerance test (MMTT) was conducted only if the glucometer fasting glucose level was between 60 and 300 mg/dL and ketone levels were <0.6 mmol/L. Participants were instructed to withhold short-acting insulin up to 4 h before the test. Participants on an insulin pump were instructed to continue their normal basal rate without bolusing within 4 h before the test. A fasting blood sample was drawn for measurement of HbA1c, glucose, C-peptide, and ketones. The modified MMTT, which consists of a standardized liquid meal (Boost® High Protein; Nestle Health Care Nutrition, Inc., Florham Park, NJ), 6 mL/kg to a maximum of 360 mL, was ingested within 5 min, and a blood sample for C-peptide was collected at 60 min. HbA1c was measured in-house on the DCA™ 2000 Vantage analyzer (Siemens Medical Solutions, Tarrytown, NY), a DCCT-aligned device, and C-peptide (in ng/mL) was measured by enzyme-linked immunosorbent assay (Alpco, Salem, NH) in the Clinical Immunology Laboratory at the Barbara Davis Center. Glucometer downloads were assessed to determine the average number of blood glucose tests performed daily.
CGM
Participants were asked to complete a 7-day period of CGM immediately after the visit. For this study, the SEVEN Plus® system (DexCom, San Diego, CA) CGM, which is Food and Drug Administration approved for 7 days of use, was used. A minimum of 72 h of CGM data was required for inclusion of CGM data into the analysis.
Statistical analysis
Statistical analyses were performed using SAS software version 9.2 (SAS Institute, Cary, NC). Because we have matched case-control sets, we used paired analysis to evaluate differences in means and a matched χ2 statistic to assess for differences in proportions, with both approaches allowing for correlation within matched case-control sets. The Wilcoxon rank-sum test was used to test differences in medians for skewed variables. IDAA1C, an alternate measure of residual β-cell function,14 was calculated as HbA1c (%)+(4×insulin dose [in units/kg/day]).
Participants enrolled 2 months from diagnosis were compared separately from those enrolled an average of 2.6±1.5 years from diagnosis. Longitudinal data were available at 6 months and 12 months from diagnosis in the former group. A mixed model was used for longitudinal analyses,15 with a covariance structure accounting for correlation within the matched pair and within individual across time (0, 6, or 12 months).
CGM data were analyzed for those participants who agreed to wear a CGM device. The Wilcoxon–Mann–Whitney test was used to determine if differences existed between DAISY and community children at enrollment. To account for an initial period of calibration, the first 12 h of data was excluded from each tracing. Subjects with fewer than 72 h of data were excluded. If more than 20% of recordings were missing on any given day, the data for that day were also excluded. Measures of glycemic control included the overall mean of glucose values, percentage of time spent >200 mg/dL, percentage of time spent <70 mg/dL, and area under the curve of glucose calculated by the trapezoidal rule. Primary variables to characterize glycemic variability included glucose range, the overall SD, and the coefficient of variation. A two-tailed P value with an α level for significance was set at 0.05. Although multiple additional computed measures have been proposed in this dynamically evolving analytical field of CGM analysis, they do not appear to offer a particular advantage, and we limited the number of comparisons performed to minimize the chance of type 1 error.
Results
Of the 31 eligible DAISY participants, a total of 21 agreed to participate in this study, and an additional 21 community children have enrolled to date (Supplementary Fig. S1; Supplementary Data are available at www.liebertonline.com/dia). There were no differences in demographic characteristics (age, gender, body mass index, family history of T1D, HbA1c at onset) between DAISY children who enrolled or declined this substudy. Table 1 lists demographic data and enrollment characteristics of the overall study cohort. HbA1c differences between DAISY compared with community children in this study were noted at diagnosis: 7.5±2.3% (58±25 mmol/mol) versus 12.9±2.1% (117±23 mmol/mol) (P<0.001). As expected, a greater number of DAISY children, based on DAISY selection criteria, had a family history of T1D and the high-risk HLA DR3/4, DQB1*0302 genotype. Community participants overall tested blood glucose levels more frequently than DAISY children (5.6 vs. 3.7 tests/day; P=0.02). However, there were no significant differences in HbA1c, IDAA1c, or C-peptide measures at time of study enrollment in the overall study cohort.
Table 1.
Comparison of Demographic and Baseline Data in the Entire Study Cohort and the Cohort Enrolled at Diagnosis
| Entire cohort (n=42) | Cohort enrolled at diagnosis (n=18) | |||||
|---|---|---|---|---|---|---|
| DAISY (n=21) | Community (n=21) | P value | DAISY (n=9) | Community (n=9) | P value | |
| Age (years) | 13.0±2.3 | 12.4±3.7 | 0.62 | 12.1±1.7 | 10.3±3.9 | 1.00a |
| Male [n (%)] | 12 (57) | 12 (57) | 1 | 6 (66.7) | 7 (77.8) | 1.00 |
| BMI z-score | 0.03±0.9 | 0.3±1.0 | 0.33 | 0.2±0.7 | 0.08±1.4 | 0.73a |
| Family history of type 1 diabetes [n (%)] | 12 (57) | 2 (9.5) | 0.002b | 5 (55.6) | 0 (0) | NA |
| HLA DR 3/4, DQB1*0302 [n (%)] | 11 (52) | 5 (24) | 0.03b | 3 (33) | 2 (22) | 1.00 |
| Antibody levels | ||||||
| GAD | 0.1 (0.2) | 0.2 (0.2) | 0.14 | 0.17 (0.3) | 0.12 (0.1) | 1 |
| IA-2 | 0.4 (0.4) | 0.5 (0.4) | 0.62 | 0.29 (0.3) | 0.23 (0.2) | 0.2 |
| mIAA | 0.05 (0.1) | 0.01 (0.02) | 0.12 | 0.08 (0.2) | 0.01 (0.01) | 0.1 |
| ZnT8 | NA | NA | 0.31 (0.4) | 0.06 (0.1) | 0.02 | |
| Number of positive diabetes antibodies at onset [mean (SD)] | 2.1 (1.0) | 1.8 (1.0) | 0.16 | 2.9 (0.6) | 1.7 (1.2) | 0.07 |
| HbA1c [% (mmol/mol]) at onset | 7.5±2.3 (58±25) | 12.9±2.1 (117±23) | 0.0002ab | 7.3±2.2 (56±24) | 13.7±1.7 (126±19) | 0.04ab |
| Years from onset | 1.5±1.6 | 1.6±1.7 | 0.19a | 0.1±0.1 | 0.2±0.1 | 1.00a |
| HbA1c (% [mmol/mol]) at study enrollment | 7.9±2.0 (63±22) | 9±2.3 (75±25) | 0.38a | 6.5±1.4 (48±15) | 9.2±2.9 (77±32) | 0.04ab |
| Glucose tests/day | 3.7±0.9 | 5.6±1.9 | 0.02b | 3.2±0.9 | 5.2±1.1 | 0.12a |
| Insulin dose (units/kg/day) | 0.6±0.4 | 0.7±0.3 | 0.43 | 0.2±0.2 | 0.5±0.3 | 0.04ab |
| IDAA1Cc | 10.3±3.6 | 11.7±2.9 | 0.15 | 7.4±2.1 | 11.2±3.5 | 0.02ab |
| C-peptide (ng/mL) | ||||||
| Fasting | 0.8±0.9 | 0.7±0.6 | 0.86 | 1.2±0.6 | 1.1±0.3 | 0.73d |
| 60-min | 1.5±1.4 | 1.1±0.7 | 0.78 | 2.5±0.5 | 1.6±0.2 | 0.047bd |
Data are mean±SD values unless specified otherwise.
P value for Wilcoxon rank-sum test.
Difference is significant.
Insulin dose-adjusted hemoglobin A1c (IDAA1C), calculated as hemoglobin A1c (HbA1c) (in %)+(4×insulin dose [in units/kg/day]).
P value for geometric means.
DAISY, Diabetes Autoimmunity Study in the Young; GAD, glutamic acid decarboxylase; HLA, human leukocyte antigen; IA-2, islet antigen-2; mIAA, micro-insulin autoantibody; NA, not applicable; ZnT8, zinc transporter isoform 8.
Comparisons were then performed based on time to enrollment from diagnosis, separating those enrolled within 6 months from diagnosis from those enrolled ≥6 months from diagnosis. Of the total of 42 subjects enrolled since study onset in June 2010, 18 enrolled shortly after diagnosis (average, 2.0±1.0 months; range, 0.1–0.5 years), and 24 enrolled an average of 2.6±1.5 years (range, 0.5–5.6 years) from diagnosis (subjects diagnosed between July 2006 and June 2010). For those 18 children enrolled an average of 2 months from diagnosis (Table 1), DAISY participants had a lower HbA1c level at onset (7.3% [56 mmol/mol] vs. 13.7% [126 mmol/mol]; P=0.04) and study enrollment (6.5±1.4% [48±15.3 mmol/mol] vs. 9.2±2.9% [77±31.7 mmol/mol]; P=0.0007). These research-screened children also had a higher stimulated C-peptide level (2.7±1.3 vs. 1.7±0.5 ng/mL; P=0.02), lower insulin dose (0.2±0.2 vs. 0.5±0.3 units/kg/day; P=0.007), and lower IDAA1c (7.4±2.1% vs. 11.2±3.5%; P=0.0003) compared with community participants enrolled shortly after diagnosis (Table 2). These differences between DAISY and community groups were no longer seen in children examined an average of 2.6±1.5 years from diagnosis. In this cohort (n=24), fasting C-peptide (0.3±0.3 vs. 0.3±0.2 ng/mL; P=0.98) and stimulated C-peptide (0.6±0.5 vs. 0.6±0.3 ng/mL; P=0.98) levels between DAISY versus community children were not different, and longitudinal data are not reported.
Table 2.
Comparison of Metabolic Outcomes at Baseline, 6 Months, and 12 Months in Those Enrolled Close to Diagnosis
| At enrollment | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DAISY (n=9) | Community (n=9) | P value | DAISY (n=9) | Community (n=9) | P value | DAISY (n=9) | Community (n=9) | P value | |
| BMI z-score | 0.2±0.7 | 0.1±1.4 | 0.83 | 0.1±0.7 | 0.3±1.0 | 0.65 | 0.4±0.8 | 0.4±0.7 | 0.92 |
| HbA1c (%) | 6.5±1.4 | 9.2±2.9 | 0.0007 | 6.5±1 | 7.8±2.3 | 0.075 | 7.9±0.7 | 8.3±1.5 | 0.56 |
| Glucose tests/day | 3.2±0.9 | 5.2±1.1 | 0.01 | 3.6±1.8 | 4.6±1.0 | 0.046 | 4.9±2.2 | 4.2±1.2 | 0.29 |
| Insulin dose (units/kg/day) | 0.2±0.2 | 0.5±0.3 | 0.007 | 0.3±0.2 | 0.6±0.2 | 0.004 | 0.6±0.3 | 0.8±0.2 | 0.13 |
| IDAA1Ca | 7.4±2.1 | 11.2±3.5 | 0.0003 | 7.6±1.7 | 10.2±2.8 | 0.009 | 10.4±1.5 | 11.3±1.6 | 0.25 |
| C-peptide (ng/mL) | |||||||||
| Fasting | 1.4±1.1 | 1.2±0.5 | 0.32 | 1.0±0.7 | 1.0±0.4 | 0.79 | 1.2±1.1 | 0.8±0.5 | 0.16 |
| 60-min | 2.7±1.3 | 1.7±0.5 | 0.02 | 1.3±0.8 | 1.2±0.4 | 0.78 | 1.8±1.6 | 1.2±0.6 | 0.12 |
Data are mean±SD values unless specified otherwise. P values are derived from mixed-model analysis.
Insulin dose-adjusted hemoglobin A1c (IDAA1C), calculated as hemoglobin A1c (HbA1c) (in %)+(4×insulin dose [in units/kg/day]).
BMI, body mass index; DAISY, Diabetes Autoimmunity Study in the Young.
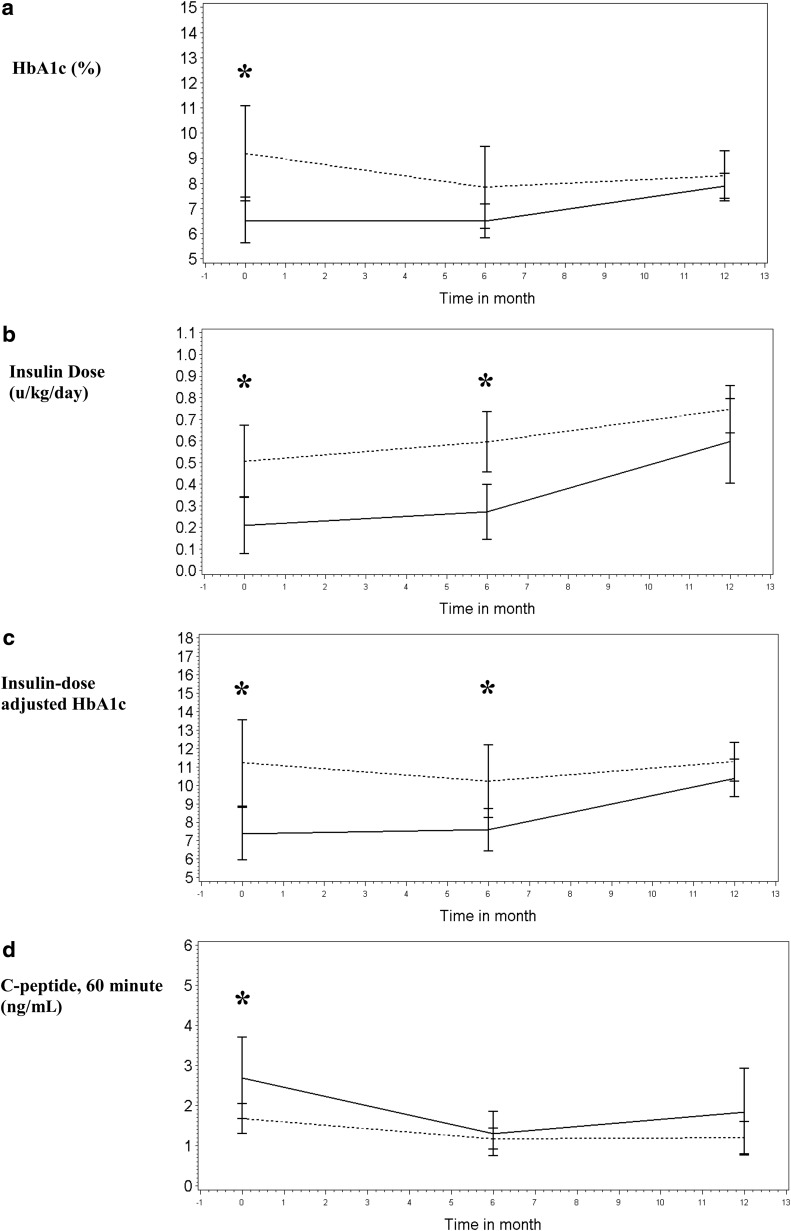
Longitudinal data were available at 6 months and 12 months following enrollment in the 18 children enrolled close to diagnosis (Table 2). At 6 months, although insulin dose and IDAA1c were significantly lower in DAISY children (P<0.01), C-peptide differences were no longer seen, and by 12 months, neither IDAA1c nor C-peptide level was significantly different in this small subgroup. Figure 1 presents means with SE bars for metabolic outcomes of interest. The decline in HbA1c level in community participants between 0 and 6 months, reflecting the honeymoon period, is not observed in DAISY participants, who maintain a stable, low HbA1c level from diagnosis. The HbA1c level also rises to a greater degree in DAISY versus community subjects so that the two groups are not significantly different at 6 and 12 months. Insulin dose similarly rises to a greater degree between 6 and 12 months in DAISY subjects, so that IDAA1c is not different between groups by 12 months. Fasting C-peptide levels (data not shown) declined at a fairly stable rate in both DAISY and community subjects and were not significantly different between the groups at any time point. The stimulated C-peptide level was higher in DAISY children at enrollment but decreased more rapidly by 6 months so that there were no differences between DAISY and community children by 6 and 12 months.
FIG. 1.
Baseline (0 months) and 6- and 12-month outcomes for DAISY (-----) versus community (- - -) participants enrolled close to diagnosis: (a) hemoglobin A1c (HbA1c), (b) insulin dose, (c) insulin dose-adjusted HbA1c, and (d) C-peptide. Data are mean±SE (error bars) values. The x-axis gives time in months; the y-axis represents variable units. *P<0.05.
Medical records of these 18 children enrolled close to diagnosis were reviewed, and the presence of the following diabetes symptoms was noted: polyuria, polydipsia, weight loss, nocturia, increased appetite, and fatigue. Of the nine DAISY children in this subgroup, five were asymptomatic at diagnosis. Four had up to three of the above-listed symptoms. All nine community children were symptomatic, with six having three or more symptoms and three having at least two diabetes symptoms at diagnosis. None of the DAISY children had diabetic ketoacidosis, whereas three of the community children were in diabetic ketoacidosis (pH<7.3; bicarbonate <18 mmol/L) at diagnosis.
Of the 42 participants, 28 agreed to wear a CGM device after the initial study visit. Analysis was limited to participants with a minimum of 72 h of complete CGM data (n=23). Of these 23 subjects, 12 were DAISY children, and 11 were community children. These participants were no longer matched pairs; 50% of the DAISY subjects and 36% of the community subjects wearing a CGM device were enrolled <6 months from diagnosis. Exactly 72 h of CGM data was compared in all subjects. CGM variables and baseline characteristics of these children are summarized in Supplementary Table S1. Time frames were selected based on typical patient lifestyles to define daytime (6 a.m.–12 a.m.) and nighttime (12 a.m.–6 a.m.) periods. CGM results showed that minimum overnight sensor glucose values were lower in community children compared with DAISY subjects (72 vs. 119 mg/dL; P=0.01). No other significant differences in CGM variables were seen.
Discussion
This is the first study in children designed to address whether earlier diagnosis and treatment of T1D through surveillance studies may preserve endogenous insulin secretion and long-term metabolic control as measured by C-peptide, IDAA1c, and CGM outcomes. The overall cohort was subdivided into two groups of DAISY and community children: those recruited an average of 2.0 months from diagnosis and those recruited an average of 2.6 years from diagnosis. This report presents enrollment characteristics of the overall cohort and longitudinal data, up to 12 months, in the subset enrolled close to diagnosis. Favorable metabolic outcomes in DAISY versus community children were only noted in this subgroup enrolled close to diagnosis—lower HbA1c level, insulin dose, and IDAA1c and higher stimulated C-peptide level—and likely reflect greater β-cell reserve, consistent with diagnosis earlier in the evolution of the Eisenbarth model of T1D.16 The presence of fewer diabetes symptoms in this cohort also indicates that these children were diagnosed earlier in the disease course. By an average 2.6 years from diagnosis, these differences between DAISY and community children are no longer seen.
Longitudinal outcomes at 6 and 12 months were analyzed in the 18 children enrolled an average of 2 months from diagnosis. Although longitudinal follow-up for the overall group is ongoing, the favorable findings in stimulated C-peptide and IDAA1c results seen in this subgroup of DAISY children at enrollment are no longer evident 1 year later. Several factors have been proposed (but not confirmed) to affect the rate of loss of β-cell function in patients with T1D, including age of onset, degree of metabolic control, immune status, genetic factors, and individual variation.17–19 Because of DAISY selection criteria, DAISY children had higher frequency of the HLA DR3/4, DQB1*0302 genotype. In the subgroup enrolled close to diagnosis, mean available ZnT8 levels were higher at onset in DAISY compared with community children. The number of positive autoantibodies (GAD, IA-2, and insulin autoantibodies) was not different between cohorts. Participant numbers here are small, and whether or not autoantibody and HLA differences affect rates of endogenous insulin loss remains unclear11,17,20 and merits further study.
Participants in this study are matched by age of onset and duration of diabetes. Data previously reported by our group found that HbA1c differences between DAISY and community children were no longer apparent 6 months after onset.5 However, the children in this previous report were not followed prospectively or matched by duration of disease from diagnosis; in addition, they had no measures of C-peptide or CGM outcomes, both of which were evaluated in this study. Although research screening decreases hospitalization rates and diabetic keotacidosis at diagnosis, the findings reported here suggest that early diagnosis and treatment do not interrupt disease progression and β-cell decline.
It is notable that there were no differences in fasting C-peptide level between DAISY and community participants at any time point in this study, in contrast to higher fasting and peak C-peptide levels at diagnosis in DPT-1 subjects compared with community controls as described in a review by Palmer.10 The preserved fasting C-peptide levels noted in DPT-1 subjects during the 30 months prior to diabetes diagnosis, despite gradually declining peak C-peptide levels during this period,21 imply that the peak C-peptide level tends to decline before changes in fasting C-peptide level are detectable. The lack of difference in fasting C-peptide in our sample suggests that our sample size is too small to detect a difference between DAISY and community controls at enrollment, that community controls were detected relatively early in the course of disease compared with the historic community controls reported by Palmer,10 or that fasting C-peptide is not as good of a measure of β-cell function as peak C-peptide. Additionally, trends in stimulated C-peptide values over time may be more important than the fasting C-peptide level alone as the DCCT analyses found a stronger association with outcomes with stimulated compared with fasting C-peptide levels.9
Through the DCCT, it has been shown that patients who have sustained production of C-peptide have lower rates of severe hypoglycemia, microalbuminuria, and retinopathy regardless of being in the intensively treated or conventionally treated cohorts.22 In addition, intensive treatment in the DCCT prolonged production of C-peptide in the cohort with C-peptide production at baseline. This observation has led to the statement that good control begets good control (i.e., that the production of C-peptide is associated with easier diabetes care), which in turn is associated with a sustained production of C-peptide. Of note is that a more recent study assessing the impact of early intensive therapy immediately after diagnosis with sensor-augmented pump therapy did not show benefit on preservation of β-cell function at 1 year.23 These recent findings may reflect the overall advances in insulin and glucose monitoring technology that have improved routine management for patients with diabetes overall.
Variable rates of C-peptide level decline from diabetes onset have been described. Most of these data are derived from the placebo arm of randomized controlled trials assessing drug interventions in newly diagnosed children with T1D. Several studies have reported modest reductions at 1 year24,25 and up to a 50% decline in stimulated C-peptide level over the first year following diagnosis.26–28 Analysis of DPT-1 C-peptide data in participants monitored prior to diabetes diagnosis found rapid decreases in C-peptide levels (14%) in the 6 months leading up to diagnosis, with an even greater decline of 24% between diagnosis and the 3 months following diagnosis. TrialNet data on C-peptide production up to 2 years from diagnosis describe a biphasic decline in C-peptide levels, with a steeper slope of decline occurring the first 12 months from diagnosis and then flattening between 12 and 24 months.11 Longer-term follow-up is ongoing, but our data to date demonstrate a stimulated C-peptide level decline in the first 6 and 12 months in both DAISY and community children, with a more rapid decline in DAISY children within the first 6 months from diagnosis. Further comparisons of the slope of C-peptide decline are ongoing as participants progress further from diagnosis.
In addition to HbA1c and C-peptide data, CGM data are collected at each visit to monitor additional aspects of metabolic control, including glucose variability and frequency of hypoglycemia. Because of the smaller sample of participants who wore CGM, these individuals were not separated into those enrolled at time of diagnosis compared with those enrolled later in the course of the disease. Overall comparisons found that variability as measured by sensor glucose SD and coefficient of variation was not significantly different between DAISY and community-diagnosed participants. Nighttime minimum glucose values were lower in community children, despite similar HbA1c levels and more frequent daily blood glucose tests. The higher C-peptide levels in DAISY children enrolled close to diagnosis, likely reflecting earlier detection of disease and greater β-cell reserve, may explain the differences detected in overnight low blood glucose levels on CGM.
Limitations of this report include the small sample size of children studied, in particular the cohort of individuals enrolled at time of diagnosis. Despite the small sample size, baseline differences in HbA1c, IDAA1c, and stimulated C-peptide levels were demonstrated between DAISY and community children enrolled close to diagnosis. However, neither the comparisons of C-peptide production in the cohort enrolled 2.6 years from diagnosis nor longitudinal comparisons in the early enrollment group demonstrated sustained differences in C-peptide production. Either a larger sample size is needed to detect differences, or earlier detection and treatment do not confer a metabolic advantage. Additionally, a modified MMTT was performed instead of the traditional 2-h MMTT. The C-peptide level usually peaks by 30–60 min on MMTT early in the course of T1D; the time to peak, however, often increases with progressive loss of β-cell function. For consistency with larger epidemiologic studies such as SEARCH and for practical reasons, a modified MMTT with fasting and 60-min stimulated C-peptide measurement was performed.
In summary, this is the first study to address if early detection of T1D through surveillance studies can preserve β-cell function in youth compared with individuals diagnosed in the community. When enrolled shortly after diagnosis, children diagnosed by research screening had more favorable patterns of IDAA1c, HbA1c, insulin dose, and C-peptide; however, these differences were no longer seen by 12 months. Therefore, any advantages of earlier diagnosis through surveillance studies on metabolic parameters appear to wane shortly after diagnosis, implying the need for early clinical intervention trials on β-cell preservation. Confirmation of these findings in larger prospective studies is required.
Supplementary Material
Acknowledgments
This research was supported by grants DK32493, DK32083, DK050979, DK57516, 5K12DK094712-04, and 5T32DK063687-09 from the National Institutes of Health and grant 17-2013-535 from the JDRF. We gratefully acknowledge the study participants and their families. We thank Laura Pyle, PhD, University of Colorado, for her statistical assistance.
Author Disclosure Statement
No competing financial interests exist.
C.L.C. takes full responsibility for the contents of the article.
References
- 1.Barker JM, Barriga KJ, Yu L, et al. : Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89:3896–3902 [DOI] [PubMed] [Google Scholar]
- 2.The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci 2008;1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. : The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 4.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. : Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JM, Goehrig SH, Barriga K, et al. : Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care 2004;27:1399–1404 [DOI] [PubMed] [Google Scholar]
- 6.Sosenko JM, Palmer JP, Rafkin-Mervis L, et al. : Glucose and C-peptide changes in the perionset period of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2008;31:2188–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Diabetes Control and Complications Trial Research Group: Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the Diabetes Control and Complications Trial. A randomized, controlled trial. Ann Intern Med 1998;128:517–523 [DOI] [PubMed] [Google Scholar]
- 8.The DCCT Research Group: Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab 1987;65:30–36 [DOI] [PubMed] [Google Scholar]
- 9.Lachin JM, McGee P, Palmer JP, et al. : Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 2014;63:739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer JP: C-peptide in the natural history of type 1 diabetes. Diabetes Metab Res Rev 2009;25:325–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenbaum CJ, Beam CA, Boulware D, et al. : Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite type 1 Diabetes TrialNet data. Diabetes 2012;61:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer JP, Fleming GA, Greenbaum CJ, et al. : C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 2004;53:250–264 [DOI] [PubMed] [Google Scholar]
- 13.Rewers M, Bugawan TL, Norris JM, et al. : Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 1996;39:807–812 [DOI] [PubMed] [Google Scholar]
- 14.Mortensen HB, Hougaard P, Swift P, et al. : New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 2009;32:1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schabenberger O, Pierce FJ: Contemporary Statistical Models for the Plant and Soil Sciences. Boca Raton, FL: CRC Press, 2002 [Google Scholar]
- 16.Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001;358:221–229 [DOI] [PubMed] [Google Scholar]
- 17.Torn C, Landin-Olsson M, Lernmark A, et al. : Prognostic factors for the course of beta cell function in autoimmune diabetes. J Clin Endocrinol Metab 2000;85:4619–4623 [DOI] [PubMed] [Google Scholar]
- 18.Petrone A, Galgani A, Spoletini M, et al. : Residual insulin secretion at diagnosis of type 1 diabetes is independently associated with both, age of onset and HLA genotype. Diabetes Metab Res Rev 2005;21:271–275 [DOI] [PubMed] [Google Scholar]
- 19.Barker A, Lauria A, Schloot N, et al. : Age-dependent decline of beta-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes Obes Metab 2014;16:262–267 [DOI] [PubMed] [Google Scholar]
- 20.Greenbaum CJ, Anderson AM, Dolan LM, et al. : Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care 2009;32:1839–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosenko JM, Palmer JP, Greenbaum CJ, et al. : Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2006;29:643–649 [DOI] [PubMed] [Google Scholar]
- 22.Steffes MW, Sibley S, Jackson M, et al. : Beta-cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 23.Buckingham B, Beck RW, Ruedy KJ, et al. : Effectiveness of early intensive therapy on beta-cell preservation in type 1 diabetes. Diabetes Care 2013;36:4030–4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook JJ, Hudson I, Harrison LC, et al. : Double-blind controlled trial of azathioprine in children with newly diagnosed type I diabetes. Diabetes 1989;38:779–783 [DOI] [PubMed] [Google Scholar]
- 25.The Canadian-European Randomized Control Trial Group: Cyclosporin-induced remission of IDDM after early intervention. Association of 1 yr of cyclosporin treatment with enhanced insulin secretion. Diabetes 1988;37:1574–1582 [PubMed] [Google Scholar]
- 26.Herold KC, Hagopian W, Auger JA, et al. : Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 27.Herold KC, Gitelman SE, Masharani U, et al. : A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005;54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown RJ, Sinaii N, Rother KI: Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care 2008;31:1403–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.