Abstract
Objective: to investigated the circulating microRNA expression profile in sepsis and its clinical evaluation. Methods: 70 patients with sepsis and 30 patients with SIRS were selected and their blood samples were collected. Using liquid bead array with 3 statistical analysis approaches analyzed the circulating microRNA expression profiles, for confirming the data of liquid bead array, qRT-PCR was performed. The prognostic value of the changed microRNA in sepsis was determined and compared with CRP and PCT by analyzing the receiver operating characteristic (ROC) curves. To reveal whether the selected microRNAs could predict the outcome of patients, 28 d survival rate were calculated using Kaplan-Meier curves. Furthermore, the level of malondialdehyde (MDA), activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in plasma were detected and the relationship with the changed microRNA was determined. Results: By integrating data from liquid bead array, we ultimately identified 6 microRNAs that were consistently changed in both of 3 statistical analysis approaches, however, only the change of microRNA-25 was significant according to the qPCR’s result. The area under ROC curve showed that the clinical accuracy of microRNA-25 for sepsis diagnosis was better than CRP and PCT (AUG=0.806, 0.676 and 0.726, P<0.05).The decrease in level of microRNA-25 was correlated with the severity of sepsis, SOFA score, CRP and PCT level, meanwhile, microRNA-25 level can be used for predicting the prognosis of patients, the patients with microRNA-25 level ≤0.492 had a lower 28 d survival rate. Moreover, Decreased microRNA-25 level was related to the level of oxidative stress indicators in sepsis patients. Conclusions: microRNA-25 can be used as a biomarker for the diagnosis and assessment of sepsis. Meanwhile, microRNA-25 level may be associated with oxidative stress in patients with sepsis, and it is expected to become a target for anti-oxidation therapy.
Keywords: Sepsis, circulating microRNA, liquid bead array, biomarker, diagnosis, prognosis, oxidative stress
Introduction
Sepsis is a systemic inflammatory response caused by infection and can lead to multiple organ dysfunction syndromes (MODS), with a high mortality rate. Though numerous studies focus on the prevention and cure of sepsis during the past 10 years and some results have been achieved, it is undeniable that sepsis remains a major cause of death in ICU [1-3]. Therefore, it is very important for early diagnosis and accurate assessment of patients with sepsis. MicroRNAs are endogenous short non-coding RNA with the length of only 21~25 nt, exists in eukaryotic cells widely, which can realize the fine regulation of post-transcriptional gene expression [4,5]. Circulating microRNAs refer to the microRNAs that present in serum or plasma stably. There are changes in the circulating microRNAs expression profiles in some diseases, thus, circulating microRNAs are potential to become a biomarker that can be used for diagnosis and prognosis of some diseases, and provide the basis for individualized treatment [6,7]. Previous studies have revealed that changes in microRNA levels existed in some patients with sepsis [8], but the clinical value of microRNA, as a biomarker, used for diagnosis of sepsis or assessment of the prognosis of patients with sepsis remains to be further confirmed, and the relevant mechanism is to be further revealed. In this study, it was found that the microRNA-25 level in serum was lower in patients with sepsis by the liquid bead array and qRT-PCR. Further analysis showed that, changes in microRNA-25 levels were correlated with the disease condition and prognosis of patients. The results showed that, circulating microRNA-25 may be a useful biomarker with the diagnostic value and prognosis assessment value for the patients with sepsis. Previous studies have also found that, microRNA-25 may be associated with oxidative stress [9], which is a key link for multiple organ damage of patients with sepsis. Therefore, in this study, we investigated the relationship between microRNA-25 and oxidative stress of patients with sepsis.
Patients and study design
Patient selection
The study population consisted of 70 patients with sepsis and 30 patients with SIRS who were admitted to the First Hospital of Lanzhou University during the period from April 2013 to May 2014. The diagnosis of sepsis was based on the definition by the American College of Chest Physicians and the American Society of Critical Care Medicine (ACCP/SCCM) [10]. In briefly, a sepsis was defined as the presence of SIRS associated with infection. SIRS was defined in the presence of two or more of the following criteria: temperature <36.8°C or >38.8°C, heart rate >90 beats per min in the absence of a pacemaker, respiratory rate >20/min or PaCO2 less than 4.3 kPa (32 mmHg), and white blood cell count >12×109/L or <4×109/l, or >10% immature band forms. Further, 70 patients with sepsis were divided into 3 groups: sepsis group, severe sepsis group and septic shock group. According to the criteria of 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definition conference, sepsis complicated by organ dysfunction is referred to as severe sepsis, while sepsis complicated by hypotension refractory to adequate volume resuscitation in the absence of an alternate cause has been termed septic shock. The exclusion criteria in this study: (1) age <18 years; (2) taking immunosuppressive drugs; (3) malignant tumors; (4) HIV-infected patients; (5) patients in pregnancy; (6) died within 24 h after admitted in ICU; The blood samples of patients were collected at 24 h after admitted in ICU. The age, gender, sequential organ failure assessment score (SOFA) score were recorded, also, the levels of c-reactive protein (CRP) and procalcitonin (PCT) in serum were analyzed routinely. Patients with sepsis were followed up for 28 days. Information consents were obtained from all patients with protocol approved by the Ethics Committee of the First Hospital of Lanzhou University.
Analysis for circulating microRNA expression profiles by liquid bead array
microRNAs in blood sampls were extracted and isolated using the microRNA isolation kit (Roche, Germany) according to the protocol in the instruction. Using FlexmiR miRNA Human Panel array (Luminex, USA) obtained the expression profiles in SIRS and sepsis patients (n=5). In order to increase credibility of liquid bead array, the data were analyzed using three different statistical analysis approaches including (a) 2-Tailed paired t-test after background correction and normalization; (b) Hierarchical clustering and (c) Bead to bead background subtraction and quantile normalization. The thresholds were set at P-value <0.05, and a fold-change >1.5.
Quantitation of microRNA expression profiles by qRT-PCR
To confirm liquid bead array results, reverse transcription reaction and quantitative real-time PCR (qRT-PCR) were performed using TaqMan® MicroRNA Reverse Transcription Kit and TaqMan® MicroRNA assays (Applied Biosystems, USA) with the TaqMan protocol supplied by the manufacturer. Relative level changes of microRNAs were determined using the ΔΔCt approach and the data were normalized by microRNA-16. In brief, the fold changes of microRNAs in sepsis patients (n=70) relative to SIRS patients (n=30) were calculated by the formula of fold change =2-ΔΔCt, ΔCt=Ct (target microRNA)-Ct (microRNA-16), ΔΔCt=ΔCt (sepsis)-ΔCt (SIRS).
Analysis for clinical value of the selected microRNAs
According to the qRT-PCR’s result, we eventually selected microRNA-25 as an interesting microRNA and evaluate its clinical value on diagnosis of sepsis and predicting prognosis. In brief, the prognostic value of microRNA-25 was determined and compared with CRP and PCT by analyzing the receiver operating characteristic (ROC) curves. Further, we analyzed the relation between the microRNA-25 levels and severity of patients with sepsis. To reveal whether the selected microRNAs could predict the outcome of patients, 28 d survival rate were calculated using Kaplan-Meier curves.
Detection of oxidative stress level in patients with sepsis
In order to estimate the oxidative stress level in patients with sepsis, the level of malondialdehyde (MDA), activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in plasma were detected using kits provided by Nanjing Jiancheng Bioengineering Institute.
Statistical analysis
Data analysis was performed using SPSS18.0 software (SPSS Inc., Chicago, IL, USA). Normal distribution data were expressed as mean ± SD and non-normal distribution data were expressed as median. For normal data, one way-ANOVA was performed for comparison between multiple groups followed with LSD test. For non-parametric statistics, Mann-Whitney test was performed for comparison between two groups. The difference in frequency distribution between groups was determined by Chi-square test. Spearman correlation analysis was performed. The 28 d survival was calculated by Kaplan-Meier curves with log-rank test. A P value <0.05 was considered statistically significant.
Result
Patient characteristics
The demographic characteristics of patients with sepsis were shown in Table 1. Thirty patients with SIRS, 20 patients with sepsis, 35 patients with severe sepsis and 15 septic shock patients were enrolled in our study. The age, gender, site of infection and pathogen culture results between SIRS and sepsis patients were comparable without significant difference (P<0.05). The SOFA score, the level of CRP and PCT in serum (at 24 h after admitted to ICU) were highest in septic shock group (P<0.05). As anticipated, SIRS patients had the lowest SOFA score, CRP and PCT level (P<0.05). Among 70 sepsis patients, 25 patients dead within 28 d and 45 cases patients survived 28 days after admitted to ICU.
Table 1.
Clinical characteristics of the subjects enrolled
| SIRS patients (n=30) | Sepsis patients (n=70) | P value | |||
|---|---|---|---|---|---|
|
|
|||||
| Sepsis subgroup (n=20) | Server subgroup (n=35) | Septic shock subgroup (n=15) | |||
| Median age (years) | 55 (42~61) | 52 (36-62) | 55 (40~71) | 54 (42-69) | NS |
| Gender | |||||
| Male | 12 | 9 | 20 | 7 | NS |
| Female | 18 | 11 | 15 | 8 | |
| Blood culture | |||||
| Negative | - | 2 | 2 | 3 | NS |
| Bacterial (G+) | - | 6 | 10 | 3 | |
| Bacterial (G-) | - | 10 | 20 | 8 | |
| Fungi | - | 2 | 3 | 1 | |
| Primary infection site | - | ||||
| Lower respiratory tract | - | 2 | 5 | 4 | NS |
| upper respiratory tract | - | 3 | 3 | 2 | |
| Urinary tract | - | 4 | 8 | 3 | |
| Abdomen | - | 11 | 19 | 6 | |
| SOFA score | 3 (1-4) | 6 (4-8) | 7 (5-10) | 9 (6-11) | <0.05 |
| C-reacting protein (mg/l) | - | 72.3 (53.2-91.3) | 95.5 (57.5-119.9) | 135.7 (100.1~158.3) | <0.05 |
| Procalcitonin (μg/l) | - | 2.9 (0.9-5.7) | 6.8 (3.4-11.6) | 12.8 (9.9-18.3) | <0.05 |
| Median ICU stay (days) | 3 (1-5) | 10 (6-18) | 15 (15-22) | 20 (18-27) | <0.05 |
| 28 d survival | - | ||||
| Yes | 28 | 17 | 23 | 5 | <0.05 |
| No | 2 | 5 | 10 | 10 | |
NS, no significance.
Circulating microRNA expression profiles
To identify the significant difference in circulating microRNA expression between SIRS and sepsis patients (n=5), we analyzed the liquid bead array data using 3 different analytics approaches as mentioned above. In different approach, we found different microRNA expression profiles. In brief, (a) according to 2-Tailed paired t-test after background correction and normalization, 16 microRNAs were identified to be changed significantly; (b) According to hierarchical clustering, 20 microRNAs were identified to be changed significantly; (c) According to bead to bead background subtraction and quantile normalization, 22 microRNAs were identified to be changed significantly. By integrating data, we ultimately identified 6 microRNAs that were consistently changed in both of 3 statistical analysis approaches, as shown in Figure 1. Further, these 6 microRNAs’ expression changes were confirmed by qRT-PCR, as shown in Figure 2, compared with SIRS patients (n=30), only the decreased microRNA-25 level of patients with sepsis (n=70) showed statistical difference (P<0.05), the median fold change relative to SIRS patients was 0.63.
Figure 1.
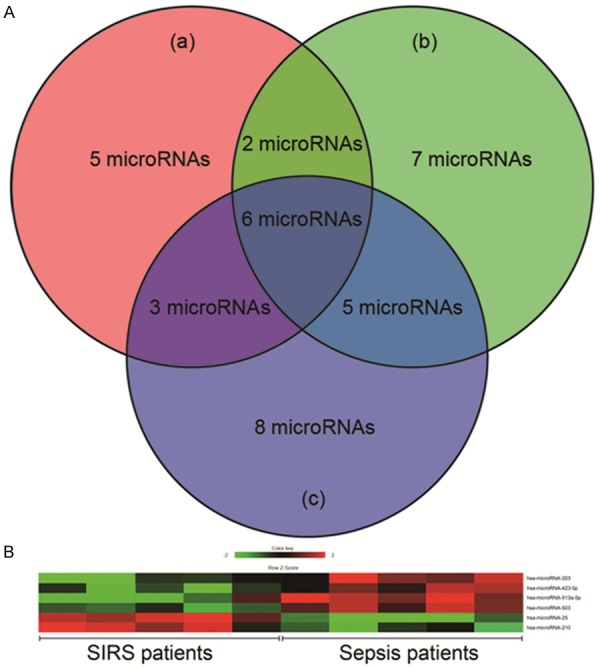
Liquid bead array results. A. microRNAs expression profiles analyzed by 3 different statistical approaches. a means 2-Tailed paired t-test after background correction and normalization; b means Hierarchical clustering; c means bead to bead background subtraction and quantile normalization. B. Heat map for 6 microRNAs that were consistently changed in both of 3 statistical analysis approaches.
Figure 2.
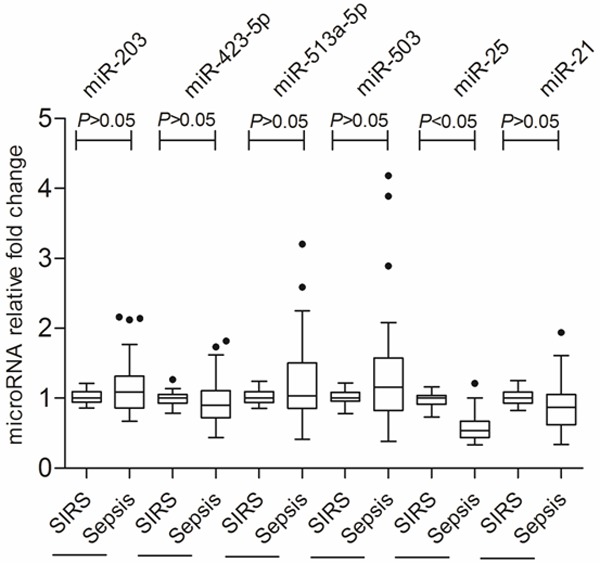
qRT-PCR analysis for confirming the data of liquid bead array.
The diagnostic significance of circulating microRNA-25
According to the qRT-PCR’s result, we eventually selected microRNA-25 as an interesting microRNA. The clinical accuracy of microRNA-25 for sepsis diagnosis was analyzed by receiver-operating characteristic (ROC) curve, as shown in Figure 3. The area under ROC curve (AUG) was 0.806 (95% CI: 0.701-0.912), which is slightly better than CRP (AUG=0.676, 95% CI: 0.54-0.810) and d PCT (AUG=0.726, 95% CI: 0.592-0.860) with statistical difference (P<0.05). It indicated that microRNA-25 can be used to distinguish sepsis from SIRS.
Figure 3.
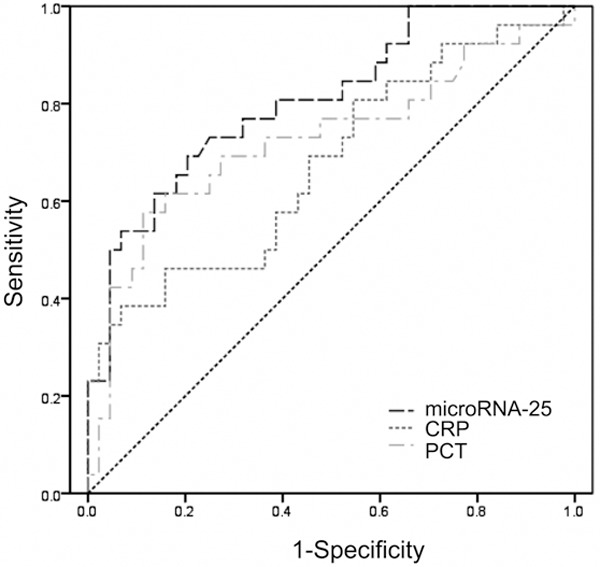
Receiver operating characteristic curve of microRNA-25, CRP, PCT level for sepsis diagnosis. CRP means C-reactive protein; PCT means procalcitonin.
The decrease in level of microRNA-25 was correlated with the severity of sepsis
At the 24 h after admitted to ICU, the median level of microRNA-25 (fold change relative to SIRS) in patients with sepsis, severe sepsis and septic shock was 0.794, 0.559 and 0.342, respectively (P<0.05, Figure 4). Obviously, compared with sepsis and severe sepsis groups, the SOFA score, CRP and PCT level in septic shock groups was more higher (P<0.05), as shown in Figure 5. Correlation analysis showed (Figure 6) that the microRNA-25 level (at 24 h) in 70 patients with sepsis was inversely proportional to the SOFA score (r=-0.691, P<0.05), as well as , negatively related to CRP and PCT level in serum (r=-0.712 and -0.611, P<0.05). These results suggested that the level of microRNA was correlated with the severity of sepsis.
Figure 4.
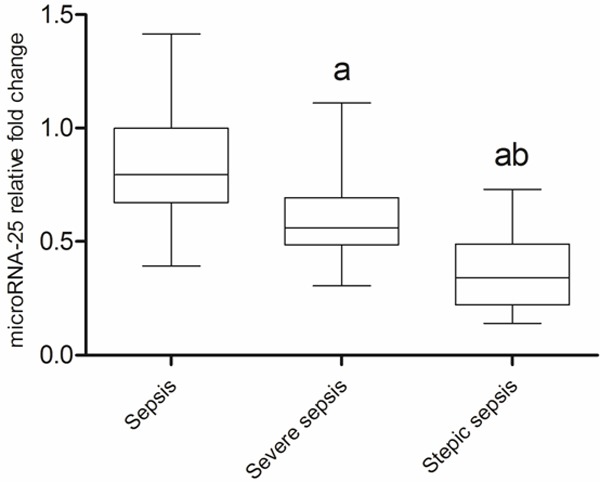
The microRNA-25 relative fold change of patients with different severity of sepsis. a means compared with sepsis P<0.05; b means compared with severe sepsis P<0.05.
Figure 5.
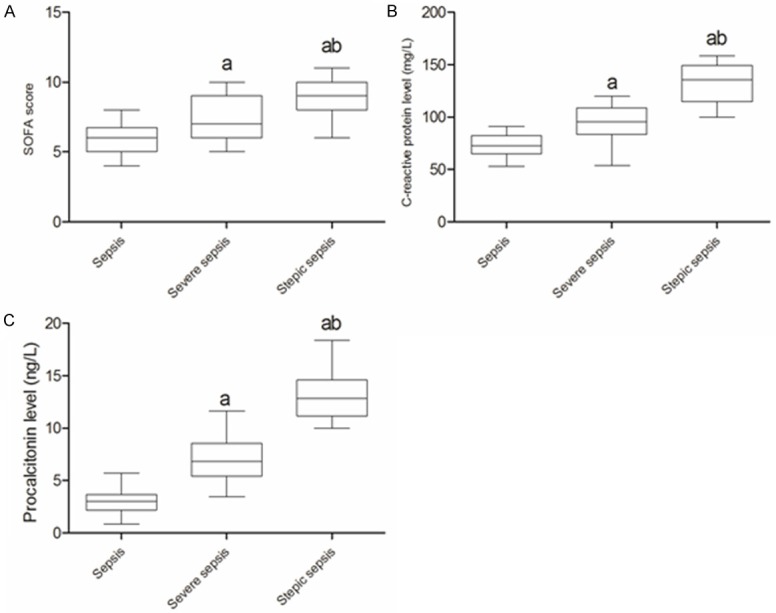
The SOFA score, CRP and PCT level of patients with different severity of sepsis. A. SOFA score in patients with different severity of sepsis. B. CRP level in patients with different severity of sepsis. C. PCT level in patients with different severity of sepsis. SOFA, sepsis-related organ failure assessment; CRP, c-reactive protein; PCT, procalcitonin. a, compared with sepsis P<0.05; b, compared with severe sepsis P<0.05.
Figure 6.
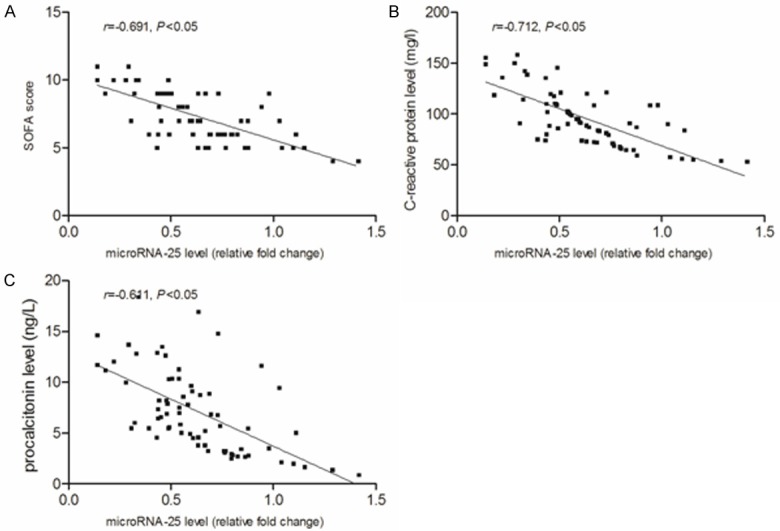
The decreased microRNA-25 was inversely associated with SOFA score, CRP and PCT level in patients with sepsis. A. Correlation between microRNA-25 level and SOFA score. B. Correlation between microRNA-25 level and CRP level. C. Correlation between microRNA-25 level and PCT level. SOFA, sepsis-related organ failure assessment; CRP, c-reactive protein; PCT, procalcitonin.
The Prognostic significance of circulating microRNA-25
As shown in Figure 7, the microRNA-25 level (fold change relative to SIRS) of surviving patients was significantly higher than that of the dead patients (P<0.05). Also, the clinical accuracy of microRNA-25 level for predicting 28 d mortality was analyzed by ROC. As shown in Figure 8, the AUG of microRNA-25 used for prediction of 28 d mortality of patients with sepsis (AUG) was 0.756 (95% CI: 0.569-0.833, P<0.05) and the cut-off point was 0.492. Kaplan-Meier survival curve showed that (Figure 9), the patients with microRNA-25 level ≤0.492 had a lower 28 d survival rate, compared with patients with microRNA-25 level >0.492 (44.5% vs. 73.9%, P<0.05). The above results suggested that the microRNA-25 level can be used for predicting the prognosis of patients.
Figure 7.
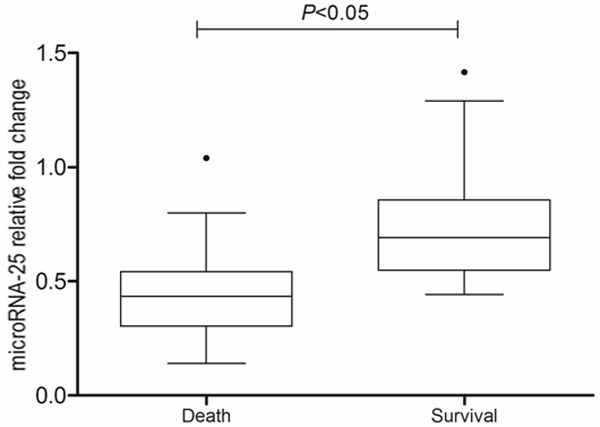
The decreased microRNA-25 was associated with mortality of septic patients.
Figure 8.
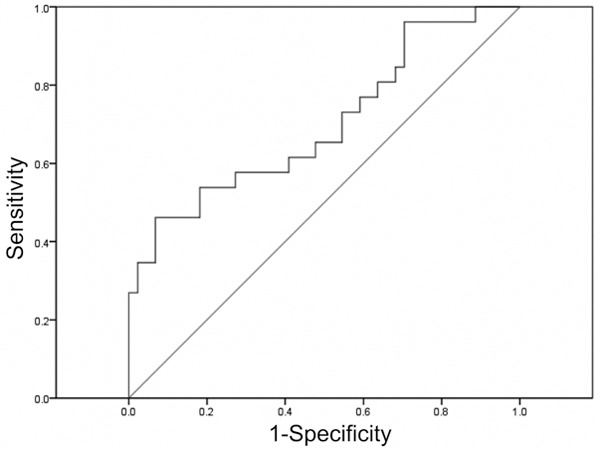
Receiver operating characteristic curve for microRNA-25 used as a predictor of 28 d survival in patients with sepsis.
Figure 9.
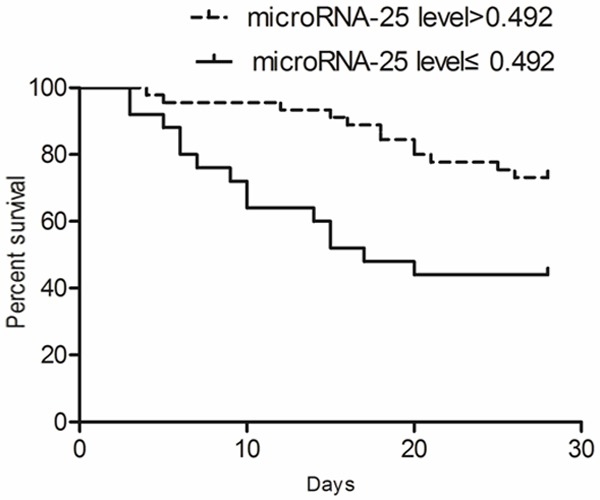
Kaplan-Meier survival curves showed that patients with lower microRNA-25 level had an increased mortality.
Decreased microRNA-25 level was related to the level of oxidative stress in sepsis patients
As shown in Figure 10, the compared with sepsis and severe sepsis groups, the MDA level in septic shock groups was more higher but with lower SOD and GSH-Px activity (P<0.05). Correlation analysis showed (Figure 11) that the microRNA-25 level in sepsis patients with sepsis was inversely proportional to the MDA level (R=-0.754, P<0.05), however, positively correlation with SOD and GSH-Px activity (r=0.768 and 0.694, P<0.05).
Figure 10.
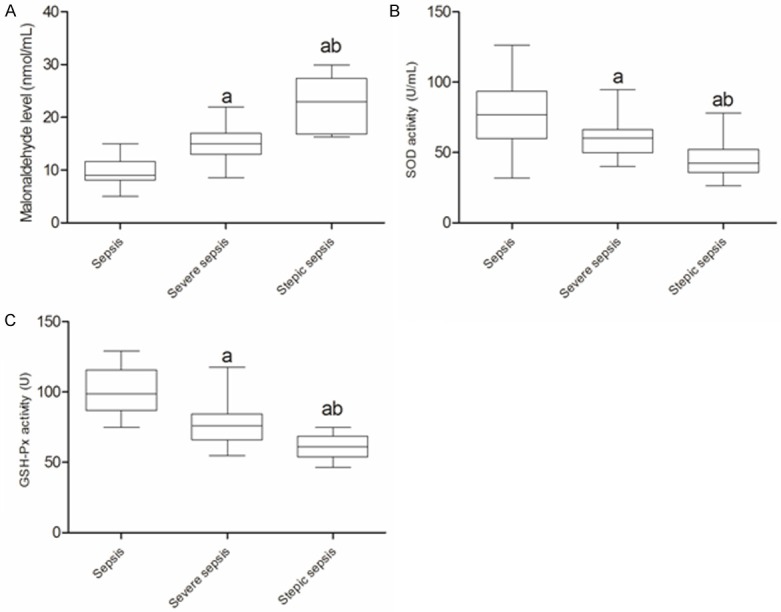
The oxidative stress indicators of patients with different severity of sepsis. A. MDA level in patients with different severity of sepsis. B. SOD activity in patients with different severity of sepsis. C. GSH-Px activity in patients with different severity of sepsis. MDA, malonaldehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase. a, compared with sepsis P<0.05; b, compared with severe sepsis P<0.05.
Figure 11.
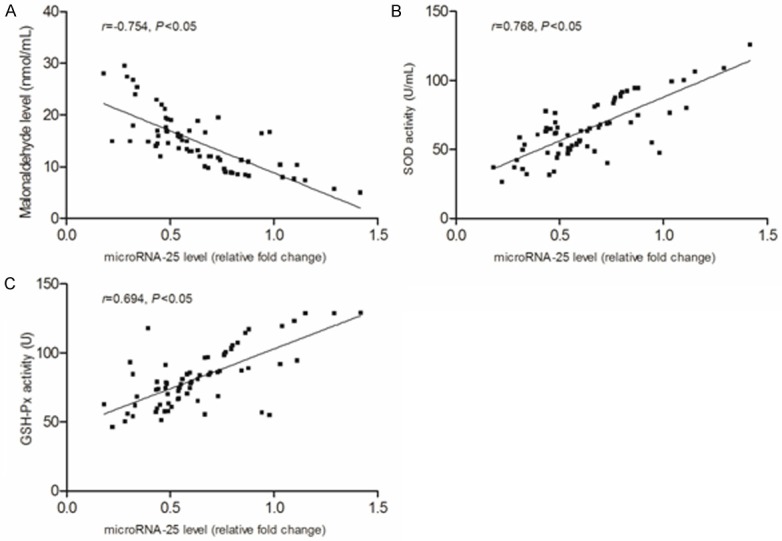
The correlation between microRNA-25 and oxidative stress indicators. A. Correlation between microRNA-25 level and MDA score. B. Correlation between microRNA-25 level and SOD activity. C. Correlation between microRNA-25 level and GSH-Px activity. MDA, malonaldehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase.
Discussion
Sepsis is a common disease and one of the major causes of death in ICU worldwide, which requires a high medical cost. The treatment guidelines recommend early intervention in patients with sepsis [11,12]. Studies showed that the early intervention can reduce mortality and improve the prognosis of patients, and reduce the medical costs. The early intervention of sepsis mainly depends on the early diagnosis and accurate assessment of the disease condition [13]. The application of biomarkers will play an important role in the early diagnosis and assessment of disease condition of sepsis. Currently the biomarkers used in the early diagnosis of sepsis include C-reactive protein (CRP), interleukin, procalcitonin (PCT), N-terminal proatrial natriuretic peptide, etc. [14,15], but they have some limitations and their accuracy is to be enhanced, for example, the CRP has a high sensitivity but low specificity for the diagnosis of sepsis, not suitable for using alone. PCT has a high specificity than CRP, but it has obvious change in the bacterial infectious diseases. Some studies have shown PCT cannot be used to distinguish sepsis from SIRS not caused by bacterial infection, having inadequate diagnostic efficacy in severe sepsis when used alone [16,17]. Therefore, it is very necessary to develop new biomarkers.
microRNAs are evolutionarily conserve, which can be stable in the blood. The techniques of quantitative detection for microRNA are relatively mature and trace detection also can be achieved [18,20]. The circulating microRNAs are easily to collect; in addition, microRNAs also have a post-transcriptional regulation function, which is capable of forming a complex regulatory network to decide the occurrence and progression of the disease [21,22]. Therefore, microRNAs not only can be used as biomarkers for disease diagnosis, but also as a target for the treatment of disease, showing extensive clinical applications. It has been reported in some studies that the changes in microRNA expression was involved in the occurrence and progression of sepsis, for example, Moore et al [5]. investigated the changes in expressions of 351 microRNAs in rats with septic shock and found that, high expression of 17 microRNAs existed, and nine microRNAs significantly regulate the target genes of the focal adhesion pathway. Wang et al [23]. found that microRNA-27a in mice with sepsis was up regulated and the inhibition of miR-27a reduced the expression levels of TNF-α and IL-6., thereby reducing the inflammatory response of mice with sepsis. The results suggested that, miR-27a can not only be used as a diagnostic marker of sepsis, but also as a therapeutic target. Zhou et al [24]. found that dysregulated microRNAs existed in the peripheral blood mononuclear cells in patients with sepsis, and Dysregulated miRs have immunological associations with clinical disease in sepsis, especially, miR-146a decrease correlated with elevated IL-6 and monocyte proliferation. In this study, we found that, compared with SIRS patients, the levels of microRNA-25 in patients with sepsis were significantly lower, and patients with low level of microRNA-25 had more serious condition of disease and poorer prognosis. ROC curve showed that, microRNA-25 not only can be used to distinguish between SIRS and sepsis, but also predict the 28 d mortality condition of patients with sepsis. Thus, we believe microRNA-25 can be used as a biomarker of patients with sepsis.
Sepsis-induced multiple organ dysfunction syndrome (MODS) is an important cause of death for patients [25]. The MODS mechanism is very complex, and one of the crucial mechanisms is oxidative stress injury. When sepsis occurs, endotoxins, ischemia-reperfusion injury can activate nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) and xanthine oxidase that could result in generation of reactive oxygen species (ROS) and oxidative/antioxidant imbalance, and ultimately lead to oxidative stress injury of tissue cells [26,27]. Hence, attention should be paid to the oxidative stress injury of patients with sepsis. In addition, studies have shown that microRNA-25 is associated with oxidative stress; so we further investigated the relationship between microRNA-25 level and oxidative stress in patients with sepsis. We found that, the lower the microRNA-25 level, the higher the level of oxidative stress. Regarding to the regulation of oxidative stress by microRNA-25, Varga believed that [9], NADPH oxidase 4 (NOX4) is the target gene of microRNA-25; when the microRNA-25 expression is inadequate, NOX4 expression would increase, which will lead to increased generation of ROS and enhance the oxidative stress injury. Further study is to be carried out to investigate how microRNA-25 to regulate the oxidative stress in patients with sepsis.
In conclusion, our studies showed that microRNA-25 can be used as a biomarker for the diagnosis and assessment of sepsis. Meanwhile, microRNA-25 level may be associated with oxidative stress in patients with sepsis, and it is expected to become a target for anti-oxidation therapy.
Acknowledgements
This study are supported by the grant from the National High Technology Research and Development Program (“863”Program) of China (No. 2011AA02A111).
Disclosure of conflict of interest
None.
References
- 1.Karapetsa M, Pitsika M, Goutzourelas N, Stagos D, Tousia Becker A, Zakynthinos E. Oxidative status in ICU patients with septic shock. Food Chem Toxicol. 2013;61:106–111. doi: 10.1016/j.fct.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 2.Leng FY, Liu JL, Liu ZJ, Yin JY, Qu HP. Increased proportion of CD4+CD25+Foxp3+ regulatory T cells during early-stage sepsis in ICU patients. J Microbiol Immunol Infect. 2013;46:338–344. doi: 10.1016/j.jmii.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Skrobik Y, Laverdiere M. Why candida sepsis should matter to ICU physicians. Crit Care Clin. 2013;29:853–864. doi: 10.1016/j.ccc.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Flowers E, Froelicher ES, Aouizerat BE. MicroRNA regulation of lipid metabolism. Metabolism. 2013;62:12–20. doi: 10.1016/j.metabol.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore CC, McKillop IH, Huynh T. MicroRNA expression following activated protein C treatment during septic shock. J Surg Res. 2013;182:116–126. doi: 10.1016/j.jss.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 6.Essandoh K, Fan GC. Role of extracellular and intracellular microRNAs in sepsis. Biochim Biophys Acta. 2014;1842:2155–2162. doi: 10.1016/j.bbadis.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid G, Kirschner MB, van Zandwijk N. Circulating microRNAs: Association with disease and potential use as biomarkers. Crit Rev Oncol Hematol. 2011;80:193–208. doi: 10.1016/j.critrevonc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Puskarich MA, Nandi U, Shapiro NI, et al. Detection of microRNAs in patients with sepsis. Journal of Acute Disease. 2015;4:101–106. [Google Scholar]
- 9.Varga ZV, Kupai K, Szűcs G, Gáspár R, Pálóczi J, Faragó N, Zvara A, Puskás LG, Rázga Z, Tiszlavicz L, Bencsik P, Görbe A, Csonka C, Ferdinandy P, Csont T. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol. 2013;62:111–121. doi: 10.1016/j.yjmcc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 11.Gattas DJ, Cook DJ. Procalcitonin as a diagnostic test for sepsis: Health technology assessment in the ICU. J Crit Care. 2003;18:52–58. doi: 10.1053/jcrc.2003.YJCRC11. [DOI] [PubMed] [Google Scholar]
- 12.Levesque E, Saliba F, Ichaï P, Samuel D. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol. 2014;60:570–578. doi: 10.1016/j.jhep.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Keegan J, Wira Iii CR. Early Identification and management of patients with severe sepsis and septic shock in the emergency department. Emerg Med Clin North Am. 2014;32:759–776. doi: 10.1016/j.emc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Parente JD, Chase JG, Shaw GM, Blakemore AJ, Lecompte AJ, Pretty C, Razak NN, Lee DS, Hann CE, Wang SH. Development of a model-based clinical sepsis biomarker for critically ill patients. Comput Methods Programs Biomed. 2011;102:149–155. doi: 10.1016/j.cmpb.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Ren H, Li Y, Han C, Hu H. Serum procalcitonin as a diagnostic biomarker for sepsis in burned patients: A meta-analysis. Burns. 2015;41:502–509. doi: 10.1016/j.burns.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Guven H, Altintop L, Baydin A, Esen S, Aygun D, Hokelek M, Doganay Z, Bek Y. Diagnostic value of procalcitonin levels as an early indicator of sepsis. Am J Emerg Med. 2002;20:202–206. doi: 10.1053/ajem.2002.33005. [DOI] [PubMed] [Google Scholar]
- 17.Lavrentieva A, Papadopoulou S, Kioumis J, Kaimakamis E, Bitzani M. PCT as a diagnostic and prognostic tool in burn patients. Whether time course has a role in monitoring sepsis treatment. Burns. 2012;38:356–363. doi: 10.1016/j.burns.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Xu H, Baloda M, Gurung AS, Xu LP, Wang T, Zhang X, Liu G. Visual detection of microRNA with lateral flow nucleic acid biosensor. Biosens Bioelectron. 2014;54:578–584. doi: 10.1016/j.bios.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong X, Zhou W, Li D, Chai Y, Xiang Y, Yuan R. RNA-regulated molecular tweezers for sensitive fluorescent detection of microRNA from cancer cells. Biosens Bioelectron. 2015;71:98–102. doi: 10.1016/j.bios.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Miao P, Meng F, Wang B, et al. Highly sensitive microRNA quantification with zero background signal from silver nanoparticles. Electrochemistry Communications. 2015;51:89–92. [Google Scholar]
- 21.Louise Hull M, Nisenblat V. Tissue and circulating microRNA influence reproductive function in endometrial disease. Reprod Biomed Online. 2013;27:515–529. doi: 10.1016/j.rbmo.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Huang W, Yang Y, Wang Y, Peng T, Chang J, Caldwell CC, Zingarelli B, Fan GC. Loss of duplexmiR-223 (5p and 3p) aggravates myocardial depression and mortality in polymicrobial sepsis. Biochim Biophys Acta. 2014;1842:701–711. doi: 10.1016/j.bbadis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y, Yin N, Jiang L. miR-27a is up regulated and promotes inflammatory response in sepsis. Cell Immunol. 2014;290:190–195. doi: 10.1016/j.cellimm.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Chaudhry H, Zhong Y, Ali MM, Perkins LA, Owens WB, Morales JE, McGuire FR, Zumbrun EE, Zhang J, Nagarkatti PS, Nagarkatti M. Dysregulation in microRNA expression in peripheral blood mononuclear cells of sepsis patients is associated with immunopathology. Cytokine. 2015;71:89–100. doi: 10.1016/j.cyto.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimbler N, Campbell A. Sepsis, SIRS and MODS. Surgery (Oxford) 2004;22:73–76. [Google Scholar]
- 26.Lee I, Hüttemann M. Energy crisis: The role of oxidative phosphorylation in acute inflammation and sepsis. Biochim Biophys Acta. 2014;1842:1579–1586. doi: 10.1016/j.bbadis.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Dessauer B, Bongain J, Molina V, Quilodrán J, Castillo R, Rodrigo R. Oxidative stress as a novel target in pediatric sepsis management. J Crit Care. 2011;26:103.e1–103.e7. doi: 10.1016/j.jcrc.2010.05.001. [DOI] [PubMed] [Google Scholar]


