Abstract
Prior corticosteroid therapy presents a major challenge in the diagnosis of CNS lymphomas, particularly in stereotactic biopsies. In this study we analysed the cytological, histopathological and immunohistochemical features in stereotactic biopsies of 25 primary CNS lymphoma cases pre-treated with corticosteroids. We documented the extent and the frequency of each finding. We also investigated the significance of subjectivity in evaluation of these biopsies in 3 seperate sessions including the final diagnostic decision. In 48% of our cases the diagnosis was straightforward. These cases were characterized by prominent blasts either in diffuse paranchymal infiltrates or in perivascular regions. The remaining 52% demonstrated some degree of variability among pathologists. Lymphoid atypia other than the typical blastic morphology appeared as a subjective finding and this was more pronounced in cytology preparations. In our study, corticosteroid pre-treatment in primary CNS lymphoma was associated with a large spectrum of histopathological, immunohistochemical and cytological findings. Combined use of an extended immunohistochemical panel would increase the possibility of conclusive diagnosis. Nevertheless some of these findings and therefore the diagnosis are open to subjectivity.
Keywords: PCNSL, interobserver, histopathology, cytology, immunohistochemistry
Introduction
Primary central nervous system lymphoma (PCNSL) is defined as extranodal malignant lymphoma in the CNS in the absence of lymphoma outside the nervous system [1]. Histopathologically, the vast majority of PCNSLs are diffuse large B-cell lymphomas (DLBCL) and CNS DLBCL is the prefered term in WHO Classification of Haematopoietic tumors [2]. Since lymphomas are aggressive malignancies, early diagnosis and appropriate treatment is extremely important. In many cases, however, diagnosis of PCNSL is associated with a distinct delay [3,4]. Although it is a routine technique in the initial evaluation of cranial lesions, findings in magnetic resonance imaging (MRI) are usually non-specific and thus, differentiation from other radiological mimickers such as metastases, glioblastoma (GMB) and demyelinating lesions (DL) can be difficult [5]. Cerebrospinal fluid analysis is suggested to be useful for staging and long-term follow-up, but its initial diagnostic value is usually limited. For these reasons diagnosis of PCNSL depends largely on histopathological evaluation. The current gold standard method for establishing the tissue diagnosis of CNS lymphoma is the stereotactic biopsy, since these lesions are usually deep seated and their resections were shown to be associated with worse prognosis [6-8]. However histopathological evaluation of stereotactic biopsies also has some limitations, not only because of small sample sizes, but also due to large spectrum of differential diagnoses, including inflamatory conditions such as vasculitis, multiple sclerosis and infection [9]. At this point, prior administration of corticosteroids (CS) presents the major diagnostic challenge. Because of the high sensitivity of lymphoma cells to corticosteroid-induced apoptosis, administration of CS can mask the morphology and it was even reported to cause tumour vanishing [7,10]. A complex neuroimmune network accompanies the neoplastic population in PCNSL although the details of interactions in between still remain to be elucidated [11]. Application of CS also interferes with the assesment of this neuroimmune response. The majority of these accompanying lymphocytes are in T-cell origin. T-cells are demostrated in brain parenchyma and perivascular region in a concentric arrengement. In a retrospective study, reactive perivascular T-cell infiltrates (RPVI) were demonstrated in 36% of patients. In addition to T-cells, some residual neoplastic B-cells and blasts are also known to be detected after CS administration [12,13].
Although demonstration of B cell phenotype and high Ki67 index is quite helpful in differential diagnosis, as an important ancillary method IHC has also some restrictions [7,8]. Because in such small specimens adequate sections for multiple markers can not be obtained and presence of CD3 positive lymphocytes does not exclude lymphoma. Molecular biological analysis of monoclonality, on the other hand, can produce false negative results in tissues with limited number of B-cells [7,14]. Furthermore this is a costly analysis and considering the T-cell clonality of undetermined significance, the results usually need to be supported by morphology and IHC [15].
In a recent study of Bruck et al., pre-treatment with CS has been shown to prevent histopathological diagnosis of PCNSL up to 50% of patients [16]. On the other hand a study of Porter et al. did not demonstrate a significant radiographic change in PCNSL patients who received CS and also they did not report any significant increase in subsequent biopsy rates [17]. Thus the relative impact of CS on the diagnosis of PCNSL still seems controversial and more importantly the significance of related histopathological findings remains somewhat unclear. Furthermore although it is widely accepted that the CS pre-treatment presents a diagnostic problem in biopsy evaluation untill now there are no published data about the interobserver variability in the diagnosis of such cases.
In this study we investigated the cytological, histopathological and immunohistochemical features in 25 CS pre-treated PCNSL cases to discover the possible effects of CS on biopsy findings in detail. We also intended to evaluate the significance of certain findings by questioning the interobserver variability rates in relation with the final diagnosis.
Materials and methods
From the archives of Ankara Dışkapı Research and Training Hospital we retrospectively reviewed the stereotactic biopsies between 2007-2015 and 25 cases of PCNSL was included to the study. Twelve out of 25 was the cases diagnosed at first biopsy. The remaining 13 was the first inconlusive or non-diagnostic biopsies of the patients in whom the PCNSL was proven with re-biopsy, resection or PCR. All patients gave their informed consents prior to their inclusion in the study. All cases had a history of CS administration at time of biopsy. The data about the presenting radiological findings and CS therapy were all collected. The dosages and length of CS administration were not available in 3 patients. Presence of a systemic lymphoma and HIV positivity were the exclusion criteria. In order to confirm the diagnosis of PCNSL, the resection materials and repeat biopsies were histopathologically re-evaluated.
All of the cases had tissue samples from at least 10 stereotactic targets (ranged between 10 to 15 targets) and 2 cytological preparations. Biopsy sizes ranged between 0.5 mm to 3 mm with a mean of 1.75 mm. The cytological preparations (imprint and squash) were prepared from the fresh tissues during the stereotactic sampling. IHC panel was composed of CD3, CD5, CD20, CD79a, CD68 and Ki67 antibodies. However since some samples were inadequate for further sectioning the basic immunophenotype of lymphoid cells was identified mainly in CD3 and CD20 stained sections from the targets with highest amount of lymphoid population. IHC positivities were evaluated according to the cytoplasmic staining and specified as (-) in case of no staining, (+) in case of a positivity not more than 25% of the lymphoid population, (++) in case of a positivity between 25 to 75% of the lymphoid population, and (+++) in case of a positivity more than 75% of the lymphoid population.
In order to provide a detailed analysis, the haematoxyline and eosin (HE) stained tissue slides from the paraffin-embedded blocks, May Grünwald-Giemsa (MGG) stained cytological preparations and IHC sections were simultaneously re-evaluated by two pathologists experienced in the field. The discrepancies were resolved by consensus.
Then all the slides were examined by three other experienced pathologists in a blinded fashion and this examination was carried out in 3 seperate and blinded sessions: 1) Examination of HE slides, 2) Examination of imprints/squashes, 3) Examination of tissue sections in combination with cytological and IHC preparations for final diagnosis. In session 1 and session 2, the pathologists were asked to evaluate each slide with respect to presence of blasts or any suspicious lymphoid atypia. After a washing out period of 4 weeks the pathologists were provided with all the slides for each case and asked to make a diagnosis. In this session the pathologists were also informed about the clinical and radiological backgrounds of the cases.
Results
The patient ages ranged between 31 and 76 with a mean age of 53,5. The female to male ratio was 2:3. The CS pre-treatment was 4 mg dexamethasone with 6 hours intervals in 22 of the patients. The duration of treatment ranged between 2 to 30 days with a median of 5 days. The interval between the cessation of the CS and the biopsy ranged between 0 to 2 days with a median of 0. The specific data about dosages and duration was not available in case 9, case 15 and case 22.
According to the consensus, the findings from the histopathological evaluation of 25 cases were grouped as follows: non-neoplastic glial tissue (G), sparse lymphoid cells -without forming an apparent cellularity- (SL), sparse macrophages and lymphocytes (SLM), perivascular normal appearing lymphocytes (PVL), perivascular lymphocytes intermingled with blasts (PVLB), perivascular lymphoid cells and macrophages (PVLM), infiltration/fragmentation of vessel wall (V), diffuse neoplastic infiltration with blasts (LB), necrosis (NE), and tissue artefacts including inadequate sectioning (TA). The targets assigned as G did not contain any lymphoid cells. The targets containing scattered lymphoid cells in association with LB, PVL, PVLB and PVLM were not assigned as SL and the targets containing PVL or PVLM in association with PVLB were assigned as PVLB. Evaluation of cytology preparations yielded 4 groups: Lymphoid cells with blasts (Bc), suspicious lymphoid atypia/monomorphysm (Ac); normal lymphocytes with or without macrophages (Lc), a few or no lymphoid cells (NLc). Only the large lymphoid tumor cells resembling typical centroblasts or immunoblasts are assigned as blast.
Out of 25 cases 23 (92%) had lymphoid cells. About 48% (12/25) of the cases demonstrated blasts as LB and/or PVLB. Another 32% (8/25) was characterized by perivascularly arranged lymphoid cells without apparent blasts (PVL and/or PVLM). The 12% (3/25) only contained SL. The distribution of the findings according to the frequencies and extents was as follows: The most frequent histopathological finding was SL with 56% (14/25). The mean number of targets involved was 4,2 [1-8]. The second most frequent finding was PVL. It was observed in 44% (11/25) of the cases. The mean number of targets involved was 2,0 (1-6). The third most frequent finding was PVLB and it was observed in 40% (10/25) of the cases. The mean number of targets involved was 2,0 [1-4]. The 36% (9/25) of the cases had obvious neoplastic infiltration as LB. The mean number of targets involved was 5,6 [3-9]. Infiltration of vessel wall by lymphoid cells was observed in 16% (4/25) of the cases with a mean number of 1,25. Three of these 4 cases had blasts in perivascular region. The 12% (3/25) of the cases had some macrophages in the perivascular cuff (PVLM). The mean number of targets involved was 1,0. Two cases (8%) had single necrotic targets and 1 case (4%) had SLM (Figure 1). The mean numbers of targets involved were 2,5 and 4,0 respectively. Out of 25 cases 17 had artefacted targets that could not be evaluated. The distribution of these findings are shown on Table 1 and Figure 2. Cytological evaluation revealed more or less crash artefact in all preparations. According to cytology findings the cases were grouped as follows; Bc: 40% (10/25), Ac: 8% (2/25), Lc: 32% (8/25), NLc: 20% (5/25) (Figure 3). The distribution of the cytological findings are shown on Table 1 and Figure 4.
Figure 1.
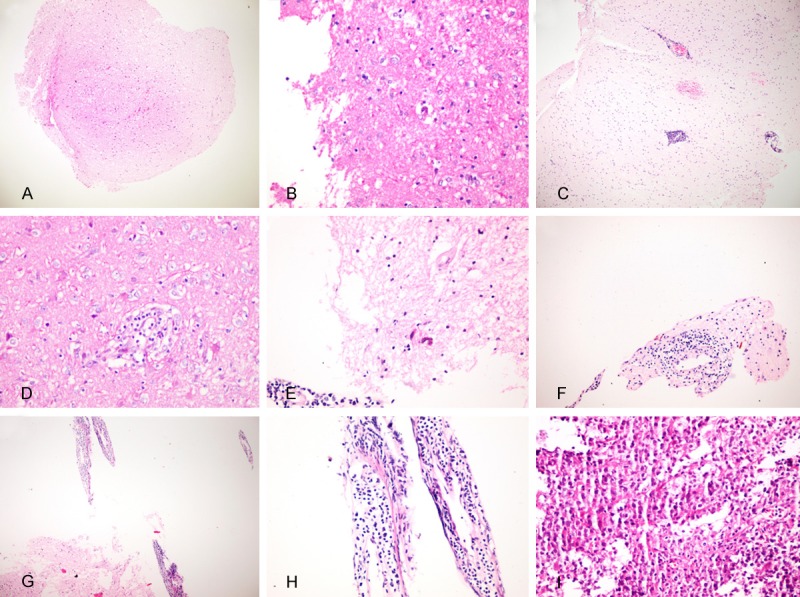
A. Scattered lymphoid cells in non-neoplastic glial tissue; example for SL target. B. Scattered macrophages and lymphoid cells; SLM target. C. Perivascularly arranged lymphocytes; PVL target. D. Macrophages accompanying perivascular lymphocytes; PVLM target. E. PVL target noted to have suspicious lymphoid atypia by 2 observers. F. PVL target noted to have lymphoid atypia by 1 observer. G, H. Perivascular lymphoid cells with blasts; PVLB targets. J. Diffuse lymphoid infiltration with blasts; LB target.
Table 1.
The results of consensus and interobserver evaluation
| CONSENSUS | INTEROBSERVER | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | MRI |
|
|||||||||||||||||||
| Histopathology** | Cytology | IHC | Tissue | Cytology | Diagnosis | ||||||||||||||||
|
|
|
||||||||||||||||||||
| G | SL | SLM | PVL | PVLM | PVLB | LB | NE | V | P1 | P2 | P3 | P1 | P2 | P3 | P1 | P2 | P3 | ||||
| 1 | Enhancing lesion with a mass effect in the left thalamus | 1 target | 7 targets | 1 target | 1 target | + | Ac | CD20: +++ | B | B | B | Ac | Ac | NDc | D | D | D | ||||
| CD3: + | |||||||||||||||||||||
| CD3: + | |||||||||||||||||||||
| 3 | Multiple enhancing lesions in deep gray structures | 2 targets | 4 targets | 2 targets | 1 target | Lc | CD20: +++ | B | B | B | Ac | Ac | NDc | D | D | D | |||||
| CD3: + | |||||||||||||||||||||
| 4 | Enhancing lesion with mass effect in left parietal lobe | 1 target | 2 targets | 3 targets | 3 targets | Bc | CD20: +++ | B | B | B | Bc | Bc | Bc | D | D | D | |||||
| CD3: + | |||||||||||||||||||||
| CD3: + | |||||||||||||||||||||
| 6 | Multiple enhancing lesions in corpus callosum, right and left parietal lobe | 4 targets | 4 targets | 2 targets | + | Bc | CD20: +++ | B | B | B | Bc | Bc | Bc | D | D | D | |||||
| CD3: + | |||||||||||||||||||||
| 7 | Periventricular enhancement | 9 targets | Bc | CD20: +++ | B | B | B | Bc | Bc | Bc | D | D | D | ||||||||
| CD3: + | |||||||||||||||||||||
| 8 | Enhancing lesion with mass effect in frontotemporal region | 2 targets | 8 targets | Bc | CD20: +++ | B | B | B | Bc | Bc | Bc | D | D | D | |||||||
| CD3: + | |||||||||||||||||||||
| 9 | Enhancing lesion with mass effect in right frontal lobe | 2 targets | 7 targets | Bc | CD20: +++ | B | B | B | Bc | Bc | Bc | D | D | D | |||||||
| CD3: + | |||||||||||||||||||||
| 10 | Multiple enhancing lesions in right temporal lobe and thalamus | 1 target | 3 targets | 4 targets | Bc | CD20: +++ | B | B | B | Bc | Bc | Bc | D | D | D | ||||||
| CD3: + | |||||||||||||||||||||
| 11 | Enhancing lesion with mass effect in right parietal lobe | 1 target | 5 targets | Bc | CD20: +++ | B | B | B | Bc | Bc | Bc | D | D | D | |||||||
| CD3: + | |||||||||||||||||||||
| 12 | Enhancing lesion with mass effect in right parietal lobe | 1 target | 2 targets | 4 targets | Bc | CD20: +++ | B | B | B | Bc | Bc | Bc | D | D | D | ||||||
| CD3: + | |||||||||||||||||||||
| 13 | Periventricular enhancement | 1 target | 1 target | 6 targets | Ac | CD20: +++ | A | A | N | Ac | Ac | NDc | D | D | S | ||||||
| CD3:+ | |||||||||||||||||||||
| 14 | Enhancing lesions in left cerebral hemisphere | 4 targets | 6 targets | Lc | CD20:+++ | N | N | N | NDc | Ac | NDc | ND | ND | ND | |||||||
| CD3: + | |||||||||||||||||||||
| 15 | Enhancing lesion in left thalamus, internal capsule, corpus callosum | 2 targets | 5 targets | 3 targets | Lc | CD20: +++ | B | A | A | Ac | Ac | NDc | D | D | S | ||||||
| CD3: + | |||||||||||||||||||||
| 16 | Left parietal mass with rim enhancement | 5 targets | 2 targets | 1 target | 1 targets | Lc | CD20: ++ | N | N | N | NDc | Ac | NDc | ND | ND | ND | |||||
| CD3: ++ | |||||||||||||||||||||
| 17 | Patchy T2 signal in both hemispheres | 4 targets | 4 targets | 2 targets | Lc | * | A | N | N | NDc | NDc | NDc | S | S | ND | ||||||
| CD20: +++ | |||||||||||||||||||||
| CD3: + | |||||||||||||||||||||
| 18 | Enhancing lesion in left-right temporal lobe, corpus callosum | 3 targets | 2 targets | 1 target | 1 target | Lc | CD20: ++ | N | N | N | NDc | NDc | NDc | ND | ND | ND | |||||
| CD3: ++ | |||||||||||||||||||||
| 19 | Multiple enhancing lesions in left thalamus and corpus callosum | 2 targets | 3 targets | 1 target | 1 target | + | Lc | CD20: + | A | N | N | NDc | NDc | NDc | ND | ND | ND | ||||
| CD3: +++ | |||||||||||||||||||||
| 20 | Enhancing lesion in left and right thalamus, internal capsule, corpus cal3losum | 3 targets | 5 targets | 1 target | NLc | CD20: ++ | N | N | N | NDc | NDc | NDc | ND | ND | ND | ||||||
| CD3: ++ | |||||||||||||||||||||
| 21 | Enhancing lesion with mass effect in right frontotemporal region | 5 targets | 5 targets | NLc | CD20: ++ | N | N | N | NDc | NDc | NDc | ND | ND | ND | |||||||
| CD3: ++ | |||||||||||||||||||||
| 22 | Multilocular enhancing lesion with mass effect in right temporal and occipital lobes | 8 targets | NLc | CD20: ++ | N | N | N | NDc | NDc | NDc | ND | ND | ND | ||||||||
| CD3: ++ | |||||||||||||||||||||
| 23 | Enhancing lesion with mass effect in left parietooccipital region | 2 targets | 3 targets | 3 targets | Lc | * | A | A | N | NDc | NDc | NDc | D | S | ND | ||||||
| CD20: +++ | |||||||||||||||||||||
| CD3: + | |||||||||||||||||||||
| 24 | Enhancement in left deep structures and contralaterally some small enhancing lesions | 8 targets | NLc | N | N | N | NDc | NDc | NDc | ND | ND | ND | |||||||||
| 25 | Left deep temporal mass with rim enhancement | 7 targets | NLc | N | N | N | NDc | NDc | NDc | ND | ND | ND | |||||||||
Co-dominance in SL targets;
Artefacted targets are not included in the table.
G: Non-neoplastic glial tissue. SL:Sparse lymphoid cells. SLM: Sparse macrophages and lymphocytes. PVL: Perivascular normal appearing lymphocytes. PVLM: Perivascular lymphoid cells and macrophages. PVLB: Perivascular lymphocytes intermingled with blasts. LB: Diffuse neoplastic infiltration with blasts. NE: Necrosis V: Infiltration/fragmentation of vessel wall. Ac: Suspicious lymphoid atypia. Lc: Normal lymphocytes with or without macrophages. Bc: Lymphoid cells with blasts. NLc: A few or no lymphoid cells. B: Blasts A: Suspicious atypia. N: Normal appearing lymphocytes. NDc: Non-diagnostic cytology. D: Diagnostic. S: Suspicious. ND: Non-diagnostic. P1: Pathologist 1. P2:Pathologist 2. P3: Pathologist 3.
Figure 2.
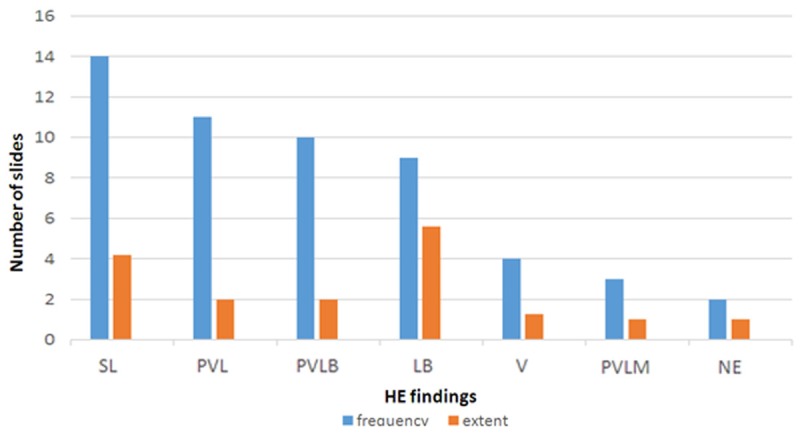
Characteristics of lymphoid infiltration in HE slides.
Figure 3.
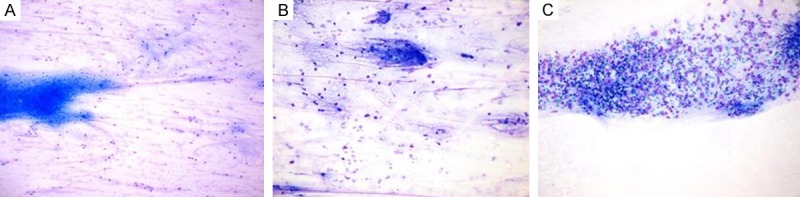
A. Normal appearing lymphocytes; example for Lc slide. B. A preparation found to have suspicious lymphoid atypia by 2 out of 3 observer. C. Lymphoid cells with obvious blasts.
Figure 4.
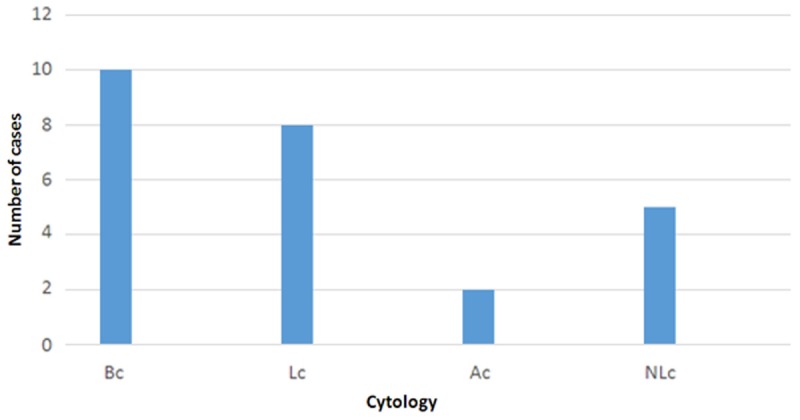
Characteristics of lymphoid infiltration in cytology slides.
In the IHC evaluation of 23 cases containing lymphoid population CD20 dominance was detected in 74% (17/23). The distribution of this dominance was as follows: All of the cases containing LB or PVLB and 8 cases with PVL. Thus in the cases characterized by PVL without any blasts or macrophages, the rate of CD20 dominance was 73%. On the other hand 3 cases containing PVL targets demonstrated mixed pattern with CD3 and CD20. Two of these cases also had PVLM targets demonstrating the same pattern. These cases were totally devoid of blasts. CD3 dominance was noted in only 1 case. Except for 2 cases, the dominant phenotype in SL targets was in concordance with the phenotype observed in LB, PVLB, PVL and/or PVLM target (s) of the corresponding case. But in these 2 cases although PVL targets demonsrated the CD20 dominance, SL targets had mixed staining. Among 3 cases which only had SL as lymphoid population, 1 had CD20 dominance and the other 2 were in mixed pattern (Figure 5). The distribution of the IHC findings are shown on Table 1 and Figure 6.
Figure 5.
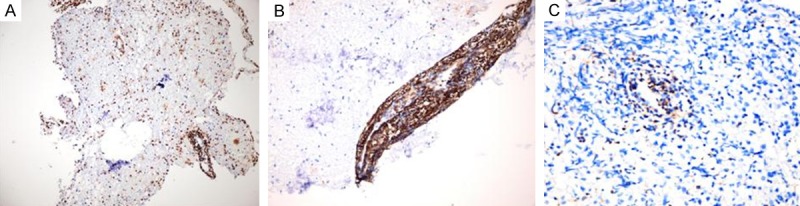
A, B. Extensive CD20 positivity. C. Scattered CD3 positivity in a LB target.
Figure 6.
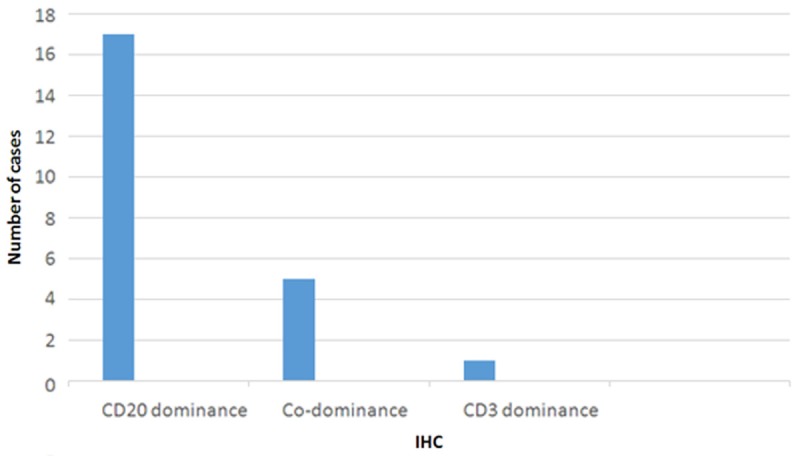
Characteristics of lymphoid infiltration in IHC slides.
Interobserver evaluation
Histopathology session; The pathologists were asked to classify the cases as containing blasts (B), lymphoid cells with suspicious atypia (A) or normal appearing lymphocytes (N). Cytology; The pathologists were asked to classify the cases as strongly suggestive with blasts (Bc), suspicious with lymphoid atypia (Ac), no lymphocytes or normal lymphocytes non-diagnostic for lymphoma (NDc). Final diagnostic session; The pathologists were asked to classify the cases as diagnostic for PCNSL (D), suspicious for PCNSL (S) or non-diagnostic (ND).
The rates of uniform agreement for histopathology, cytology and final diagnosis sessions were 80% (20/25), 72% (18/25) and 84% (21/25) respectively. In histopathology session the total rate of 80% was the sum of 48% (agreement on B) and 32% (agreement on N). None of the cases produced a uniform agreement with respect to A. In cytology the total rate of 72% was obtained by the sum of 36% (agreement on Bc) and 36% (agreement on NDc). None of the cases produced a uniform agreement on Ac. In final dignosis session the total rate of 84% was obtained by the sum of 48% (agreement on D), and 36% (agreement on ND). None of the cases produced a uniform agreement on S. The results of interobserver evaluation were shown on Table 1.
Discussion
Although it is widely accepted that CS pre-treatment presents a diagnostic problem in biopsy evaluation, the studies mostly focuses on the diagnostic yield and subsequent biopsy ratesand the related histopathological, and cytological findings remains somewhat unclear. Moreover the significance of subjectivity in the interpretation of these findings appears to be unconsidered. In this study we provided a detailed approach to histopathological, immunohistochemical and cytological findings of CS pre-treated PCNSL cases. We also investigated the role of subjectivity in 3 different sessions including the final diagnostic evaluation.
Our cohort was composed of 25 CS pre-treated PCNSL cases. By detailed evaluation of histopathological, cytological, and immunohistochemical features, three major groups emerged. The first group included the 48% of the cases and histopathologically characterized by prominent blasts either in diffuse infiltrates (LB) and/or in perivascular regions (PVLB). All cases in this group demonstrated IHC CD20 dominance and full interobserver agreement in independent histopathological evaluation with respect to presence of blasts. Accordingly interobserver testing for final diagnostic evaluation of this 48% yielded a complete agreement. That is; all the observers defined these cases as diagnostic for PCNSL and these were the only cases that were diagnosed as PCNSL with a full interobserver agreement. As a notable finding for this 48%, although PVLB was more frequent, in cases characterized by LB this was a more extensive finding involving more than the half of the targets in the mean. Thus 48% of our cohort seemed to be none or minimally affected by the CS and the diagnosis of PCNSL was straightforward. This result was in close proximity with the report of Bruck et al [16]. Nevertheless in cytological evaluation, only the LB cases demonstrated prominent blastic cytomorphology and complete interobserver agreement. On the other hand althoughthe imprint and squash preparations of PVLB cases were rich in lymphocytes they did not demonstrate obvious blasts. Likewise interobserver evaluation of these imprint and squash preparations with respect to presence of blasts or suspicious lymphoid atypia produced amarked variability.
The second group consisted of 20% of our cases and were histopathologically characterized by none (G) or sparsely scattered (SL) lymphoid cells. IHC pattern was variable (CD20 dominance or CD20/CD3 co-dominance). Interobserver testing did not produce any discrepancy in reproducibility of the negative results with respect to lymphoid atypia in HE session and final decision. So it seems that a total of 20% in our cases demonstrate the phenomenon called “tumor vanishing”. Nevertheless, independent cytological evaluation of lymphoid atypia demonstrated discrepancy in 1 case and this was the only case containing lymphoid cells in cytological preparations of G and SL cases. As another point, SL was the most frequent histopathological finding in whole cohort. However in majority of cases it was accompanied by other characterizing histopathological findings such as PVL, PVLM and PVLB.
The major challenge was in third group since this group produced a marked interobserver variability in each steps of evaluation. These were the cases characterized by PVL/PVLM and they constituted the 32% of our cohort. These cases had considerable amount of lymphoid cells but lacked an infiltration with obvious blasts. Similarly regarding the imprint and squash preparations, none of these cases contained typical blasts. Although angiocentric pattern is an important clue for PCNSL, it may also be seen in the non-neoplastic lymphoid infiltrates of other diseases such as vasculitis and meningoencephalitis [7,15]. Again accompanying macrophages, a finding observed in 12% of our cases, can raise the possibility of a demyelinating lesion. Besides CS-induced regression is not pathognomonic for PCNSL and may occur in many other diseases characterized by inflamatory cells [10]. As an helpfull finding, half of these cases demonstrated CD20 dominance. However in the interobserver evaluation, CD20 dominance did not assure the definitive diagnosis. Instead the pathologists tended to assign the cases as suspicious for lymphoma in the final diagnostic step. On the other hand as final diagnostic decision, a full interobserver agreement was observed in the other half that do not demonstrate CD20 dominance. In this agreement all the pathologists diagnosed the cases as non-diagnostic even without a suspicion. Of note, in final diagnostic session 16% of our cases were found to be suspicious for PCNSL at least by 1 observer. No agreement was observed in this diagnosis and all of these suspicious cases were in the third group. At this point the number of targets involved by PVL/PVLM and IHC dominance of CD20 appeared as the major determiners in this group. We think that particularly for this group of cases, the use of additional IHC markers, such as demonstration of high Ki67 index, would yield a great benefit. However because of sectioning inadequacy in several cases, our IHC panel was limited by CD20 and CD3.
It should be emphasized that the high subjectivity rate in independent histopathological and cytological evaluation of atypia appears as a striking finding of our study. The rate of total histopathological agreement was 80% and the variability was distinctly associated with the PVL/PVLM cases. This situation was more pronounced in the cytological evaluation. Only 72% of cases demonstrated a full cytological agreement and 36% of this rate was composed of the cases demonstrating LB in tissue sections. Although in consensus evaluation only 2 cases were indicated to have suspicious atypia in cytology, in interobserver evaluation 7 cases were found to have such atypia at least by 1 pathologist. Still none of these cases demonstrated a uniform agreement with respect to this parameter. Cytological evaluation is an important step for diagnosis in stereotactic samples. The diagnostic accuracy rate of smear preperations from intracranial lesions was reported up to 93%. It was also reported that combined use of histopathological and cytological analysis increased the diagnostic accuracy from 87% to 91%. Furthermore evaluation of imprint preparations have great value in intraoperative diagnosis [9,18]. However according to our findings from detailed cytological evaluation and interobserver comparison, in case of CS treated CNS lymphoma, cytological preparations present further limitations and it seems quite difficult, if not impossible, to make a diagnosis solely by cytological evaluation. To our knowledge this is the first study in English literature investigating the cytological findings in relation with detailed histopathological evaluation. Likewise our study appears to be the first study about the interobserver variability rates in stereotactic biopsy evaluation of CS pre-treated PCNSL cases.
In conclusion, corticosteroid pre-treatment in primary CNS lymphoma was observed to be associated with a large spectrum of histopathological, immunohistochemical and cytological findings. The diagnosis was straightforward in only 48% of our cases. The rest of our cases represented the great challenge in diagnosis of the lymphoma under corticosteroid effect. The vague lymphoid atypia appears as the most challenging finding because of the marked interobserver variability observed in its interpretation. Nevertheless we think that combined use of histopathological and cytological findings with an extended immunohistochemical panel would increase the possibility of conclusive diagnosis. Still it should be kept in mind that the majority of these findings are subtle and quite open to subjectivity.
Acknowledgements
This retrospective study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Authors declare that they do not have any conflict of interest.
Disclosure of conflict of interest
None.
References
- 1.Louis D, Ohgaki H, Wiestler O, Cavenee W. WHO Classification of Tumours of the Central Nervous System. 4th edition. Lyon: IARC; 2007. pp. 188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Wardiman JW. WHO Classification of tumors of Haematopoietic and Lymphoid Tissues. 4th edition. Lyon: IARC; 2008. pp. 240–241. [Google Scholar]
- 3.Doolittle ND, Abrey LE, Shenkier TN, Tali S, Bromberg JEC, Neuwelt EA, Soussain C, Jahnke K, Johnston P, Illerhaus G, Schiff D, Batchelor T, Montoto S, Kraemer DF, Zucca E. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2008;111:1085–1093. doi: 10.1182/blood-2007-07-101402. [DOI] [PubMed] [Google Scholar]
- 4.Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni R, Rossi A, Vavassori V, Conconi A, Devizzi L, Berger F, Ponzoni M, Borish B, Tingulely M, Cerati M, Milani M, Orvieto E, Sanchez J, Chevreau C, Dell’ Oro S, Zucca E, Cavali F. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experince. J. Clin. Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 5.Haldorsen IS, Espeland A, Larson JL, Mella O. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol. 2005;44:728–34. doi: 10.1080/02841860500256272. [DOI] [PubMed] [Google Scholar]
- 6.Küker W, Nägele T, Korfel A, Hecki S, Bamberg M, Weller M, Herrlinger U. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72:169–77. doi: 10.1007/s11060-004-3390-7. [DOI] [PubMed] [Google Scholar]
- 7.Baraniskin A, Deckert M, Schulte-Altedorneburg G, Schlegel U, Schroers R. Current strategies in the diagnosis of diffuse large B-cell lymphoma of the central nervous system. Br J Haematol. 2012;156:421–32. doi: 10.1111/j.1365-2141.2011.08928.x. [DOI] [PubMed] [Google Scholar]
- 8.Deckert M, Brunn A, Montesinos-Rongen M, Terreni MR, Ponzoni M. Primary lymphoma of the central nervous system--a diagnostic challenge. Hematol Oncol. 2014;32:57–67. doi: 10.1002/hon.2087. [DOI] [PubMed] [Google Scholar]
- 9.Weller M, Martus P, Roth P, Thiel E, Korfel A. German PCNSL Study Group Surgery for primary CNS Lymphoma? Challenging a paradigm. Neuro Oncol. 2012;14:1481–4. doi: 10.1093/neuonc/nos159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosal N, Hegde AS, Murthy G, Furtado SV. Smear preperation of intracranial lesions: a retrospective study of 306 cases. Diagn Cytopathol. 2011;39:582–92. doi: 10.1002/dc.21432. [DOI] [PubMed] [Google Scholar]
- 11.Bromberg JE, Siemers MD, Taphoorn MJ. Is a “vanishing tumor” always a lymphoma? Neurology. 2002;59:762–764. doi: 10.1212/wnl.59.5.762. [DOI] [PubMed] [Google Scholar]
- 12.Tun HW, Personett D, Baskerville KA, Menke DM, Jaeckle KA, Kreinest P, Edenfield B, Zubair AC, O’Neil BP, Lai WR, Park PJ, McKinney M. Pathways analysis of primary central nervous system lymphoma. Blood. 2008;111:3200–210. doi: 10.1182/blood-2007-10-119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponzoni M, Berger F, Chassagne-Clement C, Tinguely M, Jouvet A, Ferreri AJ, Dell’ Oro S, Terreni MR, Doglioni C, Weis J, Cerati M, Milani M, Luzzulino P, Motta T, Carbone A, Pedrinis E, Sanchez J, Blay JY, Reni M, Conconi A, Bertoni F, Zucca E, Cavalli F, Borisch B. International Extranodal Lymphoma Study Group, Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. Br J Haematol. 2007;138:316–323. doi: 10.1111/j.1365-2141.2007.06661.x. [DOI] [PubMed] [Google Scholar]
- 14.Venetz D, Ponzoni M, Schiraldi M, Ferreri AJM, Bertoni F, Doglioni C, Uguccioni M. Perivascular expression of CXCL9 and CXCL12 in primary central nervous system lymphoma: T-cell infiltration and positioning of malignant B-cells. Int J Cancer. 2010;127:2300–12. doi: 10.1002/ijc.25236. [DOI] [PubMed] [Google Scholar]
- 15.Shaw A, Iyer V, Rooney N, Wragg R, Waits P, Roberts E, Haynes HR, Kurian KM. Diagnosis of primary cerebral lymphoma: possible value of PCR testing in equivocal cases requiring rebiopsy. Br J Neurosurg. 2014;28:214–219. doi: 10.3109/02688697.2013.817531. [DOI] [PubMed] [Google Scholar]
- 16.Bruck W, Brunn A, Klapper W, Kulhmann T, Metz I, Paulus W, Deckert M. Differential dianosis of lymphoid infiltrates in the central nervous system: experience of the network lyphomas and lyphomatoid lesions in the nervous system. Pathologe. 2013;34:186–97. doi: 10.1007/s00292-013-1742-9. [DOI] [PubMed] [Google Scholar]
- 17.Porter AB, Giannini C, Kaufmann T, Luchinetti C, Wu W, Decker PA, Atkinson JLD, O’Neil BA. Primary central nervous system lymphoma can be histologically diagnosed after previous corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma. Ann Neurol. 2008;63:662–667. doi: 10.1002/ana.21366. [DOI] [PubMed] [Google Scholar]
- 18.Brommeland T, Lindal S, Straume B, Dahl IL, Henning R. Does imprint cytology of brain tumours improve intraoperative diagnosis? Acta Neurol Scand. 2003;108:153–156. doi: 10.1034/j.1600-0404.2003.00115.x. [DOI] [PubMed] [Google Scholar]


