Abstract
Objective: HOTAIR, a long intervening non-coding Hox transcript antisense intergenic RNA, negatively regulates transcription on another chromosome and is reported to reprogram chromatin organization and promote tumor progression. Nevertheless, little is known about its roles in the development of radiation therapy of lung cancer. In this study, we established a xenografed model of Lewis lung carcinoma in C57BL/6 mice and investigated the possible involvement of HOTAIR in this radiotherapy. Methods: C57BL/6 mice were subcutaneously transplanted with Lewis lung carcinoma cells and locally irradiated followed by measurement in tumor volume. Levels of HOTAIR and WIF-1 mRNA expression were determined by using Quantitative Real-Time PCR. Levels of WIF-1 and β-catenin were determined by using western blot assay. Cell viability was evaluated by MTT assay. Cell apoptosis was examined by using TUNEL assay. Results: In mice bearing Lewis lung carcinoma tumor, local radiotherapy suppressed tumor growth and it also reduced level of HOTAIR but increased WIF-1 expression. When HOTAIR was overexpressed, radio-sensitivity was reduced. In vitro experiments, irradiation inhibited HOTAIR transportation to the nucleus. However, it was reversed by over-expressed HOTAIR. Cells transfected with pcDNA-HOTAIR or siRNA-HOTAIR resulted in decline or increase in radiosensitivity, which was abrogated by co-tansfected with siRNA-β-catenin. Conclusion: Radiotherapy induced Lewis lung cancer cell apoptosis via inactivating β-catenin mediated by upregulated HOTAIR.
Keywords: β-catenin, lewis lung cancer, radiotherapy, xenograft model, WIF-1
Introduction
At present, lung cancer is still one of the most prevalent malignancies worldwide and is the leading cause of death in both men and women [1]. Despite advances made in therapy, the median overall survival is only 15 months, indicating an urgently need for new strategies to improve curative effect. Traditionally, for conveniently be located, approximately 70% of patients with lung cancer receive external beam radiation treatment as one component of their treatment [2]. However, individuals respond to radiotherapy differently and the efficacy of irradiation treatment is often impaired by the emergence of radioresistance. Thus, a better understanding the molecular mechanisms underlying the development of radiotherapy would promote our understanding of lung cancer development and treatment failure.
Recently, evidences have been accumulating to suggest a significant relationship between radiotherapy and epigenetic alterations exists [3-5]. Both microRNA (miRNAs) and long non-coding RNAs (lncRNAs) are the major regulatory noncoding RNAs that regulate gene expression at epigenetic, transcriptional, and post-transcriptional processing levels [6,7]. An increasing number of contemporary studies have shown that altered microRNA expression may play an important role in the radiotherapy of cancer cells by impairing cellular responses that affect cell cycle arrest, apoptosis, and DNA damage repair [8]. The increasing prevalence of miRNA mediated radiotherapy data has led to an increase in studies focused on targeting miRNAs as strategies for therapeutic intervention. Unfortunately, the correlations between lncRNAs and tumor chemoresistance are rarely reported, and need to be more clearly elucidated before these therapeutic strategies can be fully developed and undergo clinical assessment. HOTAIR is one of the few biologically well-documented lncRNAs, with a length of 2158 bp and a functional role in the mediation of trans-silencing [9]. HOTAIR has been found to be overexpressed in a variety of human cancers and determined to be a negative prognostic indicator in breast, colon, liver, and pancreatic cancer patient survival, evidencing a close association with increase in cancer cell metastasis [9-12]. Zhuang and his colleagues showed that tumor-promoting Col-1 up-regulates the expression of HOTAIR in NSCLC cells, suggesting that HOTAIR might play critical roles in lung tumorigenesis [13]. Although numerous studies have demonstrated the critical functions of HOTAIR in tumor development and metastasis, whether HOTAIR plays an import role during development of radiotherapy in lung cancer is still unclear.
In the present study, we established mice bearing tumor model. qRT-PCR assay was performed to detect the expression of HOTAIR in radio-cured tumor and irradiated Lewis lung cancer cells. Then, functional relationship analyses between HOTAIR and β-catenin nuclear translocation was conducted to evaluate the molecular signaling on the radiotherapy of lung cancer both in vivo and in vitro. This study explores the validity of HOTAIR as a valid therapeutic target for the reversal of radiotherapy in lung cancer.
Material and method
Mice, cells and reagents
C57BL/6 male mice (aged 6 weeks; n=20) were obtained from Institute of Laboratory Animal Science (Chinese academy sciences, Shanghai) and housed in polycarbonate cages containing woodchip bedding in a room with controlled temperature (20 to 24°C) and relative humidity (50% to 65%). Mice were fed rodent maintenance diet and provided with water ad libitum. The protocol was approved by the Guide for the Care and Use of Laboratory Animals.
The Lewis cancer cell line was cultured in RPMI 1640 (Gibco, Invitrogen Company, USA) supplemented with 10% FCS and human insulin (10 μg/ml). The cell cultures were maintained in a water humidified 37°C incubator with 5% CO2.
Quantification of lncRNAs and real-time PCR reactions
Total RNA isolated from plasma samples were used for cDNA synthesis using the First Strand cDNA Synthesis kit (Thermo Scientific, USA) according to the manufacturers’ instructions. The real-time amplification of lncRNA molecules was performed using the Light Cyler 480 (Roche, Germany). SYBR Green (Roche) was used as the fluorescent molecule. The endogenous reference gene for real-time PCR reactions was selected by comparing the basal expression levels of the GAPDH (glyceraldehyde-3-phosphate). The GAPDH gene was chosen in view of its higher stability and consistency in the control plasma samples.
The PCR program included an initial “hot start” for 10 min, followed by 45 cycles of amplification. Each cycle consisted of a denaturation step at 95°C for 10 s, annealing starting at 60°C for 20 s and decreasing 2°C every 2 cycles until 55°C, and amplification at 72°C for 30 s. For quantification of lncRNAs, the ΔΔCt method was used by coamplifying GAPDH for each sample and by comparing the Ct values. All experiments were performed triplicate and the mean values were calculated. Primers sequences are as followed: HOTAIR: sense, 5’-CAGTGGGGAACTCTGACTCG-3’; and reverse, 5’-GTGCCTGGTGCTCT-CTTACC-3’. WIF-1: sense, 5’-AGTGTCCTGATGGGTTCCAC-3’; and reverse, 5’-TGGTTGAGCAGTTTGCTTTG-3’.
Tumor volume assessment
Lewis lung cancer cells were used in a xenograft model in female C57BL/6 mice. A suspension of 1×106 cells in 100 μL volume was injected subcutaneously into the right flank of mice using a 1-cc syringe with 27½-gauge needle. Tumors were grown for 7 days. Tumors on the flanks of the mice were irradiated using an X-ray irradiator (Therapax, Agfa NDT, Inc., Lewis Town, PA). The non-tumor parts of the mice were shielded by lead blocks. Tumors were measured 2 or 3 times weekly in 3 perpendicular dimensions using a Vernier caliper. Tumor volumes were calculated using the modified ellipse volume formula (Volume = (Height × Width × Depth)/2). Growth delay was calculated as the number of days required to reach a tumor volume of 1.75 cm3 for treatment groups relative to the control.
Protein extraction and Western blot
Proteins in Lewis lung cancer cells and tumors were extracted by inM-PER mammalian protein extraction reagent (Pierce Biotechnology, Rockford, IL, USA). The nuclear protein fraction was extracted using the NE-PER Nuclear and cytoplasmic protein was extracted using Cytoplasmic Extraction Kit (Pierce Biotechnology, Rockford, IL, USA) from Lewis lung cancer cells and Lewis lung cancer -HOTAIR cells, according to the manufacturer’s instructions. Protein concentration of cell lysates was measured by using DC protein assay kit (Bio-Rad). Proteins (10-20 mg) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to PVDF membrane (Milipore, Bedford, MA, USA). Two hours after blocking in 10% nonfat milk, the protein blots were then incubated with primary antibodies in 5% bovine serum albumin at 4°C overnight, followed by incubation with secondary antibodies at room temperature for 2 h. The protein signals were detected by ECL method. For Western blot, antibodies against WIF-1 (1:500; Santa Cruz, CA, USA), β-catenin (1:1000; Millipore, Bedford, MA, USA) and GAPDH (1:5000; D16H11, CST) were used.
TUNEL assay
The TUNEL reaction, based on labeling of DNA strand breaks, was conducted to detect cells apoptosis and performed with an in situ cell detection kit (Roche Applied Science, Indianapolis, IN, USA), according to the manufacturer’s instruction. In brief, sections in 2 changes of xylene for 5 minutes each, and hydrate with 2 changes of 100% ethanol for 3 minutes each, and 95% ethanol for 1 minute followed by rinsing in distilled water. After pretreated with proteinase K, cells were cultured in TdT Reaction Buffer for 10 minutes and then conducted TdT Reaction in TdT Reaction Mixture for 1-2 hours at 37-40°C in humidified chamber. The reaction was stopped with washing buffer and the sample was resuspended in PBS containing FITC-Avidin D for 30 minutes at room temperature. The reaction mixture was counterstained with PI or DAPI for 20 minutes. TUNEL-positive nuclei (stained brown) were counted by Motic Images Advanced 3.2 in 10 random fields (×200), and then averaged.
Transfection of siRNAs and plasmid vectors
The cells were seeded into 6-well plates and transfected with 50 nM siRNAs targeting HOTAIR (siRNA/HOTAIR1: 5’-UUUUCUACCAGGUCG-GUAC-3’; siRNA/HOTAIR2: 5’-AAUUCUUAAAUUGGGCUGG-3’) (GenePharma, Shanghai, China) or 50 nM siRNAs targeting siRNA/β-catenin (siRNA/β-catenin, 5’-AGCUGAUAUUGAUGGACAG-3’; 5’-CAGUUGUGGUUAAGCUCUUdAdC-3’) or nM siRNAs targeting β-catenin siRNA/control (5’-CUACAACAGCCACAACGUCdTdT-3’) (GenePharma, Shanghai, China) using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturers’ instructions.
Overexpression HOTAIR in Lewis lung cancer cells was performed by retrovirus mediated gene transfer. Briefly, the HOTAIR gene was subcloned into pcDNA3.1 (+) (Invitrogen, USA) by PCR method using the following primers: HOTAIR, sense, 5’-CATGGATCCACATTCTGCCCTGATTTCCGGAACC-3’; reverse, 5’-ACTCTCGAGCCACACACACACACAACCTACAC-3’. The PCR products were separated by electrophoresis on a 1% agarose gel and the gel was stained with ethidium bromide and imaged. The identity of the PCR product was confirmed by direct sequence analysis. The recombinant vector was confirmed by the digestion analysis of restriction endonuclease and DNA sequencing. The stable HOTAIR-expressing cells were generated by transfection with either pcDNA/HOTAIR or pcDNA/control vectors using Lipofectamine 2000, followed by selection with G418.
MTT assay
Lewis lung cancer cells were seeded at a density of 1400 cells/well. On the next day, cells were irradiated with dose 5 Gy for 24 h. After irradiation, 50 mL of 5 mg/mL 3-(4,5-Dimethylthiazol-2-yl) 22,5-diphenyltetrazolium bromide (MTT) was added to wells for 4 h, and then the reaction was stopped by media replaced with DMSO. Optical density analysis was performed by using an mQuant Microplate Spectrophotometer (BioTek, UK) at a wavelength of 540 nm.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software (Chicago, IL). One-way Analysis of Variance (ANOVA) was used to analyze the significance differences between control groups and irradiation treatment groups as well as different time points. Student’s test was used to test the significance of the mRNA and protein expression. Error bars represent SD. P-Values <0.05 were considered statistically significant.
Results
HOTAIR expression in lung tumor radiotherapy
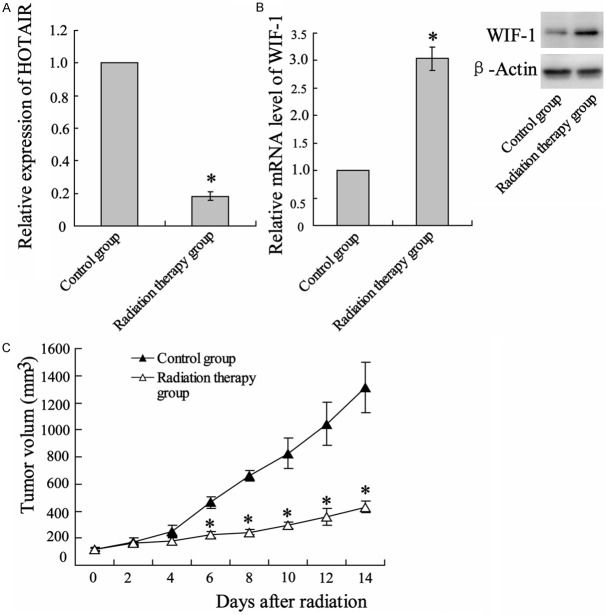
We subcutaneously transplanted C57BL/6 mice with Lewis lung carcinoma cells and locally irradiated tumor with a single dose of 12 Gy1f/1d for 14 days. After radiation treatment, HOTAIR expression levels were assessed in xenografted tumor by qRT-PCR normalized to the GAPDH. Compared with no treatment, irradiation substantially attenuated HOTAIR expression (Figure 1A). Inversely, WIF-1 expression in levels of both mRNA and protein was enhanced (Figure 1B). During radiotherapy, tumor size was regularly measured and the result showed a time-dependent inhibition on tumor growth by irradiation (Figure 1C).
Figure 1.
Radiotherapy suppressed tumor growth in mice bearing Lewis lung tumor. C57BL/6 mice were abdominal subcutaneous injected with Lewis lung cancer cells. Seven days after xenograftion, mice were locally irradiated for 14 days with 12 Gy/1f/1d of radiation (detailed in Materials and Methods). After radiotherapy, the mice were euthanased, and the tumors were removed for analysis. A. Uantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis on expression of HOTAIR in irradiated tumors and corresponding non radiotherapy tumors. B. qRT-PCR and western blot assay were performed to determined levels of WIF-1 mRNA and protein, respectively. C. In the period of irradiation, tumors were measured regularly and volume was plotted vs. time. Columns, mean of three individual experiments in each clone; bars, SD; *P<0.05 as compared with no irradiated tumor.
HOTAIR is essential for lung cancer radiotherapy
To identify the role of HOTAIR in radiotherapy, the Lewis lung cancer cells were transfected with pcDNA-HOTAIR before tumor xenograft. After Normal breeding for 7 days, tumor-bearing mice were locally irradiated with 12 Gy/1f/1d for 14 days and tumors were measured regularly per 2 days. As showed in Figure 2, over-expressed HOTAIR substantially reduced tumor radiosensitivity, which causes tumors to grow normally.
Figure 2.
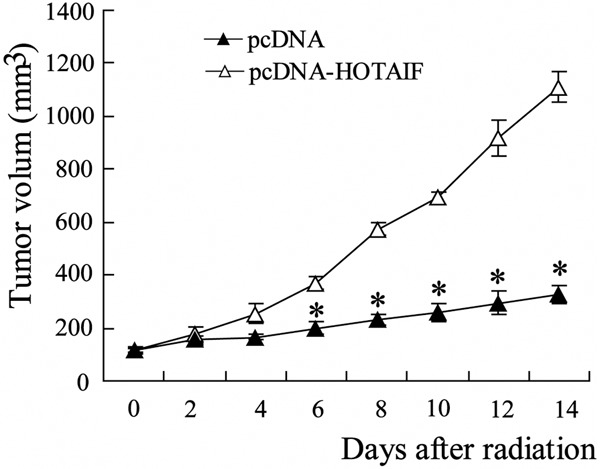
Over-expressed HOTAIR reduced radiosensitivity in xenografted Lewis lung tumor. Lewis lung cancer cells were pretreated with pcDNA-HOTAIF or control pc-DNA, followed by being transplanted to C57BL/6 mice. After Normal breeding for 7 days, tumor-bearing mice were locally irradiated with 12 Gy/1f/1d for 14 days and tumors were measured regularly and volume was plotted vs. Time. Columns, mean of three individual experiments in each clone; bars, SD; *P<0.05 as compared with no irradiated tumor.
Characterization of HOTAIR and β-catenin expression after irradiation in Lewis lung cancer cells
The above results suggested the essential role of HOTAIR for lung cancer radiotherapy. To ascertain the underlying mechanism, Lewis lung cancer cells were irradiated with 5 Gy. Level of HOTAIR expression was assessed by using western blot assay. In according with in vivo, the result showed a much lower level of HOTAIR in cells exposed to irradiation (Figure 3A). Consistently, levels of WIF-1 mRNA and protein were promoted by x ray irradiation (Figure 3B). WIF-1 is an inhibitor for Wnt/β-catenin signaling pathway and its combination to Wnt proteins can leads to β-catenin degradation. Thus, we respectively evaluated β-catenin expression level in whole-cell, cytoplasm and nuclear, respectively, by using western blot. As represented in Figure 3C, irradiation did not alter total β-catenin expression, but prevented protein transportation to the nucleus, which indicating inactivation action of β-catenin by irradiation.
Figure 3.
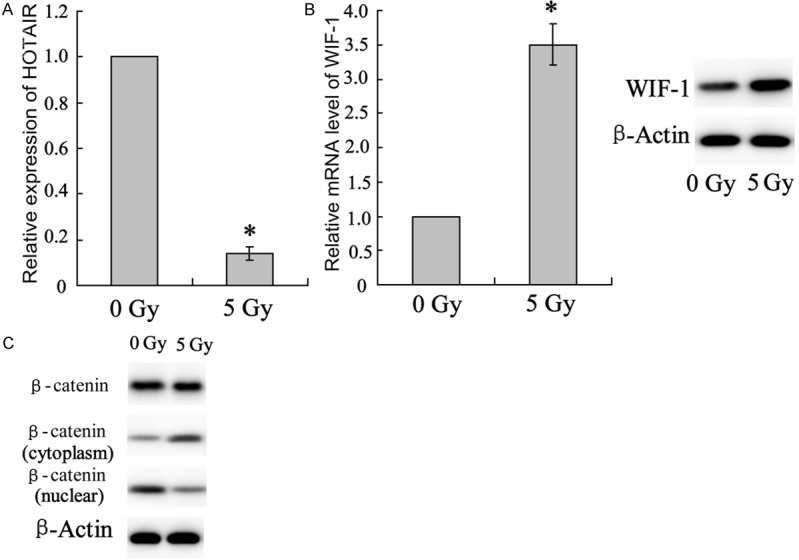
Changes in signaling molecule expression in irradiated Lewis lung cancer cells. Lewis lung cancer cells were irradiated with 5 Gy for 24 hours. A. Expression of HOTAIR was determined by using qRT-PCR. B. Detection on levels of WIF-1 mRNA and protein expression using qRT-PCR and western blot assay, respectively. C. Total, cytoplasm and nuclear β-catenin were represented by using western blot assay. Columns, mean of three individual experiments in each clone; bars, SD; *P<0.05 as compared with no irradiated cells.
HOTAIR promoted β-catenin transportation to the nucleus
To verify the regulator role of HOTAIR on β-catenin activity, the cells were transfected with si-HOTAIR or pcDNA-HOTAIR and expression of WIF-1 and β-catenin was represented by using western blot assay. Consequently, silenced HOTAIR up-regulated WIF-1 expression but inhibited nuclear translocation of β-catenin protein (Figure 4A). While HOTAIR over-expression by pcDNA-HOTAIR attenuated WIF-1 expression but increased nuclear β-catenin concentration (Figure 4B).
Figure 4.
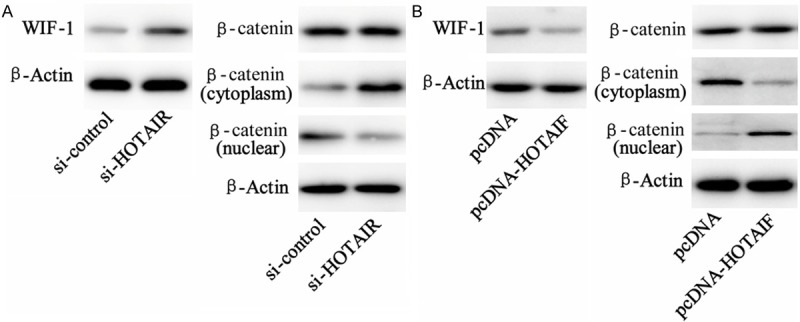
Effect of HOTAIR on expression of WIF-1 and β-catenin in Lewis cancer cells. A. Lewis lung cancer cells were treated with si-HOTAIR or its control RNA. Western blot analysis on WIF-1 and β-catenin (both in cytoplasm and unclear) expression was performed 24 hours after transfection. B. Lewis lung cancer cells were treated with pcDNA-HOTAIF or its pcDNA control, Western blot analysis on WIF-1 and β-catenin (both in cytoplasm and unclear) expression was performed 24 hours after transfection.
β-catenin interference reversed HOTAIR-reduced radiosensitivity
Irradiation treatment decreased cell viability. While pcDNA-HOTAIR transfection reduced cancer cells radiosensitivity and increased cell viability, which was reversed by cells co-transfected with pcDNA-HOTAIR and si-β-catenin (Figure 5A). Additionally, apoptosis of cancer cells were evaluated by TUNEL assay. The result showed that irradiation promoted lung cancer cells apoptosis but the radiation effect was suppressed by HOTAIR over-expression. While cell apoptosis responding to radiotherapy was recovered when cells was combining treated with pcDNA-HOTAIR and si-β-catenin (Figure 5B).
Figure 5.
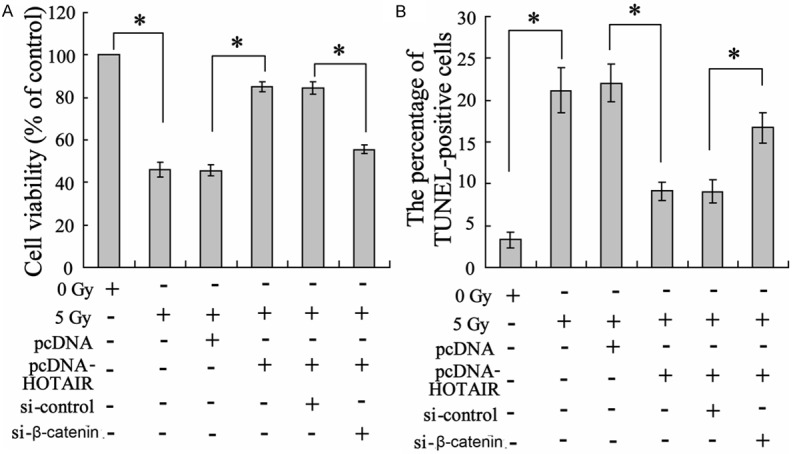
Effect of β-catenin on radiosensitivity of Lewis lung cancer cells. Lewis lung cancer cells were treated with pcDNA-HOTAIR, β-catenin or their control lentivirus, followed by irradiation with 5 Gy. Twenty-four hours after radiation, (A) MTT assay was conducted to assess cell viability and (B) TUNEL was performed to evaluate cell apoptosis. Columns, mean of three individual experiments in each clone; bars, SD; *P<0.05 as compared with the corresponding control group.
Discussion
Radiotherapy, based on its activation effect on DNA-damaged cellular signaling molecular, is proved to be the major therapeutic option for cancer treatment. However, many tumors have shown radioresistance which greatly limits the effect of radiotherapy. Cancer cells tend to be resistant to radiotherapy due to defects in apoptosis, overexpression of genes related to cell survival [14]. Therefore, fully exploration of the therapeutic targets and mechanisms underlying radiotherapy might provide better curative effect. In our current studies, we demonstrated that long non-coding RNAs-HOTAIR are the negative regulator for lung cancer radiosensitivity inhibits and functioned as an inhibitor for nuclear localization of β-catenin molecule signaling.
As an important regulator for chromosome remodeling, transcription and post-transcriptional processing, long noncoding RNAs (lncRNAs) have received increased attention, especially in cancers [15]. HOTAIR has been found to be overexpressed in a variety of human cancers and determined to be a negative prognostic indicator in breast, colon, liver, and pancreatic cancer patient survival, supporting a close association with increase in cancer cell metastasis [9-12]. In lymph node metastasis, HOTAIR is overexpressed and was showed as an promoter for lung cancer cell motility and invasion [16]. HOTAIR is also relevant to cellular proliferation, invasiveness, and clinical relapse in small-cell lung cancer [17,18]. Importantly, recent findings suggest that upregulation of HOTAIR contributes to the chemoresistance of human lung adenocarcinoma cells through its target gene expression [19] suggesting the might therapeutic target for cancer therapy. In this study, we are the first to show that irradiation downregulated HOTAIR expression in xenografted tumor.
HOTAIR is one of long noncoding RNAs and plays an important role in carcinogenesis via being associated with storage or modification of the pre-mRNA of gene function regulation [20]. Our data showed that, along with HOTAIR downregaltion, radiotherapy increased both Wnt inhibitory factor 1 (WIF-1) mRNA level and WIF-1 protein expression in irradiation-treated tumor tissue. WIF-1, as a key inhibitor of the Wnt/β-catenin signaling pathway, binds directly to extracellular Wnt ligands, preventing their interaction with the receptors and leading to degradation of cytosolic β-catenin by the APC/Axin1 destruction complex [21]. Thus, we evaluated nuclear β-catenin in irradiated-Lewis lung cancer cell and the data showed an inhibition for β-catenin transportation to the nucleus from cytoplasm. Being the key transcriptional activation factor of Wnt/β-catenin signal pathway, upon upstream activation, β-catenin often translocates to the nucleus from cytoplasm to activate its target genes, which in turn play pivotal role in tumor initiation and development [22-25]. These data indicated that the underlining mechanism of radiotherapy may be through a repression of β-catenin signaling, via down-regulation of HOTAIR expression. This was further demonstrated in vitro by Lewis lung cancer cells transfected with pcDNA-HOTAIR that resulted in accumulation of β-catenin in nuclear and in vivo by co-transfection of siRNA-β-catenin and pcDNA-HOTAIR reduced cancer cell apoptosis.
In conclusion, our results uncovered the mechanisms by which radiotherapy down-regulates HOTAIR, in turn alters the nuclear localization of β-catenin, resulting in lowered β-catenin signaling and eventual inhibition of proliferation. This discovery will contribute to a better understanding of not only the molecular mechanisms of radiotherapy in lung cells, but also the development of combination between new gene therapies and radiotherapies for types of malignancies.
Disclosure of conflict of interest
None.
References
- 1.Esposito L, Conti D, Ailavajhala R, Khalil N, Giordano A. Lung Cancer: Are we up to the Challenge? Curr Genomics. 2010;11:513–518. doi: 10.2174/138920210793175903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amini A, Yeh N, Gaspar LE, Kavanagh B, Karam SD. Stereotactic body radiation therapy (SBRT) for lung cancer patients previously treated with conventional radiotherapy: a review. Radiat Oncol. 2014;9:210. doi: 10.1186/1748-717X-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He J, Hua J, Ding N, Xu S, Sun R, Zhou G, Xie X, Wang J. Modulation of microRNAs by ionizing radiation in human gastric cancer. Oncol Rep. 2014;32:787–793. doi: 10.3892/or.2014.3246. [DOI] [PubMed] [Google Scholar]
- 4.Mao A, Liu Y, Zhang H, Di C, Sun C. microRNA expression and biogenesis in cellular response to ionizing radiation. DNA Cell Biol. 2014;33:667–679. doi: 10.1089/dna.2014.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cellini F, Morganti AG, Genovesi D, Silvestris N, Valentini V. Role of microRNA in response to ionizing radiations: evidences and potential impact on clinical practice for radiotherapy. Molecules. 2014;19:5379–5401. doi: 10.3390/molecules19045379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipovich L, Johnson R, Lin CY. MacroRNA underdogs in a microRNA world: evolutionary, regulatory, and biomedical significance of mammalian long non-protein-coding RNA. Biochim Biophys Acta. 2010;1799:597–615. doi: 10.1016/j.bbagrm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Harries LW. Long non-coding RNAs and human disease. Biochem Soc Trans. 2012;40:902–906. doi: 10.1042/BST20120020. [DOI] [PubMed] [Google Scholar]
- 8.Ma H, Rao L, Wang HL, Mao ZW, Lei RH, Yang ZY, Qing H, Deng YL. Transcriptome analysis of glioma cells for the dynamic response to gamma-irradiation and dual regulation of apoptosis genes: a new insight into radiotherapy for glioblastomas. Cell Death Dis. 2013;4:e895. doi: 10.1038/cddis.2013.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu L, Zhu G, Zhang C, Deng Q, Katsaros D, Mayne ST, Risch HA, Mu L, Canuto EM, Gregori G, Benedetto C, Yu H. Association of large noncoding RNA HOTAIR expression and its downstream intergenic CpG island methylation with survival in breast cancer. Breast Cancer Res Treat. 2012;136:875–883. doi: 10.1007/s10549-012-2314-z. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi M, Kogo R, Shibata K, Sawada G, Takahashi Y, Kurashige J, Akiyoshi S, Sasaki S, Iwaya T, Sudo T, Sugimachi K, Mimori K, Wakabayashi G, Mori M. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 2013;29:946–950. doi: 10.3892/or.2012.2219. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 13.Zhuang Y, Wang X, Nguyen HT, Zhuo Y, Cui X, Fewell C, Flemington EK, Shan B. Induction of long intergenic non-coding RNA HOTAIR in lung cancer cells by type I collagen. J Hematol Oncol. 2013;6:35. doi: 10.1186/1756-8722-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94:3127–3134. doi: 10.1002/cncr.10591. [DOI] [PubMed] [Google Scholar]
- 15.Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, An Y, Liang Y, Xie XW. Role of HOTAIR long noncoding RNA in metastatic progression of lung cancer. Eur Rev Med Pharmacol Sci. 2014;18:1930–1936. [PubMed] [Google Scholar]
- 17.Ono H, Motoi N, Nagano H, Miyauchi E, Ushijima M, Matsuura M, Okumura S, Nishio M, Hirose T, Inase N, Ishikawa Y. Long noncoding RNA HOTAIR is relevant to cellular proliferation, invasiveness, and clinical relapse in small-cell lung cancer. Cancer Med. 2014;3:632–642. doi: 10.1002/cam4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W, De W, Wang Z, Wang R. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21(WAF1/CIP1) expression. PLoS One. 2013;8:e77293. doi: 10.1371/journal.pone.0077293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagawa T, Endo H, Yokoyama M, Abe J, Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T, Satoh K. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun. 2013;436:319–324. doi: 10.1016/j.bbrc.2013.05.101. [DOI] [PubMed] [Google Scholar]
- 20.Wilusz JE, Freier SM, Spector DL. 3’ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell. 2008;135:919–932. doi: 10.1016/j.cell.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 22.Li ZR, Wu YF, Ma CY, Nie SD, Mao XH, Shi YZ. Down-regulation of c-Myc expression inhibits the invasion of bile duct carcinoma cells. Cell Biol Int. 2011;35:799–802. doi: 10.1042/CBI20110099. [DOI] [PubMed] [Google Scholar]
- 23.Villar J, Cabrera NE, Valladares F, Casula M, Flores C, Blanch L, Quilez ME, Santana-Rodriguez N, Kacmarek RM, Slutsky AS. Activation of the Wnt/beta-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS One. 2011;6:e23914. doi: 10.1371/journal.pone.0023914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeilstra J, Joosten SP, Dokter M, Verwiel E, Spaargaren M, Pals ST. Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Res. 2008;68:3655–3661. doi: 10.1158/0008-5472.CAN-07-2940. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Gill AJ, Issacs JD, Atmore B, Johns A, Delbridge LW, Lai R, McMullen TP. The Wnt/beta-catenin pathway drives increased cyclin D1 levels in lymph node metastasis in papillary thyroid cancer. Hum Pathol. 2012;43:1044–1050. doi: 10.1016/j.humpath.2011.08.013. [DOI] [PubMed] [Google Scholar]