Abstract
Objective: To observe the anti-inflammatory effects of honokiol in primary cultures of peripheral blood mononuclear cells of rheumatoid arthritis patients, the pro-inflammatory cytokines and potential targets were investigated. Methods: The levels of GM-CSF, IL-1β, TNF-α and IL-8 were determined by ELISA assay. The genes and proteins expression were analyzed by real-time PCR and Western blotting respectively. Results: The serum IL-1β, TNF-α and GM-CSF levels were 1.76-, 2.16- and 3.57-fold increased in patients with RA as compared to those of control group. Honokiol inhibited the expression levels of IL-1β, TNF-α, GM-CSF and IL-8 in PBMCs with a dose-dependent manner. Measurements obtained from supernatants were positively correlated between TNF-α and IL-1β, moreover, similar results found TNF-α levels positively correlated with GM-CSF and IL-8 activity in the supernatants of PBMCs isolated from RA patients. Furthermore, the mRNA and protein expression of IL-1β, GM-CSF and IL-8 were up-regulated when the PBMCs exposure to TNF-α, however, honokiol treatment significantly reversed the expression of IL-1β, TNF-α and GM-CSF in response to TNF-α with a dose-dependent manner. Conclusions: This study demonstrates that honokiol could possess potential anti-inflammatory effects and inhibits TNF-α-induced IL-1β, GM-CSF and IL-8 production in PBMCs from rheumatoid arthritis patients.
Keywords: Rheumatoid arthritis, TNF-α, IL-1β, GM-CSF, IL-8
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease with chronic inflammatory of peripheral joints and abnormal immune responses, about 1% of the world population who have suffered from this progressive joint destruction disease [1,2]. The pathogenesis of RA is thought that the interaction of environmental factors, genetic and immuno- inflammatory responses contributes to the progression of RA [3]. In addition to the deposition of immune complexes (IC) of autoantigens and their antibodies, however, the interaction between rheumatoid factor and pro-inflammatory cytokines play a vital roles in the pathophysiology of RA [4]. Active pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) or IL-6 are involved in the synovial inflammation of RA [5,6]. Macrophages are the major cells involved in the pathogenesis of inflammatory arthritis. These cells are abundant in the inflamed synovial tissue and their number in the synovial sublining layer is correlated with disease activity and response to treatment [7,8]. RA macrophages directly contribute to the degradation of articular cartilage and subchondral bone [9]. Furthermore, the activation of RA monocyte-macrophages is not locally restricted at the synovial level, but involves also those of the peripheral circulation [10,11].
Previous studies have shown that macrophage colony-stimulating factor (M-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF), main factors of monocytes/macrophages survival, are expressed in the synovial fluid and membrane of RA patients [12,13]. In vivo, M-CSF loss-of function is protected against antigen induced arthritis [10]. Furthermore, GM-CSF has been shown to exacerbate arthritic disease in animals, meanwhile, lacking functional GM-CSF is protected from collagen-induced arthritis in mice [11,12]. Intriguingly, synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in RA [14]. These studies demonstrate that M-CSF and GM-CSF can exacerbate the inflammatory response and support the role of monocytes/macrophages in the pathogenesis of inflammatory arthritis. However, the pro-inflammatory and immunomodulatory effects of GM-CSF appear to depend on the dose and the presence of other relevant cytokines in the context of an immune response [15]. A recent study reveals that pro-inflammatory cytokines (IL-1β and TNF-α) promote monocyte viability via GM-CSF [16]. The results suggest that there could be interaction between GM-CSF and IL-1β, as well as TNF-α.
Honokiol, a small molecular weight natural product isolated and purified from the Magnolia officinalis, has been shown to possess potent anti-oxidation [17] and anti-inflammatory [18]. Functional studies reveal that honokiol can prevent cartilage matrix degradation in human osteoarthritis chondrocytes [19] and inhibit the progression of collagen-induced arthritis [20]. Further research shows that honokiol as an effective inhibitor can prevent tumor necrosis factor-α-induced up-regulation of inflammatory cytokine and chemokine production in human synovial fibroblasts [21]. There has been no study on the effect of honokiol on human peripheral blood mononuclear cells-derived macrophages, major cells involved in the pathogenesis of inflammatory RA. Therefore, we explored the effects of honokiol in primary cultures of human monocyte-derived macrophages, isolated from whole blood of patients with RA, in terms of proinflammatory cytokines (IL-1β, IL-8 and TNF-α).
Materials and methods
Serum samples and cell culture
Human blood samples were obtained with written informed consent from Department of Immunology and Rheumatology, Affiliated Hospital of Weifang Medical University. The study was approved by the Ethics Committee of the Department of Immunology and Rheumatology, Affiliated Hospital of Weifang Medical University. 14 serum samples of RA patients and 13 cases of healthy control were collected between May 2014 and Jan. 2015.
The peripheral blood mononuclear cells (PBMCs) were separated from erythrocytes by density centrifugation in the blood samples of RA patients and healthy control, and maintained in RPMI-1640 (Invitrogen, USA) supplemented with 10% FBS (Invitrogen, USA) at 37°C in a humidified incubator (Thermo, USA), 5% CO2, 95% air atmosphere. The medium was replenished two days.
Quantitative real-time PCR
The PBMCs RNA extraction was performed according to the TRIzol manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Synthesis of cDNAs was performed by reverse transcription reactions with 1 μg of total RNA using moloney murine leukemia virus reverse transcriptase (Promega, Switzerland) with oligo dT (15) primers (Fermentas) as described by the manufacturer. Real-time PCR was carried out with the Applied Biosystems 7300 Real-Time PCR System. A total of 20 μL reactions were prepared with the TaqMan Universal PCR Master Mix. The Ct (cycle threshold fluorescence values) was automatically given by SDS 2.1 software (Applied Biosystems), and the relative expression levels of VEGF mRNA were calculated using the 2-ΔΔCt method. GAPDH as an internal control was used to normalize the data to determine the relative expression of the target genes. PCR with the following primers: IL-1β, Forward 5’-ATGCGATTGTGTAATGTCCT-3’ and Reverse 5’-GTCCGGTCCACAGTCGG-3’; GM-CSF, Forward 5’-ATGCGAATCTGTGCTGTGCT-3’ and Reverse 5’-CTGCGGTCCTGACTGTGGG-3’; IL-8, Forward 5’-CTGCGTATCTGGCATCGTGCT-3’ and Reverse 5’-GTGCGGTCCGCTCATTGGG-3’; GAPDH, Forward 5’-ACAGGGGAGGTGATAGCATT-3’ and Reverse 5’-GACCAAAAGCCTTCATACATCTC-3’.
Western blotting
The PBMCs were homogenized and extracted in NP-40 buffer, followed by 5-10 min boiling and centrifugation to obtain the supernatant. Samples containing 50 μg of protein were separated on 10% SDS-PAGE gel, transferred to PVDF Transfer Membrane (Millipore). After saturation with 5% (w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with the following antibodies, IL-1β, GM-CSF and IL-8 at dilutions ranging from 1:500 to 1:2,000 at 4°C over-night. After three washes with TBST, membranes were incubated with secondary immunoglobulins (Igs) conjugated to IRDye 800CW Infrared Dye (LI-COR), including donkey anti-goat IgG and donkey anti-mouse IgG at a dilution of 1:10,000-1:20,000. After 1 hour incubation at 37°C, membranes were washed three times with TBST. Blots were visualized by the Odyssey Infrared Imaging System (LI-COR Biotechnology). Signals were densitometrically assessed (Odyssey Application Software version 3.0) and normalized to the β-actin signals to correct for unequal loading using the mouse monoclonal anti-β-actin antibody (Bioworld Technology, USA).
Statistical analysis
The data from these experiments were reported as mean ± standard errors of mean (SEM) for each group. All statistical analyses were performed by using PRISM version 5.0 (GraphPad). Inter-group differences were analyzed by one-way ANOVA, and followed by Tukey’s multiple comparison test as a post test to compare the group means if overall P < 0.05. Differences with P value of < 0.05 were considered statistically significant.
Results
Physiological and biochemical parameters of patients with rheumatoid arthritis and healthy controls
To investigate the biochemical parameters of inflammation, we results shown that CRP and ESR were significantly higher in patients with RA than that of the healthy control (Table 1). The serum IL-1β, TNF-α and GM-CSF levels were 1.76-, 2.16- and 3.57-fold increased in patients with RA as compared to those of control group (Table 1).
Table 1.
Physiological and biochemical parameters of patients with rheumatoid arthritis and healthy controls
| Age (Years) | Sex ratio (M/F) | CRP (mg/L) | ESR (mm/h) | IL-1β (pg/mL) | TNF-α (pg/mL) | GM-CSF (pg/mL) | |
|---|---|---|---|---|---|---|---|
| Control (n = 13) | 56 ± 14 | 1.06 | 3.7 ± 1.4 | 12.8 ± 4.6 | 325 ± 30 | 113 ± 27 | 35 ± 9.4 |
| RA (n = 14) | 58 ± 13 | 0.53 | 14.8 ± 9.2 | 27.4 ± 14.2 | 572 ± 126 | 244 ± 56 | 125 ± 30.7 |
| P value | 0.49 | 0.27 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Effect of honokiol on pro-inflammatory cytokines production in PBMCs
IL-1β, TNF-α, GM-CSF and IL-8 levels were detected in supernatants of the pathological cells after treatment with honokiol or untreated cells (controls). As shown in Figure 1, honokiol inhibited the expression levels of IL-1β, TNF-α, GM-CSF and IL-8 in PBMCs with a dose-dependent manner (Figure 1A-D). These observations suggested that IL-1β, TNF-α, GM-CSF and IL-8 plays an important role in the pathological courses of RA, and honokiol played an anti-inflammatory role and might have beneficial effects in preventing and treating RA.
Figure 1.
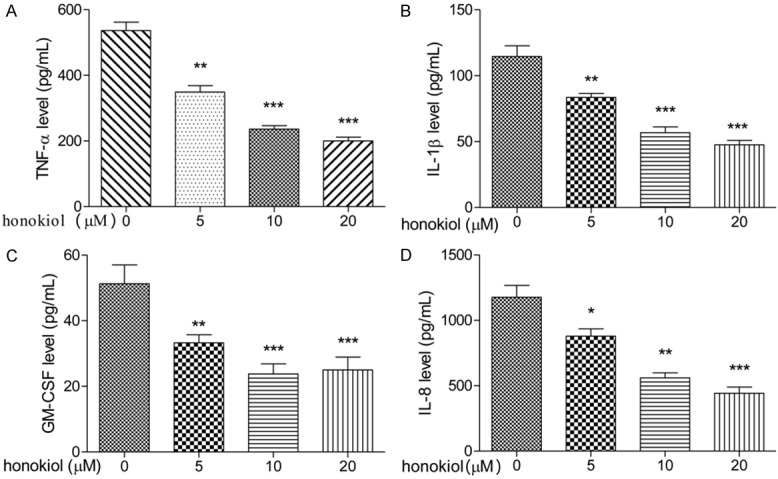
Effect of honokiol on pro-inflammatory cytokines production in PBMCs. PBMCs were treated with honokiol (0, 5, 10 or 20 μM) for 48 h, the levels of TNF-α (A), IL-1β (B), GM-CSF (C) and IL-8 (D) were determined by ELISA assay. Values are expressed as mean ± SD, n = 5 in each group. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group.
TNF-α levels positively correlated with IL-1β, GM-CSF and IL-8 activity in PBMCs isolated from RA patients
To test whether there was a relationship between TNF-α and IL-1β levels, as well as GM-CSF and IL-8, the levels of TNF-α, IL-1β level, GM-CSF and IL-8 were measured in the cell culture supernatants by ELISA assay. As shown in Figure 2, measurements obtained from supernatants were positively correlated between TNF-α and IL-1β (r = 0.823, Figure 2A). Moreover, similar results found TNF-α levels positively correlated with GM-CSF and IL-8 activity in the supernatants of PBMCs isolated from RA patients (Figure 2B and 2C).
Figure 2.
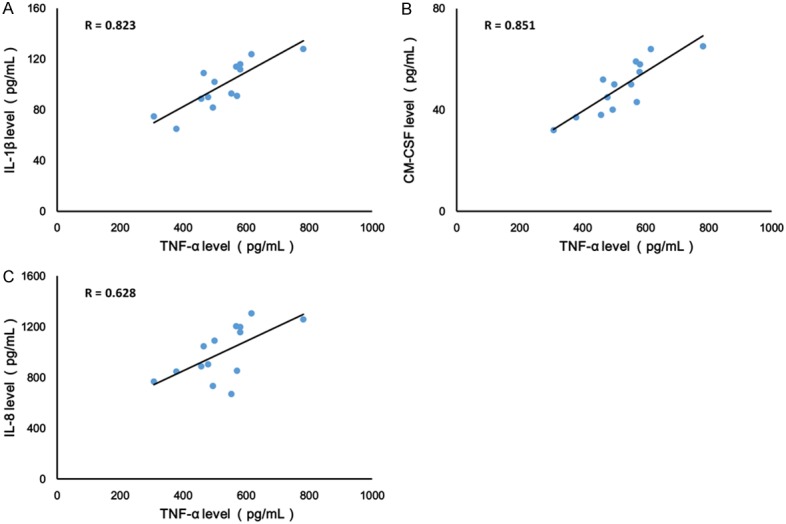
TNF-α levels positively correlated with IL-1β (A), GM-CSF (B) and IL-8 (C) activity in PBMCs isolated from RA patients. Values are expressed as mean ± SD, n = 14 in each group.
Honokiol inhibits TNF-α-induced IL-1β, GM-CSF and IL-8 production
IL-1β and IL-8 are critical proinflammatory cytokines in pathogenesis of RA. To explore the effect of TNF-α on PBMCs isolated from RA patients, the expression of IL-1β, GM-CSF and IL-8 were evaluated after exposure to TNF-α for 24 h. In our study, we shown that the mRNA and protein expression of IL-1β increased when the PBMCs exposured to TNF-α (Figure 3A-C). However, honokiol treatment significantly reversed the expression of IL-1β in response to TNF-α with a dose-dependent manner. Moreover, similar results found that honokiol could inhibit the mRNA and protein expression of IL-8, however, the inhibitory effect of honokiol on IL-8 was independent of the drug concentration when the PBMCs exposured to TNF-α (Figure 4A-C). Furthermore, the mRNA and protein expression of GM-CSF significantly increased when the PBMCs exposured to TNF-α. Intriguingly, honokiol treatment significantly reversed the expression of GM-CSF in response to TNF-α with a dose-dependent manner (Figure 5A-C).
Figure 3.
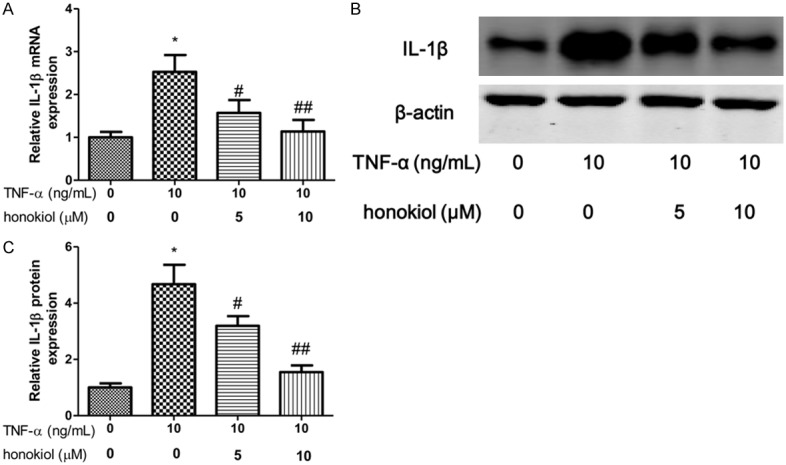
Honokiol inhibited TNF-α-induced IL-1β production. mRNA (A) and protein (B) expression were measured by real-time PCR and western blotting respectively. Densitometric quantification for western blotting (C). Values are expressed as mean ± SD, n = 4 in each group. *P < 0.05 versus control group; #P < 0.05, ##P < 0.01 versus TNF-α-treated group.
Figure 4.
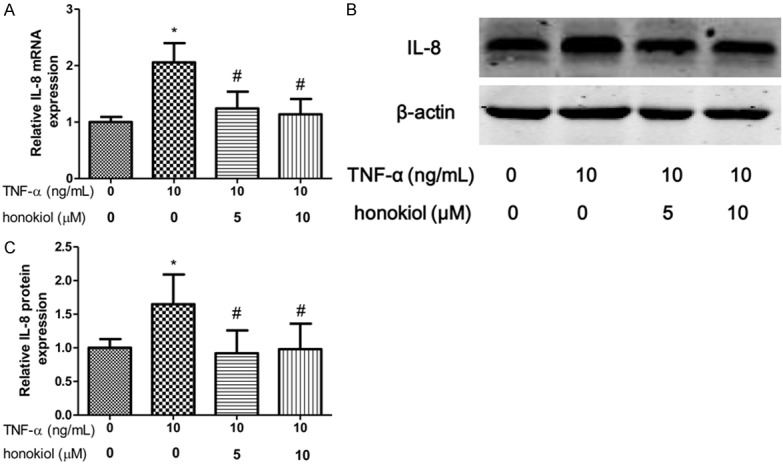
Honokiol inhibited TNF-α-induced IL-8 production. mRNA (A) and protein (B) expression were measured by real-time PCR and western blotting respectively. Densitometric quantification for western blotting (C). Values are expressed as mean ± SD, n = 4 in each group. *P < 0.05 versus control group; #P < 0.05 versus TNF-α-treated group.
Figure 5.
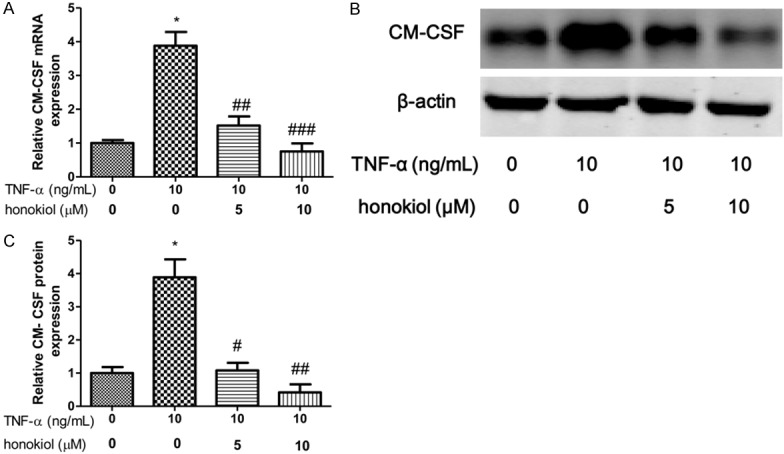
Honokiol inhibited TNF-α-induced GM-CSF production. mRNA (A) and protein (B) expression were measured by real-time PCR and western blotting respectively. Densitometric quantification for western blotting (C). Values are expressed as mean ± SD, n = 4 in each group. *P < 0.05 versus control group; #P < 0.05, ##P < 0.01, ###P < 0.001 versus TNF-α-treated group.
Discussion
Honokiol has been demonstrated to possess anti-inflammatory activities in rat aortic smooth muscle cells [22] and murine macrophages [23]. In human synovial fibroblasts, TNF-α-induced expression of these inflammatory factors, such as monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1a (MIP-1a), is inhibited in a dose-dependent manner by pre-treatment with honokiol [21]. However, the role of honokiol in RA and its contribution to resistance against inflammation has so far remained unclear. In this study, the anti-inflammatory effect of honokiol and its underlying mechanisms in PBMCs treated with TNF-α were explored.
Our results indicated that serum IL-1β, TNF-α and GM-CSF levels were increased in RA patients compared to HC. We observed a positive correlation between TNF-α levels and IL-1β, as well as GM-CSF and IL-8, in PBMCs of RA patients. Moreover, the mRNA and protein expression of IL-1β, GM-CSF and IL-8 were up-regulated when the PBMCs exposure to TNF-α, however, honokiol treatment significantly reversed the expression of IL-1β, TNF-α and GM-CSF in response to TNF-α with a dose-dependent manner. Altogether, our results indicated that active pro-inflammatory cytokines TNF-α, IL-1β, GM-CSF and IL-8 played an important role in the progression of RA, and honokiol could possess potential anti-inflammatory effects and inhibited TNF-α-induced IL-1β, GM-CSF and IL-8 production in PBMCs from rheumatoid arthritis patients. Anti-inflammatory drugs are a vital element of RA therapeutic strategies. Conventional anti-inflammatory drugs, such as methotrexate, leflunomide and glucocorticoids, show significantly more side effects in the treatment of RA, which include serious infections, expensive and inducible malignant tumors [2,24]. However, more effective, safe and affordable drugs are needed to be exploited to the treatment of RA. Many natural compounds exhibit anti-inflammatory properties and have the potential for treating inflammatory disorders. In human rheumatoid arthritis fibroblast-like synoviocytes, resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production via modulation of PI3kinase/Akt pathway [2]. Moreover, genkwa flos flavonoids have an antioxidant effects on Freund’s adjuvant-induced rheumatoid arthritis in rats [25]. Moreover, vitamin D down-regulates proinflammatory mediators in monocyte-derived macrophages, and RA cells appear more sensitive than normal cells [11]. In our study, we found that honokiol was a potential inhibitor of TNF-α-induced expression of inflammatory factors in PBMCs, which held promise as a potential anti-inflammatory drug.
In this study, we identified that GM-CSF as one of the growth factor involved in TNF-α-induced inflammatory effect in PBMCs from RA patients. Interestingly, this effect is observed using conditioned media from TNF-α and IL-1β pre-stimulated synovial fibroblasts form RA patients [16]. These results were in line with previous studies that show the importance of TNF-α in the regulation of growth factor expression in PBMCs, and it confirmed that GM-CSF could be an interesting target in the treatment of RA.
In conclusion, the preliminary data presented in this report demonstrated for the anti- inflammatory activity of honokiol under these experimental conditions and pointed out its inhibitor potential in peripheral RA PBMCs mediators, including the proinflammatory cytokines and growth factor.
Disclosure of conflict of interest
None.
References
- 1.Wendling D, Abbas W, Godfrin-Valnet M, Kumar A, Guillot X, Khan KA, Vidon C, Coquard L, Toussirot E, Prati C, Herbein G. Dysregulated Serum IL-23 and SIRT1 Activity in Peripheral Blood Mononuclear Cells of Patients with Rheumatoid Arthritis. PLoS One. 2015;10:e0119981. doi: 10.1371/journal.pone.0119981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian J, Chen JW, Gao JS, Li L, Xie X. Resveratrol inhibits TNF-alpha-induced IL-1beta, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol Int. 2013;33:1829–1835. doi: 10.1007/s00296-012-2657-0. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Tian F, Wang F. Rheumatoid arthritis-associated microRNA-155 targets SOCS1 and upregulates TNF-alpha and IL-1beta in PBMCs. Int J Mol Sci. 2013;14:23910–23921. doi: 10.3390/ijms141223910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos Savio A, Machado Diaz AC, Chico Capote A, Miranda Navarro J, Rodriguez Alvarez Y, Bringas Perez R, Estevez Del Toro M, Guillen Nieto GE. Differential expression of pro-inflammatory cytokines IL-15Ralpha, IL-15, IL-6 and TNFalpha in synovial fluid from Rheumatoid arthritis patients. BMC Musculoskelet Disord. 2015;16:51. doi: 10.1186/s12891-015-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duvallet E, Semerano L, Assier E, Falgarone G, Boissier MC. Interleukin-23: a key cytokine in inflammatory diseases. Ann Med. 2011;43:503–511. doi: 10.3109/07853890.2011.577093. [DOI] [PubMed] [Google Scholar]
- 6.Molle C, Zhang T, Ysebrant de Lendonck L, Gueydan C, Andrianne M, Sherer F, Van Simaeys G, Blackshear PJ, Leo O, Goriely S. Tristetraprolin regulation of interleukin 23 mRNA stability prevents a spontaneous inflammatory disease. J Exp Med. 2013;210:1675–1684. doi: 10.1084/jem.20120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJ, Kraan MC, Baeten D, McInnes IB, Bresnihan B, Tak PP. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:834–838. doi: 10.1136/ard.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinne RW, Stuhlmuller B, Burmester GR. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res Ther. 2007;9:224. doi: 10.1186/ar2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hummel KM, Petrow PK, Franz JK, Muller-Ladner U, Aicher WK, Gay RE, Bromme D, Gay S. Cysteine proteinase cathepsin K mRNA is expressed in synovium of patients with rheumatoid arthritis and is detected at sites of synovial bone destruction. J Rheumatol. 1998;25:1887–1894. [PubMed] [Google Scholar]
- 10.Schulze-Koops H, Davis LS, Kavanaugh AF, Lipsky PE. Elevated cytokine messenger RNA levels in the peripheral blood of patients with rheumatoid arthritis suggest different degrees of myeloid cell activation. Arthritis Rheum. 1997;40:639–647. doi: 10.1002/art.1780400408. [DOI] [PubMed] [Google Scholar]
- 11.Neve A, Corrado A, Cantatore FP. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin Exp Med. 2014;14:275–283. doi: 10.1007/s10238-013-0249-2. [DOI] [PubMed] [Google Scholar]
- 12.Xu WD, Firestein GS, Taetle R, Kaushansky K, Zvaifler NJ. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989;83:876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seitz M, Loetscher P, Fey MF, Tobler A. Constitutive mRNA and protein production of macrophage colony-stimulating factor but not of other cytokines by synovial fibroblasts from rheumatoid arthritis and osteoarthritis patients. Br J Rheumatol. 1994;33:613–619. doi: 10.1093/rheumatology/33.7.613. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds G, Gibbon JR, Pratt AG, Wood MJ, Coady D, Raftery G, Lorenzi AR, Gray A, Filer A, Buckley CD, Haniffa MA, Isaacs JD, Hilkens CM. Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206578. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharya P, Budnick I, Singh M, Thiruppathi M, Alharshawi K, Elshabrawy H, Holterman MJ, Prabhakar BS. Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy. J Interferon Cytokine Res. 2015;35:585–99. doi: 10.1089/jir.2014.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darrieutort-Laffite C, Boutet MA, Chatelais M, Brion R, Blanchard F, Heymann D, Le Goff B. IL-1beta and TNFalpha promote monocyte viability through the induction of GM-CSF expression by rheumatoid arthritis synovial fibroblasts. Mediators Inflamm. 2014;2014:241840. doi: 10.1155/2014/241840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C, Liu ZQ. Comparison of antioxidant abilities of magnolol and honokiol to scavenge radicals and to protect DNA. Biochimie. 2011;93:1755–1760. doi: 10.1016/j.biochi.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Liu X, Zhu Y, Chen S, Zhou D, Wang Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-kappaB activation and cytokine production of glial cells. Neurosci Lett. 2013;534:123–127. doi: 10.1016/j.neulet.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 19.Chen YJ, Tsai KS, Chan DC, Lan KC, Chen CF, Yang RS, Liu SH. Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J Orthop Res. 2014;32:573–580. doi: 10.1002/jor.22577. [DOI] [PubMed] [Google Scholar]
- 20.Kim KR, Park KK, Chun KS, Chung WY. Honokiol inhibits the progression of collagen-induced arthritis by reducing levels of pro-inflammatory cytokines and matrix metalloproteinases and blocking oxidative tissue damage. J Pharmacol Sci. 2010;114:69–78. doi: 10.1254/jphs.10070fp. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Shao X, Wu L, Feng T, Jin C, Fang M, Wu N, Yao H. Honokiol: an effective inhibitor of tumor necrosis factor-alpha-induced up-regulation of inflammatory cytokine and chemokine production in human synovial fibroblasts. Acta Biochim Biophys Sin (Shanghai) 2011;43:380–386. doi: 10.1093/abbs/gmr027. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, Wang Z, Hu C, Li Z, Hu J. Honokiol suppresses TNF-alpha-induced migration and matrix metalloproteinase expression by blocking NF-kappaB activation via the ERK signaling pathway in rat aortic smooth muscle cells. Acta Histochem. 2014;116:588–595. doi: 10.1016/j.acthis.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Chao LK, Liao PC, Ho CL, Wang EI, Chuang CC, Chiu HW, Hung LB, Hua KF. Anti-inflammatory bioactivities of honokiol through inhibition of protein kinase C, mitogen-activated protein kinase, and the NF-kappaB pathway to reduce LPS-induced TNFalpha and NO expression. J Agric Food Chem. 2010;58:3472–3478. doi: 10.1021/jf904207m. [DOI] [PubMed] [Google Scholar]
- 24.Callegari PE, Schaible TF, Boscia JA. Risk of serious infections and malignancies with anti-TNF antibody therapy in rheumatoid arthritis. JAMA. 2006;296:2202. doi: 10.1001/jama.296.18.2202. author reply 2203-2204. [DOI] [PubMed] [Google Scholar]
- 25.Zhang CF, Zhang SL, He X, Yang XL, Wu HT, Lin BQ, Jiang CP, Wang J, Yu CH, Yang ZL, Wang CZ, Li P, Yuan CS. Antioxidant effects of Genkwa flos flavonoids on Freunds adjuvant-induced rheumatoid arthritis in rats. J Ethnopharmacol. 2014;153:793–800. doi: 10.1016/j.jep.2014.03.046. [DOI] [PubMed] [Google Scholar]


