Abstract
The purpose of this article is to research on whether MACC1 can serve as a potential target for gene therapy of human bladder urothelial carcinoma (BUC). In this study, the expression of MACC1 gene was knocked down by RNA interference (RNAi) in the T24 cell (human BUC cell). The transcription level of MACC1 was detected by RT-PCR. Activities of MACC1, caspase-3, caspase-8, Bax and Met (mesenchymal-epithelial transition factor) protein were measured by Western blot. The cell proliferation and apoptosis were detected by MTT and flow cytometry. The cell’s invasion ability was performed on Matrigel transwell assay. We also detect MMP2 (metalloproteinase-2) proteins by ELISA. The results showed that the level of MACC1 mRNA and protein was significantly reduced after RNAi. MTT assay showed that the proliferation of T24 cell was decreased due to RNA interference. Apoptosis studies also showed that MACC1 gene interference in T24 loses its anti-apoptotic effects. The expression of apoptosis proteins (Caspase-3, Caspase-8 and Bax) increased significantly due to the MACC1 RNAi. The level of Met protein was down-regulated obviously due to RNAi. Transwell assay showed that invasion abilities of T24 cells were reduced obviously due to MACC1 RNAi. Further studies showed that the secretion of MMP-2 was reduced by RNAi. It can conclude that the ability of proliferation and invasion in T24 cells can be inhibited by RNAi-targeting MACC1. As a result, MACC1 can serve as a potential target for gene therapy of human bladder urothelial carcinoma.
Keywords: Metastasis-associated in colon cancer 1, bladder cancer, carcinoma, RNA interference, gene therapy, metalloproteinase-2
Introduction
The incidence of bladder cancer ranks nine among malignancies in the world [1]. Bladder cancer can occur at any age, even among children. But it is more likely to occur among the middle-aged, whose incidence often increases with age [2]. Different age groups have different types of bladder cancers. In most countries, the main type is are urothelial cell carcinoma, also known as transitional cell carcinoma, which accounts for more than 90% of the bladder cancer, while the squamous cell carcinoma is the main type in Egypt, which accounts for about 75% of the bladder cancer [3]. The incidence of bladder cancer tends to increase year by year. The traditional treatments, such as surgery, chemotherapy and radiotherapy on tumor are not ideal, because of their poor sensitivity and specificity for the disease, and the serious adverse reactions as well as the low survival rate. In recent years, with the development of molecular biology, people have been putting increasing emphasis on the gene therapy of bladder cancer.
MACC1 gene is human colon cancer cell metastasis gene, which is located on human chromosome 7 (7p21.1). Stein study finds that the high expression of MACC1 is closely related to the patient’s prognosis in colon cancer. The 5 years survival rate of colorectal cancer patients can be as high as 80% with low expression of MACC1 mRNA, while 5 year survival rate of colorectal cancer patients can be only 15% with high expression of MACC1 mRNA [4]. Further studies show that overexpression of MACC1 exists in gastric cancer, colon cancer, lung cancer, hepatocellular carcinoma, colorectal cancer, breast cancer and so on. Furthermore, the prognosis of patients with high expression of MACC1 was poorly [5-12]. MACC1 can be used as a tumor ‘marker’ [13-15].
However, the biological behavior of bladder cancer cell after MACC1 expression knockdown remains unknown. RNAi which is widely applied to silence particular genes is a typical post-transcriptional regulation of gene expression [16-18]. In present studies, RNAi approach was used to knock down MACC1 expression in T24 cells, and possible antitumor mechanisms of MACC1 knockdown in T24 cells were discussed.
Materials and methods
Cell lines, cell cultures and reagents
T24 and EJ cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA). BIU87 and SV-HUC-1 cell line were obtained from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). SV-HUC-1 cells originated from human normal uroepithelium. T24, EJ and BIU87 cells derived from human urothelial cell carcinoma. All cells were cultured in RPMI-1640 media (Life Technologies, Rockville, MD, USA) containing with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells were incubated at 37°C in 5% CO2 humidified air. Technologies, Rockville, MD, USA. Rabbit monoclonal anti-human primary antibodies (against MACC1, caspase-3, caspase-8, Bax, Met and β-actin) were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA) Goat anti-rabbit secondary antibody was purchased from Santa Cruz Biotechnology. MTT and DMSO were purchased from Sigma, St. Louis, MO, USA. PI was purchased from KGI Biological Development Co. Ltd, Nanjing, China. MMP-2 ELISA kits were purchased from R&D Systems, USA.
SiRNA for MACC1
Four siRNA against MACC1 gene sequences were designed synthesized by the Wuhan Cell marker Biotechnology Co., Ltd. in Wuhan, China. Four siRNA oligonucleotides were the following sequences: p-1: 5’-ATCAACTGTCTGCTTCTAA-3’; p-2: 5’-GCCCATCAGAGTATACATA-3’; p-3: 5’-GATCCAACCCCAAACCTAA-3’; p-4: 5’-ATGTCAAGGTGATTTCAAA-3’. Sequence (5’-CAACAAGATGAAGAGCACC-3’) was designed as negative control.
RT-PCR assay for MACC1 mRNA in cells
MACC1 mRNA was detected by RT-PCR in cells MACC1 primers used in RT-PCR were as follow: 5’-TTTCACCCTTCGTGGTAATAATGCT-3’ (sense); 5’-TTAAAGGCAGGACAATCTGTTCAAG-3’ (antisense). Total RNA of MACC1 was isolated using Trizol reagent (Invitrogen, Grand Island, NY, USA) and detected concentration of MACC1 by spectrophotometry (Eppendorf biospectrometer basic, Eppendorf, Hamburg, Germany). After isolation, 2 μg total RNA was reverse transcribed (RT) utilizing the HiFi-MMLV cDNA Kit (Beijing CoWin Biotech Co. Ltd., Beijing, China) compiling with the manufacturer’s protocol. PCR was performed as pre-denaturation for 120 S at 94°C; denaturation at 94°C, for 30 S; annealing extension at 57°C, for 30 S; extension at 72°C, for 30 S; total of 35 cycles. PCR products were electrophoresed on 1.0% agarose gels and dyed by Goldview I (Beijing Solarbio Science & Technology co, LTD. Beijing China). β-actin was used as internal control. Bands of PCR products were analyzed by were analyzed by the Image J software (National Institutes of Health, Bethesda, MA, USA).
Cell transfection
According to the results of RT-PCR, the highest expression of MACC1m RNA cell line (T24 cell) was selected for other experiments. There were three groups. MACC1 RANi group was transfected with RNA interference sequences; Blank control group was transfected with an empty plasmid without any sequence while the negative control group was transfected with a negative control RNA interference sequence. Blank control group and negative control group were used for negative controls. Blank control group, MACC1 shRNA and negative control shRNA transfection T24 cells were performed according to the protocol of Lipofectamine 2000 (Invitrogen, USA). Then cells were screened by medium with 800 mg/L G418. Cells that stably transfected were harvested after 8 weeks and were used to other experiments.
RT-PCR assay for efficiency of RNAi in T24 cell
Efficiency of RNAi was also detected by RT-PCR in T24 cell. There were three groups: MACC1 RANi group, Blank control group and the negative control group. RT-PCR was performed as the previous description.
Western blot assay
Total proteins were extracted from T24 cells using radio immunoprecipitation assay (RIPA) buffer (Beyotime Institute of Biotechnology, Shanghai, China) with 25 mM NaF, 1 mM Na3VO4, and 1 × protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN, USA). Protein concentrations were quantified by spectrophotometry (Eppendorf, Hamburg, Germany). After SDS-PAGE electrophoresis, the proteins were transferred to PVDF membrane, and then blocked with 5% nonfat dry milk at room temperature for 1 h. They were incubated with different rabbit monoclonal anti-human primary antibodies (against MACC1, caspase-3, caspase-8, Bax, Met and β-actin; 1:1000) overnight at 4°C respectively. The membranes were then washed prior to incubation with goat anti-rabbit secondary antibody (1:8000), and then detected by ECL (Beyotime Institute of Biotechnology, Shanghai, China). Bands were analyzed by the Image J software. Each experiment was repeated for three times.
MTT assay
Cells were seeded with 100 μl medium in 96-well plate (5 × 103 per well). After one, two, three and four days, MTT solution (20 mg/ml, Sigma, St. Louis, MO, USA) 20 μl was added and incubated for 4 h, then added 150 μl DMSO. After being shaked for 10 minutes, the 490 nm absorbance was determined by microplate reader (Thermo Multiskan MK3; Thermoelectric Electronics Co., Ltd, Shanghai, China).
Flow cytometry for cell apoptosis
Apoptosis in T24 cells was measured by Flow cytometry. Cells were scraped, washed twice with PBS and centrifuged. Pelleted cells were incubated in 1 × binding buffer containing FITC-annexin V (Promega, USA) and PI (KGI Biological Development Co. Ltd. Nanjing, China) in dark for 15 min. Apoptotic cells were examined using a Flow cytometry (FACS Calibur flow, BD Company, America). Each sample was repeated 3 times.
Monolayer cell wound healing assay for cell migration
About 5 × 104 - 5 × 105 cells were seeded in 6-well-plate. The 100% confluent monolayer cells were obtained after cells confluence, then scraped monolayer cells with 200 μl pipette tip and cell debris was washed with PBS. 2 ml medium without FBS was added into each well. The distance from one side of the scratch to the other was measured at the 0 h and 24 h, with an inverted microscopy equipped with a digital camera (Olympus, Japan).
Matrigel transwell assay for cell invasion
Transwell chambers coated with Matrigel Basement Membrane Matrix (from BD Biosciences, USA) were used to perform cell invasion assay. Cells (2 × 105 cells/ml) were seeded in the upper compartment with 200 μl serum-free medium. while 600 μl RPMI-1640 with 10% FBS was added to the lower chamber. The chamber was incubated at 37°C and 5% CO2, for 24 h and then the non-migratory cells in the upper chamber were removed with a cotton swab. Then lower chamber cells were fixed with 4% paraformaldehyde. After 10 min, 0.1% crystal violet was used to dye cells for 30 min. Cells were counted by an inverted microscopy equipped with a digital camera.
Enzyme-linked immunosorbent assay
Cells were treated as described above. Concentrations of MMP-2 in the cell culture supernatants were quantified using MMP-2 ELISA kits (R&D Systems, USA). Each sample was repeated 3 times. Briefly, the cellular supernatants were collected and centrifuged for 5 min at 500 g. Total 100 µl supernatants or standard samples or positive control samples were added into 96 well and incubated for 1 h. Followed with 100 µl enzyme-linked antibodies incubation for 0.5 h at 4°C. After washed 9 times with washing buffer, the template was added and incubated for 0.5 h, followed with 2 M H2SO4 termination reaction. The 450 nm absorbance was determined by microplate reader. Each sample was repeated three times.
Statistical analysis
The results were expressed as mean ± standard error of the mean (S.E.M). SPSS 17.0 was used for statistical analysis. Statistical analysis was done with Student’s t-test and one-way ANOVA. Differences were considered statistically significant when P < 0.05.
Results
MACC1 mRNA was increased in human bladder urothelial carcinoma cells
RT-PCR analysis showed that the expression of MACC1 mRNA was increased in human urothelial carcinoma (EJ, BIU87 and T24) cell lines compared with SV-HUC-1, human uroepithelial cell line (Figure 1). Expression of MACC1 mRNA in T24 cell line was that of highest in all cell lines.
Figure 1.
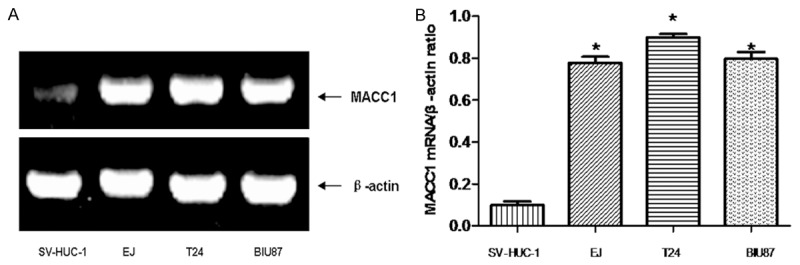
RT-PCR was used for detection the mRNA expression of MACC1 in cells (A). Data were expressed as mean ± S.E.M from three separate experiments (B). *(P < 0.05) indicates a significant difference compared with the SV-HUC-1 cells.
Expression of MACC1 inhibits evidently by RNAi
Expressions of MACC1 in cells were detected by RT-PCR and western blot after cells were cultured 48 h. The results showed that the mRNA and protein expression level in the negative control group and the blank control group was significantly higher than MACC1 RNAI group (P < 0.05). There was no significant difference between the negative control group and the blank control group (P > 0.05) (Figure 2A, 2B).
Figure 2.
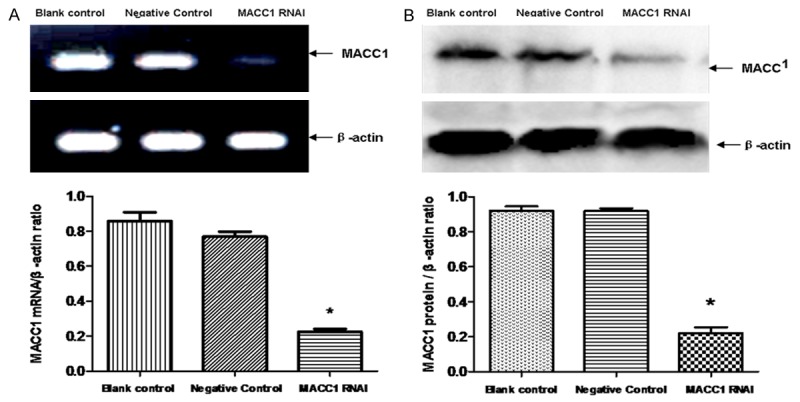
Effect of MACC1 RNAi on the expression of MACC1 in T24 cells. RT-PCR was used for detection the effect of MACC1 RNAi on the mRNA expression of MACC1. Data were expressed as mean ± S.E.M from three separate experiments (A). The protein level of MACC1 in T24 cells was detected by western blot. Data were expressed as mean ± S.E.M from three separate experiments (B). Statistical analyses were performed using the t-test and one-way ANOVA. *(P < 0.05) indicates a significant difference compared with the control groups.
Effect of RNAi on the growth of T24 cells
The rate of cell proliferation was gradually decreased in the RANi group after 48 h, and the cell proliferation is decreased significantly compared to the other groups, (P < 0.05). There were no obvious difference between the negative control group and the blank control group (P > 0.05), as is shown in Figure 3C.
Figure 3.
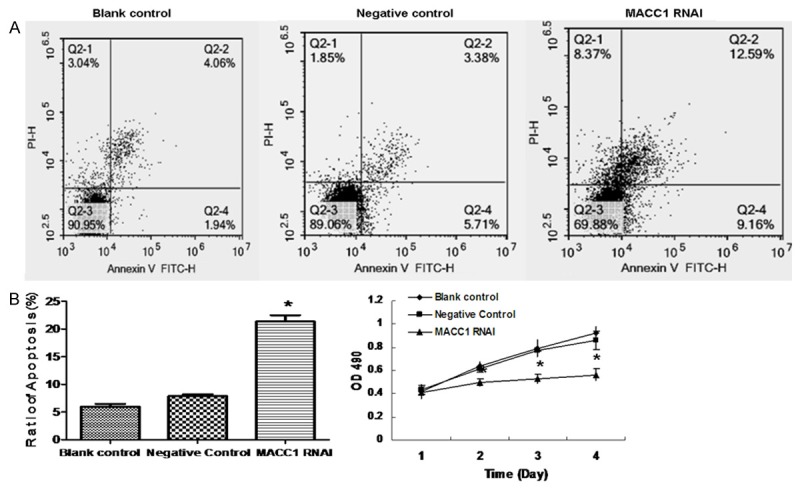
Effect of MACC1 RNAi on the apoptosis of T24 cells. The apoptosis of T24 cells were detected by FITC-Annexin V staining (A). Data were expressed as mean ± S.E.M from three separate experiments (B). Cell proliferation was monitored with MTT assay (C).*(P < 0.05) indicates a significant difference compared with the control groups.
Effect of RNAi on the apoptosis of T24 cells
Cell apoptosis was considerably increased in the RANi group compared to the other groups (P < 0.05). There was no significant difference between the negative control group and the blank control group (P > 0.05), as is shown in Figure 3A and 3B. Western blot was used to detect the expression of apoptosis proteins (Caspase-3, Caspase-8 and Bax), as is shown in Figure 4A and 4B). Results showed that the expression of Caspase-3, Caspase-8 and Bax increased significantly due to the MACC1 RNAi treatment.
Figure 4.
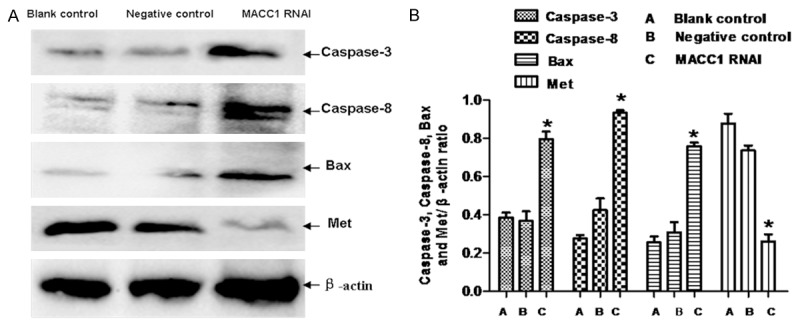
Effect of MACC1 RNAi on the proteins of T24 cells. The apoptosis proteins (Caspase-3, Caspase-8 and Bax) and Met were detected by western blot (A). Data were expressed as mean ± S.E.M from three separate experiments (B). *(P < 0.05) indicates a significant difference compared with the control groups.
Impact on invasion of T24 cells by OPN-RNAi
The number of cells going through the membrane in RANi group is significantly lower than the negative control group and blank control group (P < 0.05). This result suggests a reduced capacity of tumor invasion in the RANi group in T24 cells as is shown in Figure 5A and 5B.
Figure 5.
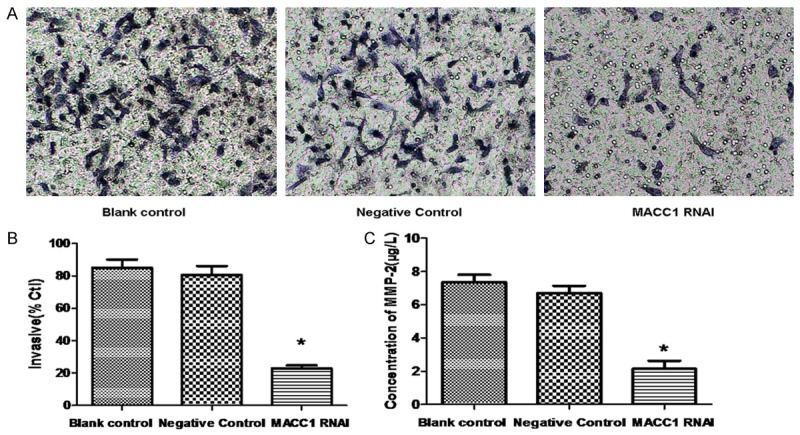
Effect of MACC1 RNAi on the invasion of T24 cells. T24 cell invasion ability was monitored with transwell assay (A) and the invasion ration was expressed as mean ± S.E.M from three separate experiments (B). The concentration of MMP2 in cell culture medium was analyzed with ELISA assay (C).*(P < 0.05 vs. control groups).
Effect of OPN-RNAi on the expression of invasion relate proteins
We also detect the expression of invasion associated proteins (MMP2). Results showed that the protein of MMP2 in the RANi group reduced significantly than the negative control group (P < 0.05) as is shown in Figure 5C.
Discussion
RNA interference was widely applied to gene therapy. Gao J used the method of RNAi to reduce the expression of MACC1 protein in Huh7 cells. The invasion abilities of the cells of the RNAi group were lower than that of the cells of the control groups [17]. Similar results were observed in ovarian cancer tissues. Expression of MACC1 in OVCAR-3 cells was significantly down-regulated by MACC1 specific small hairpin RNA and the invasion ration of the RNAi group cells were lower than that of the control group cells [18]. In our present research, we have successfully designed and synthezed four specific siRNAs, which downregulated MACC1 genes to study the effects of MACC1 inhibition on T24 cells. On the basis of construction, RNAi of MACC1 gene was studied on T24 cell by RT-PCR, Western blot, MTT, et al. After 2 days, expression of MACC1 mRNA and protein significantly decreased (P < 0.05) in the RNAi group compared with the control group. MTT assay showed that the proliferation of T24 cell was decreased due to RNA interference. Apoptosis studies also show that MACC1 gene interference in T24 loses its anti-apoptotic effects. The expression of apoptosis proteins (Caspase-3, Caspase-8 and Bax) increased significantly due to the MACC1 RNAi treatment. It indicated that MACC1 gene interference could induce the apoptosis of T24 cells through the caspase and Bax signaling pathway.
Met is a receptor for hepatocyte growth factor (HGF), and it has influence on cellular proliferation, migration and invasion [19-22]. The prognosis of patients with overexpression of Met are poor prognosis [23,24]. Studies have shown that MACC1 can increase the protein expression of Met through the HGF/Met signaling pathway, thus increasing the ability of tumor cell proliferation, and leads to the cancer’s metastasis and recurrence [25-28]. In the current study, the level of Met protein was down-regulated significantly after inhibition of MACC1 by RNAi in T24 cells. It indicated that RNAi could inhabit the proliferation of T24 cells through the HGF/Met signaling pathway.
MACC1 plays an important role in the process of cell invasion. Studies have also shown that MACC1 regulate MMP-2 secretion and promote tumor invasion and metastasis [17,18]. In the current study, transwell assay show that invasion abilities of T24 cell are reduced obviously due to MACC1 RNAi. Further studies show that the secretion of MMP-2 was reduced by RNAi in T24 cell.
Wang found that SF/HGF induced the activation of MMP-2 in two human EC lines [29]. Tsou found that HGF increased the migration and expression of MMP-2 in human chondrosarcoma cells, and MMP-2 protein could be reduced by c-Met siRNA and inhibitor [30]. These suggest that MACC1 may have an indirectly effect on the invasion of protein concerned (MMP2) by regulating the HGF/c-Met signaling pathway, and thus increase the invasive ability of BUC cell. However, its specific molecular mechanism need to be further researched.
In conclusion, we confirm that recombinant plasmid vector carries four specific shRNAs against MACC1 gene sequence, which significantly inhibit the proliferation and metastatic potential of T24 cells in vitro. Therefore, we consider that MACC1 is expected to become a new target for human BUC gene therapy.
As for the effect of MACC1 on the development of BUC, we focus more on knocking down the expression to inhibit the proliferation and invasion of tumor. We have not researched the effect of overexpression vector for MACC1 on BUC cells. It will help us to fully understand the effect of MACC1 protein in BUC cell, if it can be carried out.
Acknowledgements
This work is supported by the Natural Science Foundation of Luohe Medical College, P.R.C. (2014-S-LMC08, 2013-DF-002). This work is also supported by the 2015 basic and advanced technology research project of Henan Province, P.R.C.
Disclosure of conflict of interest
None.
References
- 1.Parkin MD, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Lynch CF, Cohen MB. Urinary system. Cancer. 1995;75:316–328. doi: 10.1002/1097-0142(19950101)75:1+<316::aid-cncr2820751314>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.El-Bolkainy MN, Mokhtar NM, Ghoneim MA, Hussein MH. The impact of schistosomiasis on the pathology of bladder carcinoma. Cancer. 1981;48:2643–2648. doi: 10.1002/1097-0142(19811215)48:12<2643::aid-cncr2820481216>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15:59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 5.Shirahata A, Sakata M, Kitamura Y, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Hibi K. MACC 1 as a marker for peritoneal-disseminated gastric carcinoma. Anticancer Res. 2010;30:3441–3444. [PubMed] [Google Scholar]
- 6.Ge Y, Meng X, Zhou Y, Zhang J, Ding Y. Positive MACC1 expression correlates with invasive behaviors and postoperative liver metastasis in coloncancer. Int J Clin Exp Med. 2015;8:1094–100. [PMC free article] [PubMed] [Google Scholar]
- 7.Chundong G, Uramoto H, Onitsuka T, Shimokawa H, Iwanami T, Nakagawa M, Oyama T, Tanaka F. Molecular diagnosis of MACC1 status in lung adenocarcinoma by immunohistochemical analysis. Anticancer Res. 2011;31:1141–1145. [PubMed] [Google Scholar]
- 8.Shirahata A, Fan W, Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y, Hibi K. MACC 1 as a marker for vascular invasive hepatocellular carcinoma. Anticancer Res. 2011;31:777–780. [PubMed] [Google Scholar]
- 9.Juneja M, Ilm K, Schlag PM, Stein U. Promoter identification and transcriptional regulation of the metastasis gene MACC1 in colorectal cancer. Mol Oncol. 2013;7:929–943. doi: 10.1016/j.molonc.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Zhang H, Cai J, Fang L, Wu J, Ye C, Zhu X, Li M. Overexpression of MACC1 and Its significance in human Breast Cancer Progression. Cell Biosci. 2013;3:16. doi: 10.1186/2045-3701-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isella C, Mellano A, Galimi F, Petti C, Capussotti L, De Simone M, Bertotti A, Medico E, Muratore A. MACC1 mRNA levels predict cancer recurrence after resection of colorectal cancer liver metastases. Ann Surg. 2013;257:1089–1095. doi: 10.1097/SLA.0b013e31828f96bc. [DOI] [PubMed] [Google Scholar]
- 12.Shimokawa H, Uramoto H, Onitsuka T, Chundong G, Hanagiri T, Oyama T, Yasumoto K. Overexpression of MACC1 mRNA in lung adenocarcinoma is associated with postoperative recurrence. J Thorac Cardiovasc Surg. 2011;141:895–898. doi: 10.1016/j.jtcvs.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 13.Qiu J, Huang P, Liu Q, Hong J, Li B, Lu C, Wang L, Wang J, Yuan Y. Identification of MACC1 as a novel prognostic marker in hepatocellular carcinoma. J Transl Med. 2011;9:166. doi: 10.1186/1479-5876-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo L, Lu W, Zhang X, Luo D, Zhang H. Metastasis-associated colon cancer-1 is a novel prognostic marker for cervical cancer. Int J Clin Exp Pathol. 2014;7:4150–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Stein U. MACC1-a novel target for solid cancers. Expert Opin Ther Targets. 2013;17:1039–52. doi: 10.1517/14728222.2013.815727. [DOI] [PubMed] [Google Scholar]
- 16.Shrivastava N, Srivastava A. RNA interference: an emerging generation of biologicals. Biotechnol J. 2008;3:339–53. doi: 10.1002/biot.200700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Ding F, Liu Q, Yao Y. Knockdown of MACC1 expression suppressed hepatocellular carcinoma cell migration and invasion and inhibited expression of MMP2 and MMP9. Mol Cell Biochem. 2013;376:21–23. doi: 10.1007/s11010-012-1545-y. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, Shi H, Chen Z, Wu Q, Ren F, Huang H. Effects of metastasis-associated in colon cancer 1 inhibition by small hairpin RNA on ovarian carcinoma OVCAR-3 cells. J Exp Clin Cancer Res. 2011;30:83. doi: 10.1186/1756-9966-30-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Zhou J, Rogers AM, Jänne PA, Benedettini E, Loda M, Hodi FS. c-Met, epidermal growth factor receptor, and insulin-like growth factor-1 receptor are important for growth in uveal melanoma and independently contribute to migration and metastatic potential. Melanoma Res. 2012;22:123–32. doi: 10.1097/CMR.0b013e3283507ffd. [DOI] [PubMed] [Google Scholar]
- 20.Jung KH, Park BH, Hong SS. Progress in cancer therapy targeting c-Met signaling pathway. Arch Pharm Res. 2012;35:595–604. doi: 10.1007/s12272-012-0402-6. [DOI] [PubMed] [Google Scholar]
- 21.Feng Y, Thiagarajan PS, Ma PC. MET signaling: novel targeted inhibition and its clinical development in lung cancer. J Thorac Oncol. 2012;7:459–67. doi: 10.1097/JTO.0b013e3182417e44. [DOI] [PubMed] [Google Scholar]
- 22.You H, Ding W, Dang H, Jiang Y, Rountree CB. c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology. 2011;54:879–89. doi: 10.1002/hep.24450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha SY, Lee J, Kang SY, Do IG, Ahn S, Park JO, Kang WK, Choi MG, Sohn TS, Bae JM, Kim S, Kim M, Kim S, Park CK, Ignatius Ou SH, Kim KM. MET overexpression assessed by new interpretation method predicts gene amplification and poor survival in advanced gastric carcinomas. Mod Pathol. 2013;26:1632–41. doi: 10.1038/modpathol.2013.108. [DOI] [PubMed] [Google Scholar]
- 24.Yun S, Koh JM, Lee KS, Seo AN, Nam KH, Choe G. Expression of c-MET in Invasive Meningioma. J Pathol Transl Med. 2015;49:44–51. doi: 10.4132/jptm.2014.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boardman LA. Overexpression of MACC1 leads to downstream activation of HGF/MET and potentiates metastasis and recurrence of colorectal cancer. Genome Med. 2009;1:36. doi: 10.1186/gm36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arlt F, Stein U. Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol. 2009;41:2356–59. doi: 10.1016/j.biocel.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Koch A, Mancini A, El Bounkari O, Tamura T. The SH2-domian-containing inositol 5-phosphatase (SHIP)-2 binds to c-Met directly via tyrosine residue 1356 and involves hepatocyte growth factor (HGF)-induced lamellipodium formation, cell scattering and cell spreading. Oncogene. 2005;24:3436–47. doi: 10.1038/sj.onc.1208558. [DOI] [PubMed] [Google Scholar]
- 28.Guo T, Yang J, Yao J, Zhang Y, Da M, Duan Y. Expression of MACC1 and c-Met in human gastric cancer and its clinical significance. Cancer Cell Int. 2013;13:121. doi: 10.1186/1475-2867-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Keiser JA. Hepatocyte growth factor enhances MMP activity in human endothelial cells. Biochem Biophys Res Commun. 2000;272:900–5. doi: 10.1006/bbrc.2000.2852. [DOI] [PubMed] [Google Scholar]
- 30.Tsou HK, Chen HT, Hung YH, Chang CH, Li TM, Fong YC, Tang CH. HGF and c-Met interaction promotes migration in human chondrosarcoma cells. PLoS One. 2013;8:e53974. doi: 10.1371/journal.pone.0053974. [DOI] [PMC free article] [PubMed] [Google Scholar]


