Abstract
The aim of this study is to investigate the expression of sarcosine metabolism related proteins according to androgen receptor (AR) and HER-2 status in estrogen receptor (ER) negative breast cancer and to analyze its clinical implications. Tissue microarray was constructed for a total of 334 cases of ER negative breast cancer. Immunohistochemical stain was conducted for sarcosine metabolism related proteins such as glycine N-methyltransferase (GNMT), sarcosine dehydrogenase (SARDH), and l-pipecolic acid oxidase (PIPOX). There were 131 AR positive, 205 AR negative cases and 143 HER-2 positive, 193 HER-2 negative cases. When subdividing into four groups according to AR and HER-2 status, there were 55 AR(+)/HER-2(-) cases, 76 AR(+)/HER-2(+) cases, 67 AR(-)/HER-2(+) cases and 138 AR(-)/HER-2(-) cases. GNMT and PIPOX expression was highest in the AR(+)/HER-2(-) group while expressed lowest in the AR(-)/HER-2(-) group (P<0.001). Stromal PIPOX expression was highest in the AR(-)/HER-2(+) group and lowest in the AR(-)/HER-2(-) group (P=0.010). GNMT and PIPOX expression was higher in the AR positive group compared with those of AR negative group (P=0.001, and P<0.001, respectively), while tumoral and stromal PIPOX expression showed a significant association with HER-2 positivity (P=0.006, and P=0.005, respectively). AR positive group had the highest ratio of low sarcosine type while the AR negative group had the highest ratio of null type (P<0.001). In conclusion, ER negative breast cancer showed different expression of sarcosine metabolism related proteins according to AR and HER-2 status. GNMT and PIPOX expression was high in the AR positive group while tumoral and stromal PIPOX expression was high in the HER-2 positive group.
Keywords: Androgen receptor, breast cancer, sarcosine
Introduction
Sarcosine (N-methylglycine) is a non-proteinogenic amino acid produced through glycine metabolism. The major enzymes that play a key role in the sarcosine metabolism pathway are glycine N-methyltransferase (GNMT), sarcosine dehydrogenase (SARDH), and l-pipecolic acid oxidase (PIPOX). Within the intracellular environment, the methyl group is transferred from S-adenosylmethionine to glycine, thereby producing sarcosine. The key enzyme that facilitates this process is GNMT, while the sarcosine-metabolizing enzymes, SARDH and PIPOX, produce glycine from sarcosine through oxidative demethylation [1]. Sarcosine is reportedly a potential oncometabolite where in prostate cancer sarcosine may serve as a possible sensitive tumor biomarker through its role in tumor progression and metastasis [2,3] (Figure 1).
Figure 1.
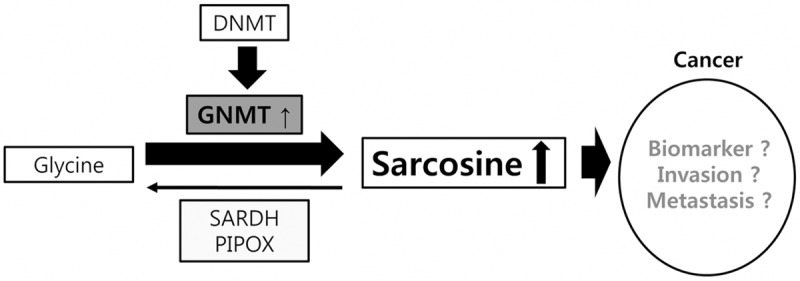
Schematic representation of sarcosine pathway.
It is well known that steroidal hormones, such as estrogen, play an important role in the pathogenesis of breast cancer, therefore evaluation of estrogen receptor (ER) and progesterone receptor (PR) is essential to the treatment and prognosis of breast cancer. Apart from the well-known ER and PR receptors, the androgen receptor (AR) is also one of the steroid hormonal subfamily with its role in carcinogenesis, yet unknown. AR expression in breast cancer is purported to be about 70% [4], while in apocrine type, lobular type breast cancers, its expression is known to be even higher [5]. In the investigation of AR and breast cancer characteristics, Farmer et al. subdivided a new subgroup of breast cancer through gene clustering analysis-the molecular apocrine breast cancer (MABC). MABC takes up about 8-14% of breast cancers characterized by apocrine histological features, androgen receptor (AR) positivity, estrogen receptor (ER) negativity [6]. MABC is frequently associated with HER-2 overexpression/amplification and is related to poor long-term survival [6]. To note, although MABC was originally described via gene clustering analysis, it may also be identified through surrogate immunohistochemical markers, in which it is ER negative and AR positive [6,7]. While taking note of previous studies that investigated the role of AR, HER-2 and sarcosine regarding prostate cancer [8], there may be a similar link amongst AR, HER-2 and sarcosine in breast cancers. This study therefore aims to investigate the expressions of sarcosine metabolism related proteins according to AR and HER-2 status in ER negative breast cancers and understand its clinical implications.
Material and methods
Patient selection and clinicopathologic evaluation
Formalin-fixed paraffin-embedded tissue samples of patients diagnosed with invasive ductal carcinoma, no specific type, from January 2010 to December 2012 at Severance Hospital were included in this study. The study was approved by the Institutional Review Board of Severance Hospital. Those cases that had undergone pre-operative chemotherapy were excluded and only those that showed ER negativity in immunohistochemical staining were selected. A cut-off value of 1% or more positively stained nuclei was used to define ER and AR positivity [9]. HER-2 staining was analyzed according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines using the following categories: 0 = no immunostaining; 1+ = weak incomplete membranous staining, in less than 10% of tumor cells; 2+ = complete membranous staining, either uniform or weak in at least 10% of tumor cells; and 3+ = uniform intense membranous staining in at least 10% of tumor cells [10]. HER-2 immunostaining was considered positive when strong (3+) membranous staining was observed whereas cases with 0 to 1+ were regarded as negative. The cases showing 2+ HER-2 expression were evaluated for HER-2 amplification by Fluorescent in situ hybridization (FISH). All cases were retrospectively reviewed by a breast pathologist (Koo JS), in which histological evaluation was based on hematoxlin and eosin (H&E) stained slides. The histological grade was assessed using the Nottingham grading system [11]. Tumor staging was based on the 7th American Joint Committee on Cancer (AJCC) criteria. Disease-free survival (DFS) was calculated from the date of the first curative surgery to the date of the first loco-regional or systemic relapse, or death without any type of relapse. Overall survival (OS) was estimated from the date of the first curative operation to the date of the last follow-up or death from any cause. Clinicopathologic parameters evaluated in each breast cancer included patient age at initial diagnosis, lymph node metastasis, tumor recurrence, distant metastasis, and survival.
Tissue microarray
After reviewing H&E-stained slides, the most suitable formalin-fixed paraffin-embedded (FFPE) tumor tissue samples were selected. The most representative tumor region on the FFPE sample was then marked and a 3-mm tissue core sample was extracted using a punch machine and planted onto a 6 × 5 recipient block. A total of 2 tissue cores were taken for all samples in this TMA construction.
Immunohistochemistry
The antibodies used for immunohistochemistry in this study are shown in Table 1. Formalin-fixed, paraffin-embedded tissue sections were used for immunohistochemical staining. FFPE blocks were sectioned at a thickness of 3 um and then deparaffinized and rehydrated using xylene and alcohol solutions. Sections were then stained using the Ventana Discoversy XT automated stainer (Ventana Medical System, Tucson, AZ, USA). Antigen retrieval was achieved by soaking sections in a CC1 buffered solution (Cell Conditioning 1; citrate buffer Ph 6.0, Ventan Medical System). The appropriate positive and negative controls were included together with the study sample for staining.
Table 1.
Source, clone, and dilution of antibodies used in this study
| antibody | company | clone | dilution |
|---|---|---|---|
| AR | Lab Vision Corp. | AR441 | 1:100 |
| HER-2 | DAKO, Glostrup, Denmark | Polyclonal | 1:1500 |
| Ki-67 | DAKO, Glostrup, Denmark | MIB-1 | 1:150 |
| Sarcosine metabolism related | |||
| GNMT | Abcam, Cambridge, UK | Polyclonal | 1:100 |
| SARDH | Abcam, Cambridge, UK | Polyclonal | 1:100 |
| PIPOX | Abcam, Cambridge, UK | Polyclonal | 1:100 |
Glycine N-methyltransferase (GNMT), sarcosine dehydrogenase (SARDH), and l-pipecolic acid oxidase (PIPOX), androgen receptor (AR).
Interpretation of immunohistochemical results
Immunohistochemical markers for GNMT, SARDH and PIPOX were accessed by light microscopy. The result was recorded as the multiplied score of the proportion of stained cell to immunostaining intensity. The proportion of stained cells was scored according to a three tiered system ranging from 0 to 2, defined as follows; 0 represented negative results, 1 represented less than 30% of cells stained, and 2 represented more than 30% of cells stained. Immunostaining intensity was scored according to a four tiered system ranging from 0 to 3 defined as follows: 0 represented negative result, 1 represented weak, 2 represented moderate, and 3 represented strong intensities. The number obtained after the multiplication of stained cell proportion by immunostaining intensity resulted in the overall interpretation score: 0-1 was defined as negative, 2-6 positive [12]. Ki-67 labeling indices (LI) were scored by counting the number of positively stained nuclei and expressed as a percentage of total tumor cells.
Sarcosine metabolism phenotype
Through immunohistochemical staining of GNMT, SARDH and PIPOX markers, sarcosine metabolism phenotypes were classified as follows; high sarcosine type: GNMT(+)/SARDH and PIPOX(-), low sarcosine type: GNMT(-)/SARDH or PIPOX(+), intermediate sarcosine type: GNMT(+)/SARDH or PIPOX(+), and null type: GNMT(-)/SARDH and PIPOX(-).
Statistical analysis
Data was analyzed using SPSS for Windows version 12.0 (SPSS Inc., Chicago, IL). Student’s t test and Fisher’s exact test were used for continuous and categorical variables, respectively. To analyze data with multiple comparisons, a corrected p-value with application of the Bonferroni method for multiple comparisons was used. Statistical significance was assumed when P<0.05. Kaplan-Meier survival curves and log-rank statistics were employed to evaluate time to tumor metastasis and time to survival. Multivariate regression analysis was performed using Cox proportional hazards model.
Results
Basal characteristics of patients according to the AR and HER-2 status in ER negative breast cancer
Within the current study, there were 131 AR positive cases, 205 AR negative cases, 143 HER-2 positive cases and 193 HER-2 negative cases (Table 2). After subdividing into four groups according to AR and HER-2 status, there were 55 cases in AR(+)/HER-2(-) group, 76 cases in AR(+)/HER-2(+) group, 67 cases in AR(-)/HER-2(+) group, and 138 cases in AR(-)/HER-2(-) group. Clinicopathologic analysis amongst the four groups revealed that AR(-)/HER-2(-) group was associated with younger age and higher Ki-67 LI, (P=0.001, and P<0.001, respectively). Direct comparisons between AR positive group and AR negative group showed that AR negative group was associated with old age and higher Ki-67 LI (P=0.002, and P<0.001, respectively). Within AR negative group, HER-2 positive group tended to have older aged patients (P=0.019) while in the HER-2 negative group, Ki-67 LI was significantly higher (P=0.001).
Table 2.
Clinicopathologic characteristics according to the AR and HER-2 status in ER negative breast cancer
| Parameters | Total N=336 (%) | AR positive group n=131 | AR negative group n=205 | p-value* | p-value† | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HER-2- n=55 (%) | HER-2+ n=76 (%) | p-value | HER-2+ n=67 (%) | HER-2- n=138 (%) | p-value | ||||
| Age (years) | 0.927 | 0.019 | 0.001 | 0.002 | |||||
| ≤35 | 34 (10.1) | 2 (3.6) | 3 (3.9) | 4 (6.0) | 25 (18.1) | ||||
| >35 | 302 (89.9) | 53 (96.4) | 73 (96.1) | 63 (94.0) | 113 (81.9) | ||||
| Histologic grade | 0.890 | 0.073 | 0.121 | 0.102 | |||||
| I/II | 138 (41.1) | 26 (47.3) | 35 (46.1) | 31 (46.3) | 46 (33.3) | ||||
| III | 198 (58.9) | 29 (52.7) | 41 (53.9) | 36 (53.7) | 92 (66.7) | ||||
| T stage | 0.731 | 0.094 | 0.202 | 0.191 | |||||
| T1 | 162 (48.2) | 28 (50.9) | 41 (53.9) | 36 (53.7) | 57 (41.3) | ||||
| T2/T3 | 174 (51.8) | 27 (49.1) | 35 (46.1) | 31 (46.3) | 81 (58.7) | ||||
| Lymph node metastasis | 0.953 | 0.328 | 0.712 | 0.533 | |||||
| No | 245 (72.9) | 41 (74.5) | 57 (75.0) | 51 (76.1) | 96 (69.6) | ||||
| Yes | 91 (27.1) | 14 (25.5) | 19 (25.0) | 16 (23.9) | 42 (30.4) | ||||
| Tumor recurrence | 0.615 | 0.824 | 0.900 | 0.582 | |||||
| No | 296 (88.1) | 50 (90.9) | 67 (88.2) | 59 (88.1) | 120 (87.0) | ||||
| Yes | 40 (11.9) | 5 (9.1) | 9 (11.8) | 8 (11.9) | 18 (13.0) | ||||
| Patient death | 0.791 | 0.393 | 0.280 | 0.088 | |||||
| No | 304 (90.5) | 52 (94.5) | 71 (93.4) | 61 (91.0) | 120 (87.0) | ||||
| Yes | 32 (9.5) | 3 (5.5) | 5 (6.6) | 6 (9.0) | 18 (13.0) | ||||
| Ki-67 LI (%) | 0.228 | 0.001 | <0.001 | <0.001 | |||||
| ≤14 | 80 (23.8) | 23 (41.8) | 24 (31.6) | 19 (28.4) | 14 (10.1) | ||||
| >14 | 256 (76.2) | 32 (58.2) | 52 (68.4) | 48 (71.6) | 124 (89.9) | ||||
| Chemotherapy | 0.865 | 0.618 | 0.170 | 0.029 | |||||
| No | 58 (17.3) | 13 (23.6) | 17 (22.4) | 8 (11.9) | 20 (14.5) | ||||
| Yes | 278 (82.7) | 42 (76.4) | 59 (77.6) | 59 (88.1) | 118 (85.5) | ||||
| Radiotherapy | 0.046 | 0.018 | 0.022 | 0.968 | |||||
| No | 156 (46.4) | 20 (36.4) | 41 (53.9) | 39 (58.2) | 56 (40.6) | ||||
| Yes | 180 (53.6) | 35 (63.6) | 35 (46.1) | 28 (41.8) | 82 (59.4) | ||||
Significant (< 0.05) P values are shown in bold.
P value was determined by comparing the four groups defined by AR and HER-2 status.
P value was determined by comparing AR+ and AR- groups by Fisher’s exact test.
The expression of sarcosine metabolism-related proteins according to the AR and HER-2 status in ER negative breast cancer
Sarcosine metabolism related protein expressions in ER negative breast cancers, according to AR and HER-2 status were as follows (Table 3 and Figure 2): GNMT and PIPOX expression was highest in the AR(+)/HER-2(-) group, while it was lowest in the AR(-)/HER-2(-) group (P<0.001). Also stromal PIPOX expression was highest in the AR(-)/HER-2(+) group and lowest in the AR(-)/HER-2(-) group (P=0.010). GNMT and PIPOX expressions were higher in AR positive group than in AR negative group (P=0.001, and P<0.001, respectively), while within the AR negative group, GNMT was highly expressed when HER-2 was positive (P=0.006). Within the AR negative group, turmoral and stromal PIPOX expressions were both higher in the HER-2 positive than in the HER-2 negative group (P=0.002, and P=0.003, respectively).
Table 3.
Expression of sarcosine metabolism-related proteins according to the AR and HER-2 status in ER negative breast cancer
| Parameters | Total N=336 (%) | AR positive group n=131 | AR negative group n=205 | p-value* | p-value† | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HER-2- n=55 (%) | HER-2+ n=76 (%) | p-value | HER-2+ n=67 (%) | HER-2- n=138 (%) | p-value | ||||
| GNMT | 0.051 | 0.006 | <0.001 | 0.001 | |||||
| Negative | 308 (91.7) | 43 (78.2) | 69 (90.8) | 60 (89.6) | 136 (98.6) | ||||
| Positive | 28 (8.3) | 12 (21.8) | 7 (9.2) | 7 (10.4) | 2 (1.4) | ||||
| SARDH | 0.811 | 1.000 | 0.689 | 0.256 | |||||
| Negative | 291 (86.6) | 47 (85.5) | 63 (82.9) | 59 (88.1) | 122 (88.4) | ||||
| Positive | 45 (13.4) | 8 (14.5) | 13 (17.1) | 8 (11.9) | 16 (11.6) | ||||
| PIPOX (T) | 0.104 | 0.002 | <0.001 | <0.001 | |||||
| Negative | 214 (63.7) | 17 (30.9) | 35 (46.1) | 44 (65.7) | 118 (85.5) | ||||
| Positive | 122 (36.3) | 38 (69.1) | 41 (53.9) | 23 (34.3) | 20 (14.5) | ||||
| PIPOX (S) | 0.802 | 0.003 | 0.010 | 0.102 | |||||
| Negative | 299 (89.0) | 48 (87.3) | 64 (84.2) | 55 (82.1) | 132 (95.7) | ||||
| Positive | 37 (11.0) | 7 (12.7) | 12 (15.8) | 12 (17.9) | 6 (4.3) | ||||
Significant (< 0.05) P values are shown in bold.
P value was determined by comparing the four groups defined by AR and HER-2 status.
P value was determined by comparing AR+ and AR- groups by Fisher’s exact test. glycine N-methyltransferase (GNMT), sarcosine dehydrogenase (SARDH), and l-pipecolic acid oxidase (PIPOX), androgen receptor (AR).
Figure 2.

Immunohistochemical results for sarcosine metabolism-related proteins according to AR and HER-2 status in ER negative breast cancer. GNMT and PIPOX expression is highest in the AR(+)/HER-2(-) group, while it is lowest in the AR(-)/HER-2(-) group (P<0.001). Also in stromal PIPOX expression is highest in the AR(-)/HER-2(+) group and lowest in the AR(-)/HER-2(-) group.
Sarcosine metabolism phenotypes according to the AR and HER-2 status in ER negative breast cancer
ER negative breast cancer was evaluated according to the sarcosine metabolism phenotype as defined through immunohistochemical staining results of GNMT, SARDH and PIPOX (Table 4). The results were analyzed according to AR and HER-2 status, in which AR positive group had the highest rate of low sarcosine type, while in the AR negative group had the highest rate of null type (P<0.001). Within the given data set, there were a total of 278 cases that had received chemotherapy (82.7%) and 180 cases that had received radiotherapy (53.6%). Statistical analysis revealed that AR positive group had received chemotherapy significantly more than the AR negative group (P=0.029) and that radiotherapy was conducted more in the HER-2 negative groups than HER-2 positive groups (P=0.046, and P=0.018, respectively).
Table 4.
Sarcosine metabolism phenotypes according to the AR and HER-2 status in ER negative breast cancer
| Parameters | Total N=336 (%) | AR positive group n=131 | AR negative group n=205 | p-value* | p-value† | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HER-2- n=55 (%) | HER-2+ n=76 (%) | p-value | HER-2+ n=67 (%) | HER-2- n=138 (%) | p-value | ||||
| Sarcosine metabolic type | 0.066 | 0.002 | <0.001 | <0.001 | |||||
| High sarcosine type | 10 (3.0) | 1 (1.8) | 2 (2.6) | 6 (9.0) | 1 (0.7) | ||||
| Low sarcosine type | 129 (38.4) | 31 (56.4) | 41 (53.9) | 24 (35.8) | 33 (23.9) | ||||
| Intermediate sarcosine type | 18 (5.4) | 11 (20.0) | 5 (6.6) | 1 (1.5) | 1 (0.7) | ||||
| Null type | 179 (53.3) | 12 (21.8) | 28 (36.8) | 36 (53.7) | 103 (74.6) | ||||
Significant (< 0.05) P values are shown in bold.
P value was determined by comparing the four groups defined by AR and HER-2 status.
P value was determined by comparing AR+ and AR− groups by Fisher’s exact test.
Correlation between clinicopathologic parameters and expression of sarcosine metabolism-related proteins in ER negative breast cancer
In the investigation between sarcosine metabolism-related proteins and clinicopathologic factors in ER negative breast cancer, GNMT expression was associated with higher Ki-67 LI (P=0.036), SARDH expression with lymph node metastasis (P=0.028), and tumoral and stromal PIPOX expression with HER-2 positivity (P=0.006, and P=0.005, respectively, Figure 3).
Figure 3.

Correlation between clinicopathologic parameters and expression of sarcosine metabolism-related proteins in ER negative breast cancer.
In the analysis according to AR status, the AR negative group showed that GNMT expression was associated with lower Ki-67 LI (P=0.024), while within the AR positive group, SARDH positivity and tumoral PIPOX negativity was associated with lymph node metastasis (P=0.004, and P=0.008, respectively). AR(+)/HER-2(+) group showed that tumoral PIPOX positivity was associated with higher histological grade (P=0.044), while SARDH expression was associated with lymph node metastasis (P=0.008, Figure 4).
Figure 4.

Correlation between clinicopathologic parameters and expression of sarcosine metabolism-related proteins in AR negative (A), AR positive (B, C), and AR(+)/HER-2(+) group (D, E).
The impact of expression of sarcosine metabolism-related proteins on disease-free survival in ER negative breast cancer
In univariate analysis of the prognostic power of sarcosine metabolism-related proteins in ER negative breast cancer showed no statistically significant findings (Table 5).
Table 5.
Univariate analysis by log-rank test of the impact of sarcosine metabolism-related protein expression in estrogen receptor negative breast cancer on prognosis
| Parameters | Disease-free survival | Overall-survival | ||
|---|---|---|---|---|
|
| ||||
| 95% CI | p-value | 95% CI | p-value | |
| GNMT | 0.417 | 0.491 | ||
| Negative | 117 (111-124) | 124 (118-130) | ||
| Positive | 90 (74-105) | 114 (95-133) | ||
| SARDH | 0.348 | 0.951 | ||
| Negative | 116 (109-123) | 124 (119-130) | ||
| Positive | 117 (105-130) | 115 (102-128) | ||
| PIPOX (T) | 0.837 | 0.519 | ||
| Negative | 119 (113-125) | 123 (117-129) | ||
| Positive | 108 (100-116) | 118 (108-128) | ||
| PIPOX (S) | 0.995 | 0.571 | ||
| Negative | 117 (109-124) | 123 (117-129) | ||
| Positive | 115 (100-129) | 122 (108-135) | ||
| Sarcosine metabolic type | 0.452 | 0.822 | ||
| High sarcosine type | 70 (50-89) | 77 (60-93) | ||
| Low sarcosine type | 110 (100-120) | 113 (103-124) | ||
| Intermediate sarcosine type | 100 (88-112) | 122 (105-140) | ||
| Null type | 120 (113-127) | 125 (118-131) | ||
Glycine N-methyltransferase (GNMT), sarcosine dehydrogenase (SARDH), and l-pipecolic acid oxidase (PIPOX), androgen receptor (AR).
In univariate analysis of the prognostic power of sarcosine metabolism-related proteins according to the status of AR and HER-2, AR negative group showed a difference in OS according to sarcosine metabolic type. The sarcosine metabolic types in order of shorter OS are as follows; intermediate sarcosine type, high sarcosine type, low sarcosine type and null type (P<0.001, and Table 6). There was no prognostic difference in sarcosine metabolism-related proteins according to HER-2 status (Table 7).
Table 6.
Univariate analysis by log-rank test of the impact of sarcosine metabolism-related protein expression in estrogen receptor negative breast cancer on prognosis according to AR status
| Parameters | AR positive | AR negative | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Disease-free survival | Overall-survival | Disease-free survival | Overall-survival | |||||
|
| ||||||||
| 95% CI | p-value | 95% CI | p-value | 95% CI | p-value | 95% CI | p-value | |
| GNMT | 0.946 | 0.757 | 0.180 | 0.122 | ||||
| Negative | 91 (83-98) | 120 (105-134) | 118 (111-126) | 122 (116-128) | ||||
| Positive | 93 (76-110) | 120 (99-141) | 71 (48-93) | 71 (50-92) | ||||
| SARDH | 0.521 | 0.366 | 0.411 | 0.574 | ||||
| Negative | 90 (82-98) | 119 (104-134) | 117 (108-125) | 123 (117-128) | ||||
| Positive | 97 (84-109) | 121 (101-140) | 118 (103-134) | 111 (94-128) | ||||
| PIPOX (T) | 0.502 | 0.152 | 0.436 | 0.483 | ||||
| Negative | 88 (78-98) | 116 (100-132) | 120 (113-127) | 122 (116-129) | ||||
| Positive | 96 (87-104) | 128 (122-135) | 106 (93-120) | 108 (94-121) | ||||
| PIPOX (S) | 0.877 | 0.999 | 0.879 | 0.508 | ||||
| Negative | 91 (84-98) | 121 (107-135) | 117 (109-125) | 121 (115-127) | ||||
| Positive | 92 (73-111) | 118 (94-142) | 116 (98-135) | 123 (109-137) | ||||
| Sarcosine metabolic type | 0.806 | N/A | N/A | <0.001 | ||||
| High sarcosine type | 77 (55-98) | N/A | N/A | 79 (60-97) | ||||
| Low sarcosine type | 88 (79-96) | N/A | N/A | 112 (100-124) | ||||
| Intermediate sarcosine type | 100 (87-112) | N/A | N/A | 22 (22-22) | ||||
| Null type | 87 (75-100) | N/A | N/A | 124 (118-130) | ||||
Glycine N-methyltransferase (GNMT), sarcosine dehydrogenase (SARDH), and l-pipecolic acid oxidase (PIPOX), androgen receptor (AR).
Table 7.
Univariate analysis by log-rank test of the impact of sarcosine metabolism-related protein expression in estrogen receptor negative breast cancer on prognosis according to HER-2 status
| Parameters | HER-2 positive | HER-2 negative | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Disease-free survival | Overall-survival | Disease-free survival | Overall-survival | |||||
|
| ||||||||
| 95% CI | p-value | 95% CI | p-value | 95% CI | p-value | 95% CI | p-value | |
| GNMT | 0.232 | 0.371 | 0.854 | 0.977 | ||||
| Negative | 112 (102-121) | 124 (114-133) | 118 (110-126) | 121 (115-128) | ||||
| Positive | 81 (57-105) | 106 (75-137) | 84 (72-96) | 84 (72-96) | ||||
| SARDH | 0.160 | 0.334 | 0.986 | 0.493 | ||||
| Negative | 83 (75-90) | 120 (109-131) | 119 (110-127) | 123 (117-129) | ||||
| Positive | 124 (112-136) | 124 (110-138) | 87 (78-96) | 106 (90-123) | ||||
| PIPOX (T) | 0.906 | 0.343 | 0.802 | 0.892 | ||||
| Negative | 110 (99-121) | 120 (108-132) | 121 (113-128) | 121 (114-128) | ||||
| Positive | 91 (79-103) | 121 (107-135) | 111 (101-120) | 114 (105-123) | ||||
| PIPOX (S) | 0.993 | 0.909 | 0.851 | N/A | ||||
| Negative | 85 (78-92) | 122 (112-133) | 118 (110-126) | N/A | ||||
| Positive | 112 (93-131) | 117 (98-135) | 89 (75-103) | N/A | ||||
| Sarcosine metabolic type | N/A | N/A | N/A | N/A | ||||
| High sarcosine type | N/A | N/A | N/A | N/A | ||||
| Low sarcosine type | N/A | N/A | N/A | N/A | ||||
| Intermediate sarcosine type | N/A | N/A | N/A | N/A | ||||
| Null type | N/A | N/A | N/A | N/A | ||||
Glycine N-methyltransferase (GNMT), sarcosine dehydrogenase (SARDH), and l-pipecolic acid oxidase (PIPOX), androgen receptor (AR).
Discussion
In the current study regarding ER negative breast cancers, sarcosine metabolism related protein expression was analyzed according to AR and HER-2 status. AR and sarcosine metabolism related protein expression was found to have significant relationships with each other. GNMT and PIPOX expression was higher in the AR positive group compared to AR negative group. Although there are no studies dealing with sarcosine metabolism-related proteins in breast cancers for direct comparison, sarcosine metabolism studies on prostate cancers may serve as a good hypothetical clue. In prostate cancers, increased sarcosine level was associated with cancer progression. When sarcosine was injected into a benign prostate cell, the cell had transformed to an invasive phenotype [1], and also reported an increased sarcosine level within prostate cancer tissues [13]. Also, in both in vitro and in vivo models sarcosine was shown to be associated with prostate cancer growth and progression [2]. In the current study, sarcosine level was not evaluated directly from breast cancer tissues, but instead, sarcosine metabolism-related proteins, such as GNMT, SARDH and PIPOX were measured semi-quantitatively through immunohistochemical staining. However, in the study based on prostate cancer showed that such sarcosine metabolism-related proteins did correlate well with sarcosine activity level; the sarcosine generating enzyme, GNMT, expression was high in the cancer tissue compared to normal prostate tissue, while sarcosine metabolizing enzymes, such as SARDH and PIPOX, expression was low [2]. In the current study, expressions of AR and sarcosine metabolism related proteins were found to be intricately related. In the previously reported study regarding prostate cancer cells, androgen had increased expression of GNMT through AR, which was attributed to the fact that androgen response element exists on the first exon coding region of GNMT [14]. Therefore AR was assumed to play a key role in the expression of sarcosine metabolism related proteins in prostate cancers, a finding that parallels with our results. However in the current study, not only did GNMT, a sarcosine increasing substance, but PIPOX, a sarcosine metabolizing enzyme had increased in AR positive cases, that calls for further investigations into the relationship between AR and sarcosine levels in breast cancer. Sarcosine phenotypes, classified according to expressions of sarcosine metabolism related proteins, revealed distinctive associations with AR status. AR positive group had a high proportion of low sarcosine types, while the AR negative group, null type had the highest proportion. Because the null type is the group where GNMT, SARDH and PIPOX are all negative, in the case when AR is positive, sarcosine metabolism related activity would be expected to be increased compared to AR negative groups.
In the current study, the relationship between HER-2 and sarcosine metabolism related protein expression was significantly different according to AR status. Within AR negative group, GNMT and PIPOX were both highly expressed in the HER-2 positive group than in HER-2 negative group. Although in breast cancers, the exact mechanism and association between HER-2 and sarcosine metabolism related protein expression is not yet known, previous studies on prostate cancer reported that sarcosine increased both HER-2 mRNA and protein expressions [8]. In this study, AR status explains the relationship between HER-2 and sarcosine metabolism related protein expressions. In previous reports that describe molecular apocrine breast cancer (MABC) as a unique entity of ER negative breast cancers with AR positivity [i.e. ER(-)/AR(+)] and apocrine histological features, about 20-50% is reported to have HER-2 overexpression/amplification [6,7]. Therefore, ER(-)/AR(+) group and ER(-)/AR(-) group would be expected to have different features. In this study, the ER(-)/AR(-) group showed a difference in expression between GNMT and PIPOX according to HER-2 status, while in the ER(-)/AR(+) group (MABC), there was no significant difference according to HER-2 status. Although HER-2 status is an important biomarker that characterizes breast cancers, in MABC, there was no significant difference between HER-2 positive and negative groups [6], consistent with the findings of this study.
In conclusion, in ER negative breast cancers, expression of sarcosine metabolism related proteins is different according to AR status; in the AR positive group, GNMT and PIPOX expression was higher than in those of the AR negative group. Within the AR negative group, GNMT and PIPOX were both highly expressed in the HER-2 positive group than in HER-2 negative group.
Acknowledgements
This study was supported by a grant from National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1420080). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2015R1A1A1A05001209).
Disclosure of conflict of interest
None.
References
- 1.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 2.Khan AP, Rajendiran TM, Ateeq B, Asangani IA, Athanikar JN, Yocum AK, Mehra R, Siddiqui J, Palapattu G, Wei JT, Michailidis G, Sreekumar A, Chinnaiyan AM. The role of sarcosine metabolism in prostate cancer progression. Neoplasia. 2013;15:491–501. doi: 10.1593/neo.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum CE, Price DK, Figg WD. Sarcosine as a potential prostate cancer biomarker and therapeutic target. Cancer Biol Ther. 2010;9:341–342. doi: 10.4161/cbt.9.5.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, Look MP, Liem GS, Klijn JG, Henzen-Logmans SC. The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer. 1996;32A:1560–1565. doi: 10.1016/0959-8049(96)00112-8. [DOI] [PubMed] [Google Scholar]
- 5.Riva C, Dainese E, Caprara G, Rocca PC, Massarelli G, Tot T, Capella C, Eusebi V. Immunohistochemical study of androgen receptors in breast carcinoma. Evidence of their frequent expression in lobular carcinoma. Virchows Arch. 2005;447:695–700. doi: 10.1007/s00428-005-0003-6. [DOI] [PubMed] [Google Scholar]
- 6.Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, Duss S, Nicoulaz AL, Brisken C, Fiche M, Delorenzi M, Iggo R. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 7.Banneau G, Guedj M, MacGrogan G, de Mascarel I, Velasco V, Schiappa R, Bonadona V, David A, Dugast C, Gilbert-Dussardier B, Ingster O, Vabres P, Caux F, de Reynies A, Iggo R, Sevenet N, Bonnet F, Longy M. Molecular apocrine differentiation is a common feature of breast cancer in patients with germline PTEN mutations. Breast Cancer Res. 2010;12:R63. doi: 10.1186/bcr2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl M, Bouchelouche P, Kramer-Marek G, Capala J, Nordling J, Bouchelouche K. Sarcosine induces increase in HER2/neu expression in androgen-dependent prostate cancer cells. Mol Biol Rep. 2011;38:4237–4243. doi: 10.1007/s11033-010-0442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 11.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 12.Won KY, Kim GY, Kim YW, Song JY, Lim SJ. Clinicopathologic correlation of beclin-1 and bcl-2 expression in human breast cancer. Hum Pathol. 2010;41:107–112. doi: 10.1016/j.humpath.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Jentzmik F, Stephan C, Lein M, Miller K, Kamlage B, Bethan B, Kristiansen G, Jung K. Sarcosine in prostate cancer tissue is not a differential metabolite for prostate cancer aggressiveness and biochemical progression. J Urol. 2011;185:706–711. doi: 10.1016/j.juro.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 14.Lee CM, Yen CH, Tzeng TY, Huang YZ, Chou KH, Chang TJ, Arthur Chen YM. Androgen response element of the glycine N-methyltransferase gene is located in the coding region of its first exon. Biosci Rep. 2013;33:e00070. doi: 10.1042/BSR20130030. [DOI] [PMC free article] [PubMed] [Google Scholar]


