Abstract
The hobnail variant of papillary thyroid carcinoma (PTC) is a rare, aggressive variant in which > 30% of the tumor cells have hobnail features. The clinical behavior and pathologic characteristics of these tumors are still unclear due to the rarity of the entity. The present study aimed to investigate cytologic, clinical, pathological, and molecular features of the hobnail variant from our data and from the literature. We retrospectively retrieved 10 cases of hobnail variant from 2,904 consecutive PTC patients. Cytologic and histopathologic slides from those 10 patients were reviewed. We performed molecular analysis for BRAF, ALK, and TERT promoter mutations on paraffin blocks from surgical specimens, and further analyzed the clinicopathologic characteristics of all case reports published in the literature until now. Cytologically, all tumors were characterized by single cells with eccentric nuclei and tapering cytoplasm (comet-like cells), and syncytial or micropapillary clusters with apically placed nuclei resulting in a hobnail appearance in both conventional smears and liquid-based cytology. The BRAF V600E mutation was found in 8 cases (80%) whereas no cases had ALK fusion or TERT promoter mutations. In the literature review of 55 patients including our cases, most patients presented with advanced stage cancer, and disease-specific survival rates were 83%, 71%, and 54% at 5, 10, and 20 years after the initial surgery, respectively. Characteristic cytologic features can allow a preoperative diagnosis of the hobnail variant of PTC based on cytology specimens. Further studies should be performed to identify the molecular genetics of the variant.
Keywords: Thyroid cancer, hobnail variant, fine needle aspiration, BRAF, ALK, TERT
Introduction
Papillary thyroid carcinoma (PTC) usually displays an indolent clinical course. However, a few patients with PTC die of distant metastasis and radioactive iodine-refractory, progressive disease. The types of PTC associated with aggressive clinical behavior include tall cell, columnar cell, solid, diffuse sclerosing and hobnail variants [1].
The hobnail variant of PTC demonstrates discohesive growth and single cells with a loss of polarity and is characterized by exhibition in > 30% of the tumor cells of apically placed nuclei and a surface bulge [2,3]. This rare variant is associated with aggressive clinicopathologic features and a higher mortality rate compared with classic PTC [4-6].
The most commonly occurring mutation in patients with PTC is a BRAF V600E mutation that is associated with extrathyroidal extension, lymph node metastasis, distant metastasis, recurrence, and mortality [7-9]. The BRAF V600E mutation has been frequently found in the hobnail variant of PTC [4,10,11]. TERT promoter mutations have recently been found in the aggressive thyroid cancers [12,13]. However, these mutations have not been investigated in the hobnail variant of PTC.
Fine needle aspiration cytology (FNAC) has been widely used as the most effective preoperative evaluation tool for the screening of thyroid nodules [14]. The definitive diagnosis of the hobnail variant in FNAC specimens plays an important role in the planning of surgical management. However, the cytologic diagnosis of the hobnail variant is difficult not only because of its rarity, but because the cytologic features of the hobnail variant on conventional smears have been reported as small series of cases or single case reports.
The present study aimed to investigate the clinicopathologic characteristics and cytologic features of conventional smears and liquid-based cytology of the hobnail variant of PTC. We further performed a systematic review and analysis of studies that investigated the clinicopathologic features and prognosis of the hobnail variant of PTC.
Materials and methods
Patient selection
We retrospectively reviewed the pathology slides of all 2,904 consecutive patients who underwent thyroid surgery for PTC at Seoul St. Mary’s Hospital, The Catholic University of Korea over a three year period. A total of 10 patients were selected as having the hobnail variant of PTC according to the diagnostic criteria as previously reported [2].
Fine needle aspiration cytology
All patients underwent ultrasound-guided FNAC. Of the 10 FNAC specimens, seven were directly smeared on the slides and three were processed with ThinPrepTM (Hologic Inc., Marlborough, MA, USA) liquid-based preparations (LBPs). The specimens were stained with Papanicolaou staining and/or hematoxylin and eosin. Cell block preparations (CBPs) were made from the LBPs. All cytologic slides were reviewed by two independent pathologists (CKJ. and YSL). The cytologic diagnosis on the FNAC specimens was performed according to the Bethesda system for reporting thyroid cytopathology [15].
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded tissue sections of surgical specimens. The following antibodies were used: ALK antibody (clone p80, Novocastra Laboratories Ltd., Newcastle upon Tyne, UK), p53 (clone DO-7, Ventana Medical Systems, Inc., Tucson, AZ, USA), cyclin D1 (clone SP4, Thermo Scientific, Fremont, CA, USA), HBME1 (clone HBME-1, Dako Corporation, Carpinteria, CA, USA), and CD56 (clone 1B6, Novocastra). The p53 expression was measured as the percentage of positive nuclear immunostaining among tumor cells. For other markers, immunostaining was categorized as negative, focally positive (< 50% of the tumor cells), or diffusely positive (≥ 50% of the tumor cells).
Mutational analysis
Genomic DNA was isolated from the microdissected paraffin-embedded tissue sections from surgical resection specimens using the QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany). The presence of BRAF V600E and TERT promoter mutations was investigated by Sanger sequencing, as described earlier [13,16]. Fluorescence in situ hybridization (FISH) for the ALK fusion gene was performed using a ZytoLight SPEC ALK Dual Color Break Apart Probe and Kit (ZytoVision GmbH, Bremerhaven, Germany) according to the manufacturer’s protocol. ALK FISH-positive cases were defined as > 15% of split signal separation and/or isolated red signal in at least 100 tumor cells as previously described [17].
Literature review
We searched the literature for hobnail variant of PTC using a comprehensive PubMED search (http://www.ncbi.nlm.nih.gov/pubmed/) with a combination of the search words “thyroid” and “hobnail” or “micropapillary”. After further manual analysis, 45 cases from eight articles were determined to meet the diagnostic criteria for the hobnail variant of PTC and were selected for statistical analysis [3-6,11,18-20]. Disease-specific survival rates were obtained using the life-table method.
Results
Clinical features
The clinicopathologic characteristics of the 10 patients are summarized in Table 1. The age of the patients (6 women and 4 men) ranged from 32 to 68 years (median 50.5 years). All patients underwent ultrasound-guided FNAC and were diagnosed with PTC. Tumor size ranged from 0.6 to 4.0 cm (median 1.3 cm). All patients underwent total thyroidectomy and cervical lymph node dissection. The follow-up period after thyroidectomy ranged from 9 to 28 months (median 12 months). One patient (case 1) developed a regional lymph node recurrence 21 months after the initial surgery. No patients died from the disease during the follow-up period.
Table 1.
Demographic and clinicopathologic characteristics of patients with the hobnail variant of papillary thyroid carcinoma in the present study and the literature review
| Characteristic | Number of cases (%) | ||
|---|---|---|---|
|
| |||
| Present study (n = 10) | Review of the literature (n = 45) | Total cases (n = 55) | |
| Female:male ratio | 6:4 (1.5:1) | 32:13 (2.5:1) | 38:17 (2.2:1) |
| Mean age (years) (range) | 50.4 (32-68) | 56.9 (21-86) | 55.7 (21-86) |
| Mean tumor size (cm) (range) | 1.63 (0.6-4) | 3.81 (0.5-11) | 3.41 (0.5-11) |
| Multifocality | 3/10 (30%) | 12/28 (43%) | 15/38 (40%) |
| Tumor site | |||
| Right lobe | 6/10 (60%) | 0/14 (0%) | 6/24 (25%) |
| Left lobe | 4/10 (40%) | 0/14 (0%) | 4/24 (17%) |
| Right and left lobe | 0/10 (0%) | 14/14 (100%) | 14/24 (58%) |
| Results of fine needle aspiration cytology | |||
| Atypia of undetermined significance | 0/10 (0%) | 1/18 (5.6%) | 1/28 (3.6%) |
| PTC | 10/10 (100%) | 16/18 (89%) | 26/28 (93%) |
| PTC, tall cell variant | 0/10 (0%) | 1/18 (5.6%) | 1/28 (3.6%) |
| Original histologic diagnosis | |||
| PTC | 0/10 (0%) | 24/45 (53%) | 24/55 (44%) |
| PTC, classic | 2/10 (20%) | 2/45 (4.4%) | 4/55 (7.3%) |
| PTC with hobnail features | 1/10 (10%) | 1/45 (2.2%) | 2/55 (3.6%) |
| PTC, hobnail variant | 7/10 (70%) | 18/45 (40%) | 25/55 (46%) |
| Chief complaint | |||
| Incidental finding | 9/10 (90%) | 3/15 (20%) | 12/25 (48%) |
| Neck mass | 1/10 (10%) | 8/15 (53%) | 9/25 (36%) |
| Cervical lymphadenopathy | 0/10 (0%) | 2/15 (13%) | 2/25 (8.0%) |
| Dyspnea | 0/10 (0%) | 2/15 (13%) | 2/25 (8.0%) |
| Extrathyroidal extension | 7/10 (70%) | 26/44 (59%) | 33/54 (61%) |
| Tracheal invasion | 1/10 (10%) | 5/43 (12%) | 6/53 (11%) |
| Mean mitotic count (/10HPFs) (range) | 0.2 (0-1) | 2.7 (0-9) | 2.1 (0-9) |
| Necrosis | 2/10 (20%) | 3/45 (6.7%) | 5/55 (9.1%) |
| Lymyphovascular invasion | 8/10 (80%) | 27/43 (63%) | 35/53 (66%) |
| Lymph node metastasis | 8/10 (80%) | 26/41 (63%) | 34/51 (67%) |
| Lateral lymph node metastasis | 4/10 (40%) | 14/40 (35%) | 18/50 (36%) |
| Bone metastasis | 0/10 (0%) | 3/42 (7.1%) | 3/52 (5.8%) |
| Lung metastasis | 0/10 (0%) | 9/42 (21%) | 9/52 (17%) |
| AJCC staging | |||
| I | 4/10 (40%) | 13/41 (32%) | 17/51 (33%) |
| II | 0/10 (0%) | 2/41 (4.9%) | 2/51 (3.9%) |
| III | 4/10 (40%) | 9/41 (22%) | 13/51 (25%) |
| IVA | 2/10 (20%) | 8/41 (20%) | 10/51 (20%) |
| IVC | 0/10 (0%) | 9/41 (22%) | 9/51 (18%) |
| Mutational analysis | |||
| BRAF V600E mutation | 8/10 (80%) | 17/24 (71%) | 25/34 (74%) |
| RET/PTC1 rearrangement | NA | 2/10 (20%) | 2/10 (20%) |
| TERT promoter mutation | 0/10 (0%) | NA | 0/10 (0%) |
| ALK fusion | 0/10 (0%) | NA | 0/10 (0%) |
| Regional/local recurrence | 1/10 (10%) | 11/42 (26%) | 12/52 (23%) |
| Outcome | |||
| No evidence of disease | 9/10 (90%) | 24/42 (5.7%) | 33/52 (64%) |
| Alive with disease | 1/10 (10%) | 8/42 (19%) | 9/52 (17%) |
| Died of disease | 0/10 (0%) | 10/42 (24%) | 10/52 (19%) |
PTC, papillary thyroid carcinoma; HPFs, high power fields; NA, not available; AJCC, American Joint Committee on Cancer.
Cytologic features
The cytologic features of the tumors are summarized in Table 2. All seven conventional smears were highly cellular with little colloid. The hobnail features were frequently found in the papillary, micropapillary or discohesive cell clusters (Figure 1A and 1B). Isolated single cells showed eccentric nuclei with a tear-drop cytoplasm, so-called comet-like cells (Figure 1C). Multiple soap-bubble-like intranuclear inclusions were seen in all cases (Figure 1D). Mitosis was found in two cases. In three LBP samples, papillary and micropapillary structures were less frequently found than in conventional smears whereas syncytial cell clusters with apically-placed nuclei were frequently seen (Figure 2A). Comet-like cells and multiple soap-bubble-like intranuclear inclusions, as well as typical nuclear features of PTC were easily detected (Figure 2B and 2C). All cell blocks showed papillary fragments lined by hobnail cells (Figure 2D).
Table 2.
Fine needle aspiration cytologic features of hobnail variant of papillary thyroid carcinoma
| Case No. | Specimen type | Cellularity | Papillary | Micro-papillary | Syncytial fragments | Hobnail features | Single cells | Comet-like cells | Soap-bubble-like nuclear inclusions | Nuclear features of PTC | Psammoma bodies | Giant cells |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Smear | Marked | + | + | ++ | + | ++ | ++ | + | ++ | - | - |
| 2 | Smear | Marked | + | + | ++ | + | ++ | ++ | ++ | ++ | + | + |
| 3 | Smear | Marked | + | + | ++ | + | ++ | ++ | + | ++ | - | - |
| 4 | Smear | Marked | ++ | + | ++ | ++ | ++ | ++ | + | ++ | - | + |
| 5 | Smear | Marked | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | + | - |
| 6 | Smear | Marked | + | + | ++ | + | + | ++ | + | ++ | + | - |
| 7 | Smear | Marked | ++ | + | ++ | ++ | ++ | ++ | + | ++ | + | - |
| 8 | LBP | Marked | + | + | ++ | + | ++ | ++ | + | ++ | - | - |
| 9 | LBP | Marked | + | + | ++ | + | ++ | ++ | ++ | ++ | - | - |
| 10 | LBP | Marked | + | + | ++ | + | ++ | ++ | ++ | ++ | + | - |
LBP, liquid-based preparation; -, absent; +, present; ++, frequently present; PTC, papillary thyroid carcinoma.
Figure 1.
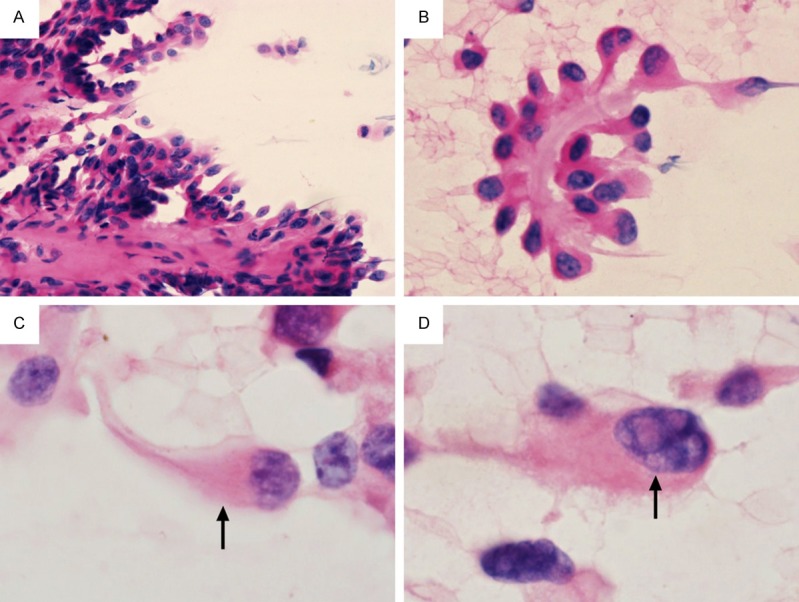
Cytologic features of hobnail variant of papillary thyroid carcinoma in a conventional smear. A. A papillary fragment lined by hobnail cells (H&E × 200). B. A micropapillary fragment showing hobnail appearance (H&E ×400). C. A comet-like cell (arrow), showing nucleus protruding toward one side and cytoplasm tapering toward the opposite side (H&E × 1000). D. Isolated single cells with multiple soap-bubble-like intranuclear inclusions (arrow) (H&E × 1000). H&E, hematoxylin and eosin.
Figure 2.

Cytologic features of hobnail variant of papillary thyroid carcinoma in a liquid-based cytology (ThinPrepTM). A. A syncytial cell cluster showing apically placed nuclei and a surface bulging (Papanicolaou stain × 400). B. A comet-like cell, characterized by eccentric nucleus and tapering cytoplasm (Papanicolaou stain × 1000). C. Multiple soap-bubble-like intranuclear inclusions (Papanicolaou stain × 1000). D. A cell block showing papillary structures with prominent hobnail features (H&E × 200).
Histopathology and immunohistochemistry
The histologic features and immunohistochemistry of the hobnail variant are summarized in Table 1. In three patients (cases 1, 6 and 7) with two tumor foci, the largest tumor was hobnail variant (Figure 3A) and the smaller was classic type, tall cell variant, and non-invasive encapsulated follicular variant, respectively. Mitosis was found only in two cases (cases 4 and 7) which also showed tumor necrosis. Extrathyroidal extension was seen in seven cases and lymph node metastases were found in eight cases. Vascular invasion was observed in only one case (case 1). The percentage of tumor cells positive for p53 immunostaining ranged from 1% to 48% (Figure 3B). All cases were diffusely positive for cyclinD1 and HBME1, and focally positive for CD56. No cases demonstrated expression of ALK.
Figure 3.
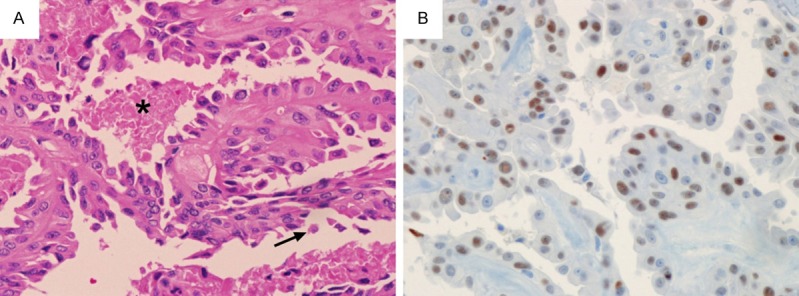
A. Histology of hobnail variant of papillary thyroid carcinoma. Thin arborizing papillae are lined by hobnail cells. Single detaching cells (arrow) and tumor necrosis (asterisk) are also noted (H&E × 200). B. Immunohistochemistry reveals positivity for p53 (× 200).
Mutational analysis
Eight cases (80%) harbored BRAF V600E mutation. No cases had ALK fusion or TERT promoter mutations (Table 1).
Analysis of cases in our study and the literature
We analyzed the clinicopathologic and molecular features of 55 patients (45 in the literature as well as our 10 cases) with the hobnail variant of PTC (Table 1). Women were affected more frequently than men (2.2:1). The age of patients ranged from 21 to 86 years (mean, 55.7 years). The patients often presented with incidental ultrasound findings or a neck mass. Mean tumor size was 3.4 cm (range, 0.5-11.0 cm). Almost all patients were diagnosed with PTC before undergoing surgery. Extrathyroidal extension and tracheal invasion were found in 61% and 11%, respectively. More than half of patients presented with stage III (25%) or IV (38%) cancer. The tumors developed bone and lung metastases in 5.8% and 17% of cases, respectively. Clinical follow-up data were available in 52 patients (follow-up period, 4-274 months). The local recurrence rate was 23% during the follow-up period. Disease-specific survival rates were 83%, 71%, and 54% at 5, 10, and 20 years after surgery, respectively (Figure 4). The BRAF V600E mutation was present in 25 (74%) of 34 cases. The rate of RET/PTC1 rearrangement was 20% (2/10) in one series [11].
Figure 4.
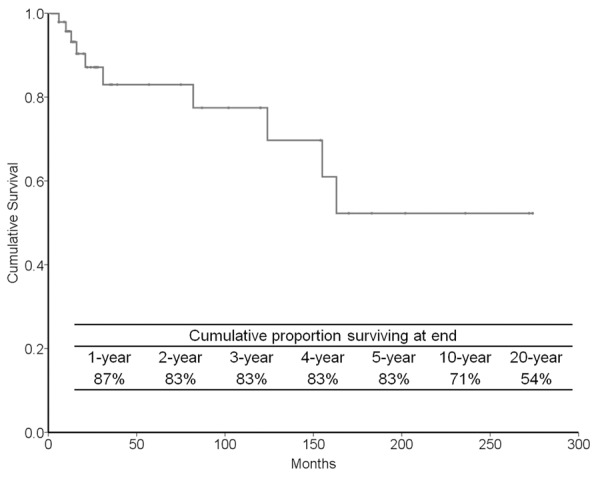
Cumulative survival probabilities of 52 patients with hobnail variant of papillary thyroid carcinoma calculated by the life-table method.
Discussion
We have shown that the hobnail variant of PTC is an aggressive variant characterized by a higher rate of initial presentation at an advanced tumor stage and poor outcome. Although the number of reported cases of hobnail PTC is small, it is important to note that the long-term survival rate of the variant is much lower than that of classic PTC.
Preoperative FNAC diagnosis of thyroid nodule is important in planning surgical strategy. When the result of FNAC is positive for PTC, National Comprehensive Cancer Network guidelines indicate a total thyroidectomy as primary treatment in patients with any one of the following: radiation history, distant metastasis, bilateral nodularity, extrathyroidal extension, tumor > 4 cm in diameter, cervical lymph node metastases, or poorly differentiated features (http://www.nccn.org/). Current guidelines for PTC do not specifically mention treating patients with hobnail PTC. We suggest that total thyroidectomy should be considered for the initial treatment of patients with hobnail PTC because the variant has a poor outcome in comparison to poorly differentiated thyroid cancer.
The preoperative diagnosis of the histologic subtypes of PTC may be challenging in FNAC samples. In our study using conventional smears and LBPs, the most characteristic cytologic features were hobnail appearance, comet-like cells and soap-bubble-like intranuclear inclusion. These findings were in concordance with previous studies reporting cytologic features of hobnail PTCs in the conventional smears, although there have been no studies investigating LBPs of the tumor [5,19,20]. These cytologic features should therefore be mentioned in FNAC results and can allow a preoperative diagnosis of the hobnail variant of PTC.
The differential diagnosis of hobnail PTC in FNAC includes tall cell variant of PTC characterized by eccentric nuclei, columnar and abundant cytoplasm, distinct cell borders, and soap-bubble-like intranuclear inclusions [21]. However, tall cell variant lacks the cytologic features of the hobnail appearance and comet-like cells. The cytologic features of other variants needing to be differentiated from hobnail PTC are well summarized in a previous study [5].
The overexpression of p53 is generally found in poorly differentiated and anaplastic thyroid cancers and associated with tumor progression from well-differentiated to undifferentiated cancer [22]. One previous study reported that p53 overexpression (> 25% of the tumor cells) was found in all of a series of 14 hobnail PTCs [3]. In our study, three of 10 cases showed > 10% immunoexpression of p53 and the highest percentage of the immunostaining was 48%. Data regarding mitotic activity were available for analysis in 40 cases from four published reports as well as the present study [3-5,20]. The mean number of mitotic figures per 10 high-power fields was 2.1 ranging from 0 to 9. These findings support the aggressive biologic behavior of the hobnail PTC.
We investigated whether molecular alterations known to be associated with aggressive thyroid cancer can also be identified in the hobnail variant of PTC. Three studies reported a 71% (17/24) mutation rate of BRAF V600E (Table 1) [4,11,19]. In our study, the 80% mutation rate falls in the range of overall prevalence found in Korean PTC patients [16,23]. ALK fusion and TERT promoter mutation are more prevalent in aggressive thyroid cancers [12,13,24]. In our study, all 10 cases were negative for ALK fusion and TERT promoter mutation. From our literature review, these mutations have not been studied in the hobnail variant of PTC. Further studies in a large number of cases are needed to confirm whether those genetic alterations are related to the hobnail variant of PTC.
In conclusion, the rare hobnail variant of PTC may require more aggressive treatment than classic PTCs due to its aggressive clinicopathologic features and poor outcome. The cytologic features of the hobnail variant are characteristic and can allow a preoperative diagnosis on the basis of FNAC specimens. The BRAF V600E mutation is the most common event frequently found in the hobnail variant. Further studies are needed to identify more precise molecular genetics.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2013R1A2A2A01068570).
Disclosure of conflict of interest
None.
References
- 1.Roman S, Sosa JA. Aggressive variants of papillary thyroid cancer. Curr Opin Oncol. 2013;25:33–38. doi: 10.1097/CCO.0b013e32835b7c6b. [DOI] [PubMed] [Google Scholar]
- 2.Albores-Saavedra J. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical, and molecular study of 8 cases. Am J Surg Pathol. 2010;34:913. doi: 10.1097/PAS.0b013e3181d85d80. author reply 914. [DOI] [PubMed] [Google Scholar]
- 3.Asioli S, Erickson LA, Righi A, Lloyd RV. Papillary thyroid carcinoma with hobnail features: histopathologic criteria to predict aggressive behavior. Hum Pathol. 2013;44:320–328. doi: 10.1016/j.humpath.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Asioli S, Erickson LA, Sebo TJ, Zhang J, Jin L, Thompson GB, Lloyd RV. Papillary thyroid carcinoma with prominent hobnail features: a new aggressive variant of moderately differentiated papillary carcinoma. A clinicopathologic, immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2010;34:44–52. doi: 10.1097/PAS.0b013e3181c46677. [DOI] [PubMed] [Google Scholar]
- 5.Asioli S, Maletta F, Pagni F, Pacchioni D, Vanzati A, Mariani S, Palestini N, Lloyd RV, Sapino A. Cytomorphologic and molecular features of hobnail variant of papillary thyroid carcinoma: case series and literature review. Diagn Cytopathol. 2014;42:78–84. doi: 10.1002/dc.23028. [DOI] [PubMed] [Google Scholar]
- 6.Motosugi U, Murata S, Nagata K, Yasuda M, Shimizu M. Thyroid papillary carcinoma with micropapillary and hobnail growth pattern: a histological variant with intermediate malignancy? Thyroid. 2009;19:535–537. doi: 10.1089/thy.2008.0271. [DOI] [PubMed] [Google Scholar]
- 7.Elisei R, Viola D, Torregrossa L, Giannini R, Romei C, Ugolini C, Molinaro E, Agate L, Biagini A, Lupi C, Valerio L, Materazzi G, Miccoli P, Piaggi P, Pinchera A, Vitti P, Basolo F. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab. 2012;97:4390–4398. doi: 10.1210/jc.2012-1775. [DOI] [PubMed] [Google Scholar]
- 8.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Clifton-Bligh R, Tallini G, Holt EH, Sykorova V. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 2015;33:42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amacher AM, Goyal B, Lewis JS Jr, El-Mofty SK, Chernock RD. Prevalence of a Hobnail Pattern in Papillary, Poorly Differentiated, and Anaplastic Thyroid Carcinoma: A Possible Manifestation of High-grade Transformation. Am J Surg Pathol. 2015;39:260–265. doi: 10.1097/PAS.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 11.Lubitz CC, Economopoulos KP, Pawlak AC, Lynch K, Dias-Santagata D, Faquin WC, Sadow PM. Hobnail variant of papillary thyroid carcinoma: an institutional case series and molecular profile. Thyroid. 2014;24:958–965. doi: 10.1089/thy.2013.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, Sun H, El-Naggar AK, Xing M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603–610. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G, Murugan AK, Guan H, Yu H, Wang Y, Sun H, Shan Z, Teng W, Xing M. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014;99:E1130–1136. doi: 10.1210/jc.2013-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lastra RR, LiVolsi VA, Baloch ZW. Aggressive variants of follicular cell-derived thyroid carcinomas: a cytopathologist's perspective. Cancer Cytopathol. 2014;122:484–503. doi: 10.1002/cncy.21417. [DOI] [PubMed] [Google Scholar]
- 15.Cibas ES, Ali SZ NCI Thyroid FNA State of the Science Conference. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132:658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 16.Cho U, Oh WJ, Bae JS, Lee S, Lee YS, Park GS, Lee YS, Jung CK. Clinicopathological features of rare BRAF mutations in Korean thyroid cancer patients. J Korean Med Sci. 2014;29:1054–1060. doi: 10.3346/jkms.2014.29.8.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park G, Kim TH, Lee HO, Lim JA, Won JK, Min HS, Lee KE, Park do J, Park YJ, Park WY. Standard immunohistochemistry efficiently screens for anaplastic lymphoma kinase rearrangements in differentiated thyroid cancer. Endocr Relat Cancer. 2015;22:55–63. doi: 10.1530/ERC-14-0467. [DOI] [PubMed] [Google Scholar]
- 18.Amacher AM, Goyal B, Lewis JS Jr, El-Mofty SK, Chernock RD. Prevalence of a hobnail pattern in papillary, poorly differentiated, and anaplastic thyroid carcinoma: a possible manifestation of high-grade transformation. Am J Surg Pathol. 2015;39:260–265. doi: 10.1097/PAS.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 19.Bellevicine C, Cozzolino I, Malapelle U, Zeppa P, Troncone G. Cytological and molecular features of papillary thyroid carcinoma with prominent hobnail features: a case report. Acta Cytol. 2012;56:560–564. doi: 10.1159/000338395. [DOI] [PubMed] [Google Scholar]
- 20.Yang GC, Fried K, Scognamiglio T. Cytological features of clear cell thyroid tumors, including a papillary thyroid carcinoma with prominent hobnail features. Diagn Cytopathol. 2013;41:757–761. doi: 10.1002/dc.22935. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Jung CK, Bae JS, Jung SL, Choi YJ, Kang CS. Liquid-based cytology improves preoperative diagnostic accuracy of the tall cell variant of papillary thyroid carcinoma. Diagn Cytopathol. 2014;42:11–17. doi: 10.1002/dc.23007. [DOI] [PubMed] [Google Scholar]
- 22.Parameswaran R, Brooks S, Sadler GP. Molecular pathogenesis of follicular cell derived thyroid cancers. Int J Surg. 2010;8:186–193. doi: 10.1016/j.ijsu.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Oh WJ, Lee YS, Cho U, Bae JS, Lee S, Kim MH, Lim DJ, Park GS, Lee YS, Jung CK. Classic papillary thyroid carcinoma with tall cell features and tall cell variant have similar clinicopathologic features. Korean J Pathol. 2014;48:201–208. doi: 10.4132/KoreanJPathol.2014.48.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly LM, Barila G, Liu P, Evdokimova VN, Trivedi S, Panebianco F, Gandhi M, Carty SE, Hodak SP, Luo J, Dacic S, Yu YP, Nikiforova MN, Ferris RL, Altschuler DL, Nikiforov YE. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A. 2014;111:4233–4238. doi: 10.1073/pnas.1321937111. [DOI] [PMC free article] [PubMed] [Google Scholar]


