Abstract
Pyruvate kinase M2 (PKM2) and vascular endothelial growth factor-C (VEGF-C) have been known to play an important role in tumorigenesis and tumor progression in breast cancer. However, the association between PKM2 and VEGF-C in breast cancer remains unclear. In the present study, a total of 218 specimens from breast cancer patients and 26 paired breast tumors with adjacent normal tissues as well as two breast cancer cell lines were enrolled to investigate the correlation between PKM2 and VEGF-C. We found that PKM2 and VEGF-C mRNA levels were both significantly increasing in breast tumors compared with adjacent normal tissues. Knockdown of PKM2 mRNA expression resulted in VEGF-C mRNA and protein down-regulated as well as cell proliferation inhibited. A positive correlation between PKM2 and VEGF-C expression was identified by immunohistochemical analyses of 218 specimens of patients with breast cancer (P=0.023). PKM2 high expression was significantly correlated with histological grade (P=0.030), lymph node stage (P=0.001), besides VEGF-C high expression was significantly associated with lymphovascular invasion (P=0.012). While combined high expression of PKM2 and VEGF-C was found to be associated with worse histological grade, more lymph node metastasis, more lymphovascular invasion, shorter progression free survival (PFS), and poorer overall survival (OS) in human breast cancer. The results of the present study suggested that PKM2 expression was correlated with VEGF-C expression, and combination of PKM2 and VEGF-C levels had the better prognostic significance in predicting the poor outcome of patients with breast cancer.
Keywords: Breast cancer, pyruvate kinase M2, vascular endothelial growth factor-C
Introduction
About 80 years ago, a German biochemist Otto Warburg who got the Nobel Prize found that cancer cells obtain most of their energy by glycolysis even in the presence of adequate oxygen [1]. This concept was called “aerobic glycolysis” or “Warburg effect”. PKM2 in the critical position of Warburg effect [2,3] catalyzed the final step of glycolysis, resulting in the conversion of phosphoenolpyruvate (PEP) to pyruvate while phosphorylating ADP to ATP [4]. PKM2 was expressed in various tumor tissues including breast cancer and demonstrated to be a key contributor to tumorigenesis and tumor progression [5,6].
VEGF-C is a member of VEGF family, and primarily responsible for inducing angiogenesis and lymphangiogenesis in tumors. It is widely known that VEGF-C bound to VEGF-C receptors in endothelial cells could stimulate the generation of microvascular and lymphatic vessels in breast cancer [7,8]. Increased angiogenesis and lymphangiogenesis can not only directly provide more nutrients to the tumor cells to facilitate tumorigenesis, but also contribute to tumor progression such as local spread or distant metastasis.
PKM2 and VEGF-C both have been known to take part in breast cancer tumorigenesis and tumor progression [9,10]. However, the association between PKM2 and VEGF-C in breast cancer remains unclear. In this study, we used twenty-six paired breast tumors with adjacent normal tissues to analyze PKM2 and VEGF-C mRNA expression levels in breast cancer patients. Then we knockdown PKM2 mRNA expression in MCF-7 and MDA-MB-231 cell lines by lentivirus-mediated RNA interference to see its influence in VEGF-C mRNA and protein expression. Next, we evaluated the correlation between PKM2 and VEGF-C expression status in 218 specimens from patients with invasive ductal carcinoma by immunohistochemistry. Finally, we analyzed the association of PKM2 and VEGF-C expression with patients’ clinicopathologic features and accessed the prognostic value of PKM2 and VEGF-C in human breast cancer.
Materials and methods
Patients and tissue samples
For PKM2 and VEGF-C immunohistochemistry
Hematoxylin and eosin stained tissue sections of 218 patients with invasive ductal carcinoma diagnosed between July 2008 and Dec 2009 were retrieved from the archive of Tianjin Medical University Cancer Hospital (Tianjin, China) and were reviewed by two pathologists. Histologic types were defined according to the WHO classification. Histologic grading was carried out using the modified Bloom and Richardson grading system [11]. The patients were followed up for 3-82 months with a median follow-up time of 48.1 months. During follow-up time, 21.8% (48/206) patients suffered from loco-regional recurrence or distant metastasis, and 18.6% (41/206) patients died of tumor.
For RNA extraction
A total of 26 paired breast tumors and adjacent normal tissues were collected from surgical specimens and stored at Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). All the cases have been diagnosed invasive ductal carcinoma by intraoperative frozen section examination before radical mastectomy. The fresh samples were frozen shortly after resection and stored at -80°C.
Patient’s consent for research was obtained prior to surgery and the study was approved by the Institutional Research and Ethical Committee.
Cell lines and cell culture
Human breast cancer cell lines MCF-7 and MDA-MB-231 were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS (Invitrogen, Carlsbad, CA) and incubated at 37°C in an atmosphere of 5% CO2.
RNA extraction and qRT-PCR
Approximately 1 g frozen tissue was directly placed in a liquid nitrogen precooled mortar. The RNA isolation reagent (Trizol, Invitrogen, Carlsbad, CA) was then added. Total RNA was isolated following the manufacturer’s protocol. qRT-PCR was carried out on an Applied Biosystems 7900 HT thermocycler using the cycling conditions recommended by the manufacturer. Double-stranded DNA-specific expression was tested by the comparative Ct method using 2-ΔΔCt. The primers were as follows: PKM2 forward, 5’-CAGAGGCTGCCATCTACCAC-3’, PKM2 reverse, 5’-CCAGACTTGGTGAGGACGAT-3’, VEGF-C forward, 5’-CGGGAGGTGTGTATAGATGTG-3’, VEGF-C reverse, 5’-ATTGGC TGGGGAAGAGTTTG-3’, GAPDH forward, 5’-GA AGGTGAAGGTCGGAGTC-3’, and GAPDH reverse, 5’-GAAGATGGTGATGGGATTTC-3’. All assays were performed in triplicate and repeated at least three times.
RNA interference
Knockdown of PKM2 was used by lentivirus-mediated RNA interference purchased from GeneChem Co., Ltd. (Shanghai, China). The sequences of PKM2-targeting RNAi nucleotides were as follows: 5’-GATCAACGCCTCACTGAAA-3’. The negative control was designed as random sequence. A total of 1×105 cells/well was seeded into six-well plates and transfected with lentivirus supernatant according to the manufacturer’s instructions.
Western blot
Total protein was isolated from cultured cell lines using RIPA lysis buffer (Beyotime Biotechnology, China) and the protein concentration was measured using the BCA protein assay kit (Beyotime Biotechnology). Equal amounts of protein (40 μg) were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto PVDF membranes. Membranes were blocked in 5% fat-free milk solution containing 0.1% Tween-20 for 1 h at room temperature. Membranes were then incubated with primary detection antibody (1:200 dilution PKM2, Cell Signaling Technology, Inc., USA; 1:100 dilution for VEGF-C, Zymed Laboratories South San Francisco, USA; 1:200 dilution for β-actin, Cell Signaling Technology, Inc., USA) at 4°C overnight followed by incubation with the secondary detection antibody (1:5000, Abgent) against rabbit or mouse IgG-HRP for 2 h at room temperature. Proteins were detected using ECL reagent (Pierce Biotechnology, USA). Quantification based on grayscale analysis was performed with Quantity One software (Bio-Rad, USA).
CCK8 assays
Cell Counting Kit-8 (CCK-8) assays were used to evaluate cell proliferation according to the manufacturer’s instructions. Briefly, cells were seeded in 96-well plates (2×103 cells/well) in triplicate. At the appropriate time (24, 48, 72, 96, 120 h), the cells were incubated with 10 μl CCK-8 solution for 2 h at 37°C. Absorbance was measured at a wave length of 450 nm on a Gen5 microplate reader (BioTek, USA).
Immunohistochemical staining
Immunohistochemistry was performed using the labeled streptavidin biotin method. Briefly, formalin-fixed, paraffin-embedded tissue sections (4 μm thick section) were dewaxed in xylene, rehydrated with distilled water, and antigen retrieval was performed using pressure cooking in EDTA buffer (pH 8.0) for 2 min. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 10 min, and normal goat serum was then applied for 10 min. After incubation with primary antibodies, including PKM2 (1:800, Cell Signaling Technology, USA), and VEGF-C (1:200, Zymed Laboratories South San Francisco, USA) at 4°C overnight, the secondary biotinylated goat anti-mouse/anti-rabbit immunoglobulin was applied for 20 min followed by incubation with peroxidase-conjugated streptavidin. Color was developed by incubation with 3, 3’-diaminobenzidine tetrahydrochloride (DAB). The sections were counterstained with hematoxylin. Primary antibody was replaced by goat serum as negative control. PKM2 and VEGF-C staining results were scored independently by two pathologists. Immunoreactivity for PKM2 was scored as follows: 0 (undetectable), 1+ (weakly positive), 2+ (moderately positive), 3+ (intensely positive). The specimens were divided into two groups: PKM2 low (0 and 1+) and PKM2 high (2+ and 3+). For VEGF-C, the percentage of tumor cells that showed cytoplasm staining was assessed. The specimens were divided into two groups: VEGF-C low (<10% positive tumor cells) and VEGF-C high (≥10%).
Statistical analysis
Statistical analysis was carried out using SPSS 16.0 software package. For continues variables, data are expressed as mean ± SD and compared using Student’s t-test. The association between PKM2/VEGF-C expression and clinicopathological features was analyzed using the Pearson’s chi-square test. The correlation between PKM2 and VEGF-C protein expression was analyzed using Spearman’s correlation coefficient test. The Kaplan-Meier method was used to estimate progression-free survival (PFS) and overall survival (OS), and survival differences between groups were compared with the log-rank test. Cox proportional hazards models were used to perform univariate and multivariable analyses. A two-tailed P value of <0.05 was considered to be statistically significant.
Results
PKM2 and VEGF-C mRNA expression were both significantly increasing in human breast cancer
Twenty-six paired breast tumors with adjacent normal tissues were analyzed for PKM2 and VEGF-C mRNA expression. The mean value of PKM2 mRNA expression was nearly 3-fold increasing in tumor tissues compared with the adjacent normal tissues (P<0.01). Besides, VEGF-C mRNA expression was also more than 2-fold increasing in tumor tissues (P<0.05). These data indicated that PKM2 and VEGF-C mRNA were both commonly elevated in human breast cancer (Figure 1A).
Figure 1.
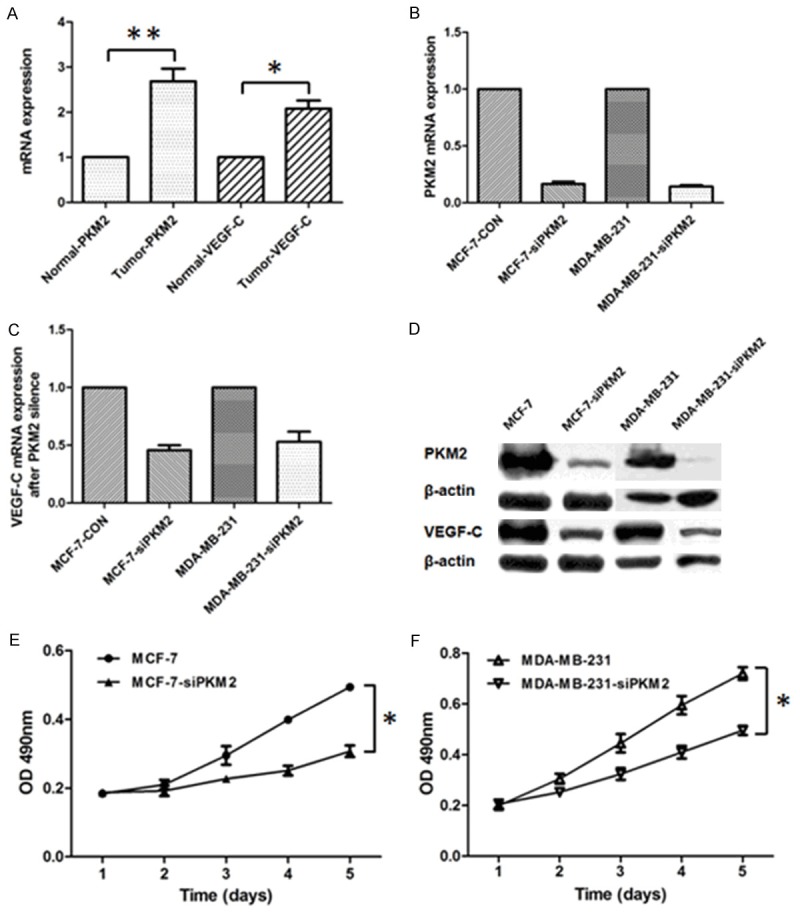
PKM2 expression was correlated with VEGF-C expression and cell proliferation. A. PKM2 and VEGF-C mRNA expression elevated in breast tumors compared with paired adjacent normal tissues. B. qRT-PCR analyzed the efficacy of PKM2 knockdown by lentivirus-mediated RNAi in MCF-7 and MDA-MB-231 cell lines. C. RT-PCR analysis of VEGF-C mRNA expression in MCF-7 and MDA-MB-231 cell lines following PKM2 knockdown. D. Western blot analysis of PKM2 and VEGF-C expression in MCF-7 and MDA-MB-231 cell lines after PKM2 knockdown. E, F. Effects of PKM2 knockdown on cell proliferation was evaluated by CCK8 assays in MCF-7 and MDA-MB-231 cell lines, respectively. *Indicates P<0.05, **indicates P<0.01.
Knockdown of PKM2 mRNA results in down-regulation of VEGF-C mRNA and protein expression as well as inhibition of cell proliferation in breast cancer cell lines
To investigate the potential association between PKM2 and VEGF-C in breast cancer, we used lentivirus-mediated RNA interference to knockdown PKM2 mRNA expression in MCF-7 and MDA-MB-231 breast cancer cell lines. The efficacy of PKM2 knockdown was confirmed by real-time PCR and Western blot (Figure 1B, 1D). Then we found that VEGF-C mRNA and protein expression were also down-regulated following PKM2 knockdown (Figure 1C, 1D). Next, by performing CCK-8 assays, we found knockdown of PKM2 and down-regulated of VEGF-C in MCF-7 and MDA-MB-231 cell lines resulted in the inhibition of cell proliferation compared with the control group, respectively (P<0.05) (Figure 1E, 1F).
The correlation of PKM2 and VEGF-C expression in human breast cancer
PKM2 and VEGF-C expression was determined by immunohistochemical analysis of 218 specimens of patients with invasive ductal carcinoma. PKM2 and VEGF-C expression were both restricted to the cytoplasm of tumor cells (Figure 2). A positive correlation between PKM2 and VEGF-C expression was identified by using the Spearman’s correlation coefficient test (rs=0.154, P=0.023) (Table 1). There were 39.4% (86/218) patients’ primary tumors with high PKM2 expression showed high VEGF-C expression at the same time.
Figure 2.
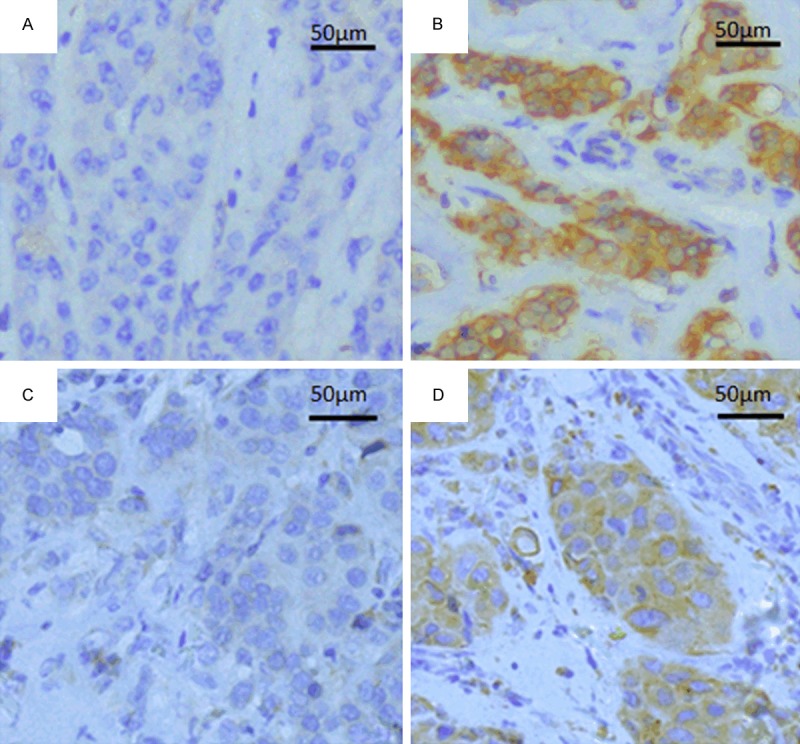
Immunohistochemical staining for PKM2 and VEGF-C expression in breast invasive ductal carcinoma. These two proteins were both restricted to the cytoplasm of tumor cells. A. PKM2 low expression (200×). B. PKM2 high expression (200×). C. VEGF-C low expression (200×). D. VEGF-C high expression (200×).
Table 1.
Correlation between PKM2 and VEGF-C expression in 218 breast cancer patients
| Tissue samples | PKM2 | P | rs | ||
|---|---|---|---|---|---|
|
| |||||
| Low | High | ||||
| VEGF-C | Low | 43 | 39 | 0.023* | 0.154 |
| High | 50 | 86 | |||
Indicates P<0.05.
Correlation between PKM2/VEGF-C expression and breast cancer clinicopathologic features
Associations of PKM2 and VEGF-C expression with clinicopathologic features are summarized in Table 2. High expression of PKM2 was significantly correlated with histological grade (P=0.030) and lymph node stage (P=0.001). Besides, high expression of VEGF-C was significantly associated with lymphovascular invasion (P=0.012). Then we separated all of the 218 patients into three groups according to PKM2 and VEGF-C expression status: Group 1, PKM2 and VEGF-C were both low expression (L/L); Group 2, only one protein high expression (PKM2 low/VEGF-C high, L/H, or PKM2 high/VEGF-C low, H/L); Group 3, PKM2 and VEGF-C were both high expression (H/H). There were significant differences in histological grade (P=0.018), lymph node stage (P=0.002) and lymphovascular invasion (P=0.001) among these three groups (Table 2). Compared with Group 1 and Group 2, Group 3 had the worst histological grade, the most lymph node metastasis and the most lymphovascular invasion (Figure 3).
Table 2.
Correlation between PKM2/VEGF-C status and clinicopathological features (n=218)
| Parameters | PKM2 | P | VEGF-C | P | PKM2 &. VEGF-C | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Low (%) | High (%) | Low (%) | High (%) | L/L (%) | L/H &. H/L (%) | H/H (%) | ||||
| Age (year) | ||||||||||
| <45 | 24 (26) | 31 (25) | 0.866 | 22 (27) | 33 (24) | 0.673 | 15 (35) | 16 (18) | 24 (28) | 0.085 |
| ≥45 | 69 (74) | 94 (75) | 60 (73) | 103 (76) | 28 (65) | 73 (82) | 62 (72) | |||
| Tumor stage | ||||||||||
| T1 (<2 cm) | 32 (35) | 38 (30) | 0.460 | 26 (32) | 44 (33) | 0.940 | 15 (36) | 28 (32) | 27 (31) | 0.876 |
| T2+T3 (≥2 cm) | 59 (65) | 87 (70) | 55 (68) | 91 (67) | 27 (64) | 60 (68) | 59 (69) | |||
| Histological grade | ||||||||||
| I | 14 (15) | 8 (6) | 0.030* | 10 (12) | 12 (9) | 0.198 | 8 (19) | 8 (9) | 6 (7) | 0.018* |
| II | 60 (65) | 76 (61) | 55 (67) | 81 (59) | 26 (60) | 63 (71) | 47 (55) | |||
| III | 19 (20) | 41 (33) | 17 (21) | 43 (32) | 9 (21) | 18 (20) | 33 (38) | |||
| Lymph node stage | ||||||||||
| negative | 53 (57) | 44 (35) | 0.001* | 39 (48) | 61 (45) | 0.697 | 21 (49) | 50 (56) | 26 (30) | 0.002* |
| positive | 40 (43) | 81 (65) | 43 (52) | 75 (55) | 22 (51) | 39 (44) | 60 (70) | |||
| Lymphovascular invasion | ||||||||||
| no | 80 (86) | 91 (73) | 0.054 | 73 (89) | 98 (73) | 0.012* | 39 (91) | 75 (84) | 57 (66) | 0.001* |
| yes | 13 (14) | 33 (27) | 9 (11) | 37 (27) | 4 (9) | 14 (16) | 29 (34) | |||
Indicates P<0.05.
L/L: Low/Low; L/H: Low/High; H/L: High/Low; H/H: High/High.
Figure 3.
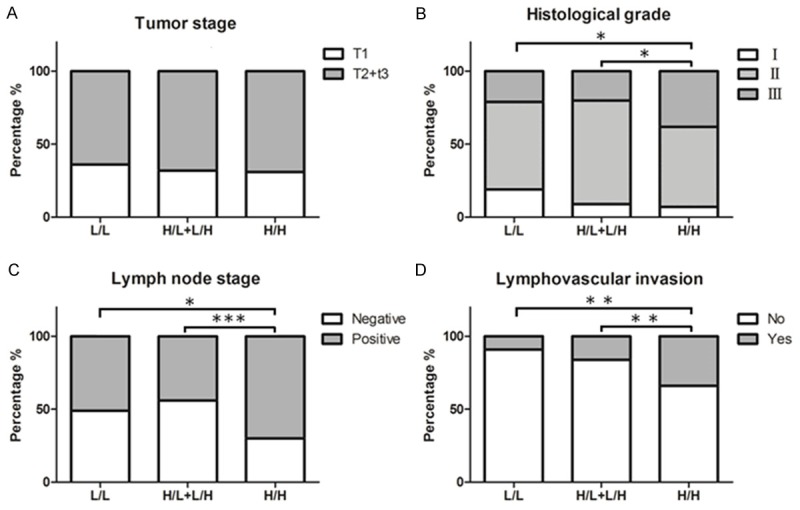
The association of combined of PKM2 and VEGF-C expression with patients’ clinicopathologic features. No significant differences in tumor stage among the three groups (A). Both high expression of PKM2 and VEGF-C had the worst histological grade (B), the most lymph node metastasis (C) and the most lymphovascular invasion (D) in the three groups. *Indicates P<0.05, **indicates P<0.01, ***indicates P<0.001.
Survival analysis and prognostic value of combined expression of PKM2 and VEGF-C in human breast cancer
Kaplan-Meier analysis with a log rank test for PFS and OS were performed to evaluate the association between tumors PKM2/VEGF-C status and patients survival. According to the grouping method before, significant differences in PFS (χ2=15.623, P<0.000) and OS (χ2=12.275, P=0.002) were observed in the three groups. Apparently, Group 1 had the best PFS and OS compared with Group 2 and Group 3, while the poorest PFS and OS were observed in Group 3 (Figure 4A, 4B).
Figure 4.
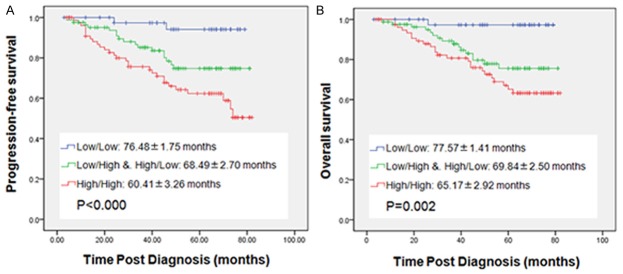
Kaplan-Meier plots with log rank test of progression-free survival (PFS) and overall survival (OS). The mean time (months) of survival was showed. PFS (A) and OS (B) based on combined PKM2 and VEGF-C status. All of the 218 patients were separated into three groups according to the grouping method before: the blue line represents PKM2 and VEGF-C both low expression; the green line represents only one protein high expression; the red line represents PKM2 and VEGF-C both high expression.
The univariate Cox proportional hazards models analysis indicated that PKM2 status, tumor stage, histological grade, lymph node status and lymphovascular invasion were significantly associated with poor PFS and OS (P<0.05), and VEGF-C status was significantly correlated with poor PFS only (P=0.028). By multivariate Cox proportional hazards models analysis, PKM2 status and lymph node stage were indicated as independent predictors for poor PFS (P=0.010, P=0.034) and OS (P=0.017, P=0.023) respectively, and lymphovascular invasion was an independent predictor for poor PFS (P=0.025) only (Table 3).
Table 3.
Univariate and multivariate analyses of PFS and OS
| Variables | PFS | OS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Univariate analysis | ||||||
| Age (<45 vs. ≥45) (years) | 0.677 | 0.371-1.234 | 0.202 | 0.664 | 0.348-1.266 | 0.213 |
| Tumor stage (TI vs. T2+T3) | 2.075 | 1.034-4.165 | 0.040* | 2.180 | 1.007-4.720 | 0.048* |
| Histological grade (I+II vs. III) | 2.647 | 1.500-4.674 | 0.001* | 2.208 | 1.197-4.074 | 0.011* |
| Lymph node stage (neg vs. pos) | 5.112 | 2.391-10.932 | <0.000* | 4.853 | 2.150-10.953 | <0.000* |
| Lymphovascular invasion (no vs. yes) | 4.002 | 2.269-7.057 | <0.000* | 3.590 | 1.942-6.635 | <0.000* |
| PKM2 status (low vs. high) | 3.726 | 1.805-7.693 | <0.000* | 3.997 | 1.771-9.017 | 0.001* |
| VEGF-C status (low vs. high) | 2.086 | 1.085-4.011 | 0.028* | 1.827 | 0.916-3.647 | 0.087 |
| Multivariate analysis | ||||||
| Tumor stage (TI vs. T2+T3) | 1.215 | 0.590-2.503 | 0.597 | 1.274 | 0.568-2.856 | 0.557 |
| Histological grade (I+II vs. III) | 1.570 | 0.853-2.889 | 0.147 | 1.225 | 0.639-2.348 | 0.540 |
| Lymph node stage (neg vs. pos) | 2.981 | 1.294-6.865 | 0.010* | 2.965 | 1.211-7.258 | 0.017* |
| Lymphovascular invasion (no vs. yes) | 2.090 | 1.099-3.975 | 0.025* | 1.883 | 0.971-3.650 | 0.061 |
| PKM2 status (low vs. high) | 2.321 | 1.066-5.053 | 0.034* | 2.696 | 1.144-6.354 | 0.023* |
| VEGF-C status (low vs. high) | 1.013 | 0.485-2.118 | 0.972 | -- | -- | -- |
Indicates P<0.05.
neg, negative; pos, positive; HR, hazard ratio; CI, confidence interval.
Discussion
Cancer is considered to be a chronic metabolic disease recently. PKM2 as the key enzyme of Warburg effect plays a critical role in tumorigenesis and progression. Pyruvate kinases (PKs) include 4 isoforms encoded by 2 paralogous gene, the PKL and PKM gene. The PKL gene generates PKL and PKR isoforms that are driven by different tissue-specific promoters [12]. The PKM gene is alternatively spliced by mutual exclusion of the 10th and 9th exons to generate PKM1 and PKM2, respectively [13]. PKM2 was initially characterized in proliferating cells during embryonic development, and was progressively replaced by the other three isozymes after birth [14]. However PKM2 was also as the predominant subtype over-expressing in multiple cancers, through adjusting cancer cells growth and proliferation contributed to Warburg effect. To some extent, PKM2 status in vivo represents the level of aerobic glycolysis in tumor microenvironment [2,3]. Previous studies have showed that PKM2 was expressed in many kinds of human cancers and predicted poor prognosis. Hu W. et al. reported PKM2 serves as a promising biomarker for poor prognosis of patients with hepatocellular carcinoma [15]. Zhang X. et al claimed that nuclear PKM2 expression predicts poor prognosis in patients with esophageal squamous cell carcinoma [16]. Li J. et al demonstrated PKM2 are a prognostic marker for poor prognosis of gallbladder cancer [17]. However, studies on PKM2 expression in clinical cases of breast cancer are rare.
Tumor growth and metastasis is an angiogenesis dependent process, and breast cancer is a typical vascular dependent tumor [18]. Angiogenesis plays a critical role in tumor growth, invasion and metastasis. VEGF-C was identified as the most important molecule in angiogenesis and lymphangiogenesis in breast cancer, and also played an important role in tumorigenesis and tumor progression. In this study, increased PKM2 mRNA expression was accompanied by VEGF-C mRNA elevated in tumor tissues compared with adjacent normal tissues. Furthermore, knockdown of PKM2 mRNA expression in MCF-7 and MDA-MB-231 cell lines resulted in both VEGF-C mRNA and protein down-regulated as well as cell proliferation inhibited. Then we found a positive correlation between PKM2 and VEGF-C expression identified by immunohistochemical analyses of 218 specimens of patients with breast cancer. Next, we found that the combined expression of high PKM2 and high VEGF-C was more significantly related to histological grade, lymph node stage and lymphovascular invasion than high PKM2 or high VEGF-C alone. At the same time, we found that combined expression of high PKM2 and high VEGF-C was significantly correlated with shorter PFS and poorer OS in human breast cancer. These results indicate that breast cancer patients who are both high expression of these two proteins in primary tumors may have the worse outcome in future. It is may be due to the fact that aerobic glycolysis which led by PKM2 increased cancer cells demand for nutrients during tumorigenesis, and induced cancer cells to secret cytokines such as VEGF-C. Increased VEGF-C bound to VEGF-C receptor could stimulate the proliferation of endothelial cells [19,20]. The endothelial cells could induce neovascularization and lymphangiogenesis. These new blood vessels provide adequate nutrients to cancer cells causing rapid proliferation. Meanwhile, Cancer cells are more readily to enter the bloodstream through these new blood vessels and lymphatic to achieve local spread or hematogenous metastasis. Previously studies showed VEGF-C levels in plasma was positively correlated with breast cancer metastasis. Wang et al. reported VEGF-C contributes to metastasis via its ability to enhance tumor-initiating cell-associated characteristics [10]. PKM2 could also promote tumor angiogenesis by increasing endothelial cell proliferation and migration [21]. Previous study showed PKM2 involved feedback loop PKM2/NF-κB/miR-148a/152 can modulate angiogenesis and tumor progression in breast cancer [22]. Moreover, Hypoxia-induced factor-1 alpha (HIF1α) could upregulate VEGF-C to promote lymphangiogenesis and angiogenesis in breast cancer patients [23]. HIF1α could also modulate cell fate reprogramming through early glycolytic shift and up-regulation of PDK1-3 and PKM2 [24]. The relationship between PKM2 and VEGF-C may be connected by the regulation of HIF1α in the molecular mechanism. Further studies are required to investigate the molecular mechanisms underlying this intriguing correlation.
In conclusion, our study is the first time to investigate the correlation between PKM2 and VEGF-C in human breast cancer. Combined high expression of PKM2 and VEGF-C was more significantly associated with histological grade, lymph node stage and lymphovascular invasion than high PKM2 expression or high VEGF-C expression alone. Combination of PKM2 and VEGF-C levels had the better prognostic significance in predicting the poor outcome of patients with breast cancer.
Acknowledgements
Research was supported by grants from the National Natural Science Foundation of China (30930038, 81202101, 81302292) and Initial Funding for Doctors of Tianjin Medical University Cancer Institute and Hospital (B1215).
Disclosure of conflict of interest
None.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–6. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 3.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 4.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43:969–980. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Shi Y, Liu S, Cao Y, Wang X, Tao Y. PKM2: the thread linking energy metabolism reprogramming with epigenetics in cancer. Int J Mol Sci. 2014;15:11435–45. doi: 10.3390/ijms150711435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamada M, Suematsu M, Saya H. Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res. 2012;18:5554–61. doi: 10.1158/1078-0432.CCR-12-0859. [DOI] [PubMed] [Google Scholar]
- 7.Mohammed RA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br J Cancer. 2007;96:1092–1100. doi: 10.1038/sj.bjc.6603678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura Y, Yasuoka H, Tsujimoto M, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat. 2005;91:125–132. doi: 10.1007/s10549-004-5783-x. [DOI] [PubMed] [Google Scholar]
- 9.Jiang K, He B, Lai L, Chen Q, Liu Y, Guo Q, Wang Q. Cyclosporine A inhibits breast cancer cell growth by downregulating the expression of pyruvate kinase subtype M2. Int J Mol Med. 2012;30:302–8. doi: 10.3892/ijmm.2012.989. [DOI] [PubMed] [Google Scholar]
- 10.Wang CA, Harrell JC, Iwanaga R, Jedlicka P, Ford HL. Vascular endothelial growth factor C promotes breast cancer progression via a novel antioxidant mechanism that involves regulation of superoxide dismutase 3. Breast Cancer Res. 2014;16:462. doi: 10.1186/s13058-014-0462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002;41:154–61. [PubMed] [Google Scholar]
- 12.Noguchi T, Yamada K, Inoue H, Matsuda T, Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem. 1987;262:14366–14371. [PubMed] [Google Scholar]
- 13.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 15.Hu W, Lu SX, Li M, Zhang C, Liu LL, Fu J, Jin JT, Luo RZ, Zhang CZ, Yun JP. Pyruvate kinase M2 prevents apoptosis via modulating Bim stabi- lity and associates with poor outcome in hepatocellular carcinoma. Oncotarget. 2015;6:6570–83. doi: 10.18632/oncotarget.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, He C, He C, Chen B, Liu Y, Kong M, Wang C, Lin L, Dong Y, Sheng H. Nuclear PKM2 expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Pathol Res Pract. 2013;209:510–5. doi: 10.1016/j.prp.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Yang Z, Zou Q, Yuan Y, Li J, Liang L, Zeng G, Chen S. PKM2 and ACVR 1C are prognostic markers for poor prognosis of gallbladder cancer. Clin Transl Oncol. 2014;16:200–7. doi: 10.1007/s12094-013-1063-8. [DOI] [PubMed] [Google Scholar]
- 18.Weidner N, Semple JP, Welch WR, Folkman J. Tumor Angiogenesis and Metastasis-Correlation in Invasive Breast Carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 19.Chien MH, Lee LM, Hsiao M, Wei LH, Chen CH, Lai TC, Hua KT, Chen MW, Sun CM, Kuo ML. Inhibition of Metastatic Potential in Breast Carcinoma In Vivo and In Vitro through Targeting VEGFRs and FGFRs. Evid Based Complement Alternat Med. 2013;2013:718380. doi: 10.1155/2013/718380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omoto I, Matsumoto M, Okumura H, Uchikado Y, Setoyama T, Kita Y, Owaki T, Kijima Y, Shinchi H, Ishigami S, Ueno S, Natsugoe S. Expression of vascular endothelial growth factor-C and vascular endothelial growth factor receptor-3 in esophageal squamous cell carcinoma. Oncol Lett. 2014;7:1027–1032. doi: 10.3892/ol.2014.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Zhang Y, Qiao J, Yang JJ, Liu ZR. Pyruvate kinase M2 in blood circulation facilitates tumor growth by promoting angiogenesis. J Biol Chem. 2014;289:25812–21. doi: 10.1074/jbc.M114.576934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Q, Liu LZ, Yin Y, He J, Li Q, Qian X, You Y, Lu Z, Peiper SC, Shu Y, Jiang BH. Regulatory circuit of PKM2/NF-κB/miR-148a/152-modulated tumor angiogenesis and cancer progression. Oncogene. 2015 doi: 10.1038/onc.2015.6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Ni X, Zhao Y, Ma J, Xia T, Liu X, Ding Q, Zha X, Wang S. Hypoxia-induced factor-1 alpha upregulates vascular endothelial growth factor C to promote lymphangiogenesis and angiogenesis in breast cancer patients. J Biomed Res. 2013;27:478–85. doi: 10.7555/JBR.27.20130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prigione A, Rohwer N, Hoffmann S, Mlody B, Drews K, Bukowiecki R, Blümlein K, Wanker EE, Ralser M, Cramer T, Adjaye J. HIF1α modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells. 2014;32:364–76. doi: 10.1002/stem.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]


