Abstract
Regeneration and repair of peripheral nerve injury has always been a major problem in the clinic. The conventional technique based on suturing the nerve ends to each other coupled with the implantation of nerve conduits outside is associated with postoperative adhesions and scar problems. Recently, a novel biodegradable poly (DL-lactic acid) (PDLLA) film has been introduced. This novel anti-adhesion film has a porous structure with better mechanical properties, better flexibility, and more controllable degradation as compared to traditional non-porous nerve conduits. However, little is known about the effects of such PDLLA films on regeneration and repair of peripheral nerve injury in vivo. In this study, we evaluated the effects of PDLLA films implantation after sciatic nerve transection and anastomosis on subsequent sciatic nerve regeneration in vivo, using a rat sciatic nerve injury model. Sciatic nerve transection surgery coupled with direct suturing only, suturing and wrapping with traditional nerve conduits, or suturing and wrapping with PDLLA films was performed on adult Wistar rats. The additional wrapping with PDLLA films inhibited the nerve adhesion after 12 weeks recovery from surgery. It also increased the compound muscle action potentials and tibialis and gastrocnemius muscle wet weight ratio following 8 weeks recovery from surgery. Regenerated nerve fibers were relatively straight and the aligned structure was complete in rats with implantations of PDLLA films. The results suggested that PDLLA films can improve the nutritional status in the muscles innervated by the damaged nerves and promote nerve regeneration in vivo.
Keywords: Biodegradable materials, poly (DL-lactic acid) film, peripheral nerve, regeneration
Introduction
Regeneration and repair of peripheral nerve injury has always been a major problem in the clinic. Whether peripheral nerve can achieve successful regeneration after peripheral nerve injury depends on the availability of suitable microenvironment for their re-growth [1]. More recently, due to rapid advances in microsurgical techniques, nerve anastomosis quality has been greatly improved [1-3]. However, the postoperative adhesions and scar problems have not been well solved. Therefore, it is highly important to prevent the nerve adhesion and provide good anastomotic microenvironment to promote nerve regeneration and repair.
One of the exciting and promising therapeutic strategies is to wrap the anastomosis with nerve conduits [1,2,4]. The ideal conduit not only gives physical support to injured nerves, but also provides anastomosis with a relatively concealed microenvironment. The conduits guide the axial growth of neuronal axons. The conduit structure with the appropriate porosity and the size of pores allows free exchange of tissue fluids and nutrients, as well as accumulation of neurotrophic factors that are required for nerve regeneration [3,5]. Over the years, the nerve conduits have evolved from silicone based materials, non-absorbable artificial materials, or biological based material (intravenous, amniotic membrane, etc.) to the modern biodegradable polymers [2,6].
Biodegradable polymers are made of either natural materials such as chitosan, chitin and cellulose, or synthetic materials such as polylactic acid (PLA). While the application of chitosan, chitin and cellulose is hindered by various issues such as their biological origin, the potential for hypersensitivity or immune rejections, non-synchronized degradation and nerve regeneration rates, high brittleness, poor permeability, or poor biocompatibility, the use of nerve conduits made of biodegradable materials provides several advantages for the peripheral nerve repair. First, nerve conduits wrap local nerve anastomosis, and effectively separate the injured nerves from the surrounding tissues, so that the local endogenous concentrations of nerve cell factors can be maintained. Thus, the chemotaxis of nerve regeneration is likely to be fully realized. Second, nerve conduits separate the microenvironment of axon regeneration from the surrounding environment, thus avoiding the incorrect growth and invasive growth of non-neural tissues. Third, the biodegradable polymeric materials are often non-toxic, non-irritating, and considered to be the ideal biomaterials for nerve repairing [6,7].
The synthetic PLA has been approved by US Food and Drug Administration (FDA). It has good biocompatibility, biodegradability and bioabsorbability [8,9]. Lactic acid, the degradation production of PLA, is an intermediate metabolite in citric acid cycles in vivo. The mechanism of lactic acid absorption and metabolism has been clearly demonstrated. Due to its reliability and biosafety, PLA polyesters are the most extensively studied and the most widely used biodegradable materials to date. Their application in the field of biomedical engineering became particularly popular in the recent years. For instance, resorbable PLA barrier film can act as an adhesion barrier to prevent posterior spinal scar formation [7]. It has been found that PLA film used as an absorbable adhesion barrier effectively reduced post-surgery adhesion and minimized safety issues [7,10].
Recently, a novel biodegradable poly (DL-lactic acid) (PDLLA) film has been prepared by using a phase transformation method with biodegradable polylactic acid polymer as starting material [11]. This novel anti-adhesion film has a porous structure, which provides better mechanical properties, better flexibility, and more controllable degradation as compared to traditional non-porous films. However, little is known about the effects of such PDLLA films on regeneration and repair of peripheral nerve injury in vivo. Therefore, in the present study, we evaluated the effects of PDLLA films implantation after sciatic nerve transection and anastomosis on the subsequent sciatic nerve regeneration in vivo.
Materials and methods
Animals
Wistar rats (n = 30), irrespective of gender, weighing 200 ± 20 g, were provided by the Animal Center, School of Basic Medicine, Jilin University, China (license No. SCXK (Ji) 2003-001). Animals were housed in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle. All animals had free access to food daily with water ad libitum. All animal experiments were approved by the local Institutional Animal Care and Use Committee. The housing and treatment of the animals followed the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China.
Sciatic nerve transection and anastomosis
Adult Wistar rats (n = 24) were randomly divided into three groups (n = 8/group). Rats were anesthetized by 10% chloral hydrate (0.3 ml/kg; i.p.). After shaving the hair from the mid back to hind limb area, rats were then placed in the prone position on the surgery table with a heating pad underneath for maintaining the body temperature at approximately 37°C. To allow the exposure of the sciatic nerve at the dorsocaudal region, an approximately 3 cm-long incision was made starting at 0.5 cm laterally from the rat midline toward the tibiofemoral articulation, followed by separations of the femoral biceps and gluteus muscles. A microsurgical scissor was used to make unilateral left side sciatic nerve transection. The nerve was then repaired by epineural microsutures using 9-0 nylon sutures (Johnson & Johnson medical equipment, Shanghai, China) under the magnification of a binocular loupe. Group 1 received sciatic nerve transection and anastomosis with no additional treatment. Group 2 received sciatic nerve transection and anastomosis with 6 mm artificial nerve conduits (Tian Xin Fu Medical Appliance, Beijing, China). Group 3 received nerve transection and anastomosis with 6 mm biodegradable PDLLA films wrapping (production batch number: 130601F; Changchun SinoBiomaterials, Jilin, China). Six non-transected rats were kept as a control group.
Gross observation
Following the surgery, the rats were observed daily. We also recorded the appearance of the left lower limb, any signs of tissue swelling and infection, as well as the healing of the wound. In some rats, the ulcers on the side toe of foot that received the sciatic surgery appeared during the early recovery. We recorded the time when the ulcers appeared and healed. Finally, after 8 or 12 weeks of recovery, the conditions of the implanted PDLLA films and the sciatic nerve adhesions were examined.
Neuroelectrophysiological examination
Eight weeks after the surgery, the transected sciatic nerves were separated from the surrounding connective tissues and exposed in each group of rats (n = 6/group). The normal sciatic nerves on the left side of non-transected control rats (sham group; n = 6) were also exposed. The stimulation electrodes (fine non-insulated platinum needles) were placed along the proximal sciatic nerve. The final position of electrodes was chosen in a manner that allowed obtaining an electrical response in the tested muscle on the weakest stimulus, usually less than 0.5 mA. Stimulating rectangular pulses of 0.05 ms in duration were delivered through a Viking IV electromyelography (EMG) machine (Keypoint, Frederiksberg, Denmark). The intensity of the stimulus used throughout the investigations was 5 mA. A platinum needle ground electrode was placed subcutaneously in the distal part of the paw. The compound muscle action potentials (CMAPs) recorded from the flexor (gastrocnemius and soleus) and extensor muscles (tibialis anterior and peroneus) were measured using two subcutaneous platinum needles, one positioned over the bulk of the muscle, and the other placed distally at the level of the ankle. The latency period and the peak amplitude were recorded, and the motor nerve conduction velocity (MNCV) was calculated on the normal and regenerated nerves.
Tibialis and gastrocnemius muscle wet weight ratio
Recovery assessment was also indexed using the weight ratio of the tibialis and gastrocnemius muscles 8 weeks after surgery. Immediately after animals were sacrificed, the tibialis and gastrocnemius muscles were dissected and carefully harvested from both intact and injured rats, and weighed while still wet using an electronic balance. All measurements were made by two blinded observers. Values were expressed as a ratio of the wet weight of the tibialis and gastrocnemius muscles to the body weight of the rat.
Histological evaluation
Following 12 weeks of recovery from surgery, the rats were euthanized and a 1 cm-long segment of the sciatic nerve containing the anastomosis site was removed. The conditions of nerve regeneration at the anastomosis site and scar tissue formations were examined.
Data analysis
The experimental data were expressed as means ± SD. The statistical significance of differences between groups was determined by a one-way analysis of variance (ANOVA) followed by Duncan’s test for multiple comparisons. A value of P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA).
Results
Gross observation
No postoperative mortality occurred. None of the rats had infections on the surgical incisions, and the incision sutures were off on their own. However, 14 rats in all three groups displayed toe or foot ulcers at about 10 days after surgery (Figure 1A and 1B). The ulcer healing time was 10.2 weeks on average in animals that received suture only. However, the ulcer healing time was 8.7 or 8.5 weeks in rats that received implantation of nerve conduits or PDLLA films, respectively (Figure 1C and 1D). In general, ulcer healing time was much shorted in animals that received either nerve conduits or PDLLA films implantation following the nerve anastomosis, as compared to those with the sutures only.
Figure 1.
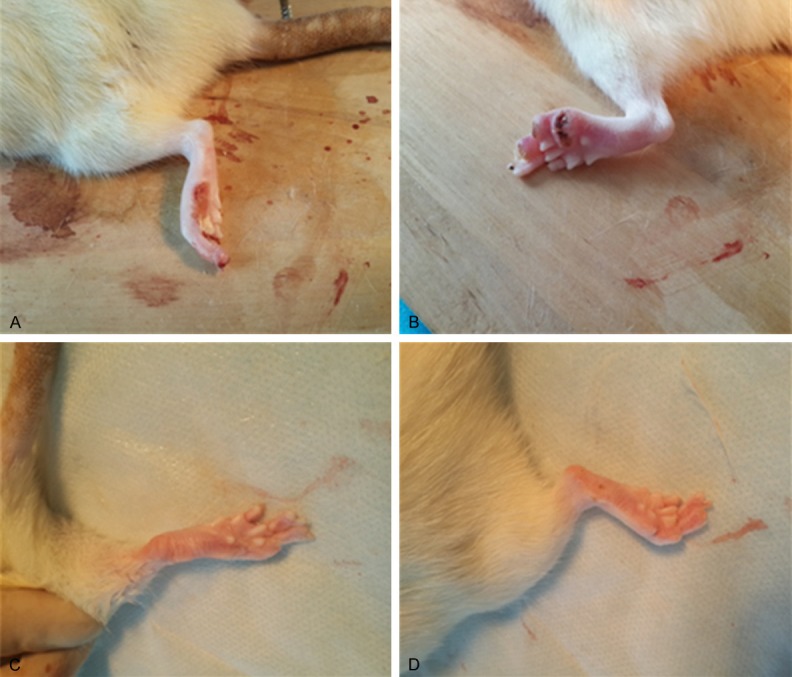
Representative photographs showing the appearance of local inflammatory response and skin ulcers due to the denervation in rats 10 days (A, B) or 10 weeks (C, D) after sciatic transection surgery.
After 8 weeks of recovery, the implanted nerve conduits or PDLLA films did not shed, and both types of implantations covered the nerve anastomosis nicely. In contrast, group 1 of rats with suture only showed significant postoperative perineural adhesions, proximal nerve enlargement, and the formation of traumatic neuromas around the site of nerve anastomosis (Figure 2A). However, the animals from groups 2 and 3 with the implantations of either nerve conduits or PDLLA films had no significant adhesion appearance between the peripheral nerve tissues and the biomaterials. Specifically, PDLLA film was clearly softened and closely attached to the nerves (Figure 2B and 2C).
Figure 2.
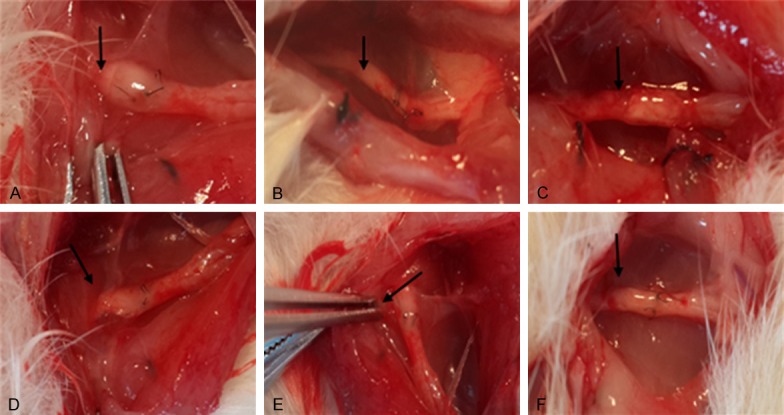
Gross observation of sciatic nerve regeneration after 8 (A) or 12 (B) weeks following sciatic nerve transection surgery in rats with direct suturing (A and D), nerve conduits (B and E), or PDLLA films (C and F). Arrows represent the anastomosis sites.
Similarly, after 12 weeks of recovery, the implanted nerve conduits or PDLLA films did not shed, and both types of implantations covered the nerve anastomosis nicely. In contrast, group 1 of rats with suture only showed significant postoperative perineural adhesions, proximal nerve enlargement, and the formation of traumatic neuromas around the site of nerve anastomosis (Figure 2D). However, group 2 or group 3 of rats with the implantations of either nerve conduits or PDLLA films had no significant adhesion appearance between peripheral nerve tissues and the biomaterials (Figure 2E and 2F). While the biomaterials were almost degraded, a thin layer of loose connective tissues formed surrounding the sciatic nerve anastomosis. There was no compression on the nerve itself.
Neuroelectrophysiological examination
Eight weeks after surgery, we measured the compound muscle action potentials (CMAPs), recorded the latency period, and calculated the motor nerve conduction velocity (MNCV).
The sciatic transection surgery reduced the amplitude of action potentials in all rats, as compared with sham rats (F(3, 20) = 9.13; P < 0.0001; Figure 3A). In the sham group, the mean amplitude of action potentials was 25.4 ± 8.9 mV. However, in the group of rats receiving suture only after sciatic nerve transection (i.e., Group 1), the mean amplitude of action potentials was 5.2 ± 1.45 mV. Furthermore, implantations of nerve conduits (i.e., Group 2; 6.4 ± 2.8 mV) failed to enhance the amplitude of action potentials, as compared with Group 1 (Figure 3A). However, implantations of PDLLA films (i.e., Group 3; 9.5 ± 4.1 mV) increased the amplitude of action potentials, as compared with Group 1 (Figure 3A).
Figure 3.
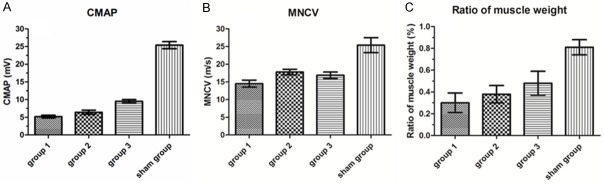
Effects of PDLLA film implantation on the compound muscle action potentials (CMAPs; A), motor nerve conduction velocity (MNCV; B), and wet weight ratio of the tibialis and gastrocnemius muscles (C) eight weeks after surgery in the group of rats receiving suture only (Group 1), implantations of nerve conduits (Group 2), implantations of PDLLA films (Group 3), or no sciatic nerve transection (Sham group).
Furthermore, the sciatic transection surgery reduced the MNCV in all rats, as compared with sham rats (F(3, 20) = 6.41; P < 0.001; Figure 3B). In the sham group, the mean nerve conduction velocity was 24.8 ± 5.12 m/s. However, in the group of rats receiving suture only after sciatic nerve transection (i.e., Group 1) the mean nerve conduction velocity was 14.5 ± 2.89 m/s. Furthermore, implantations of nerve conduits (i.e., Group 2; 17.84 ± 4.05 m/s) failed to enhance the nerve conduction velocity, as compared with Group 1 (Figure 3B). Similarly, implantations of PDLLA films (i.e., Group 3; 16.9 ± 4.28 m/s) failed to increase the nerve conduction velocity, as compared with Group 1 (Figure 3B).
Tibialis and gastrocnemius muscle wet weight ratio
Eight weeks after surgery, we also measured the wet weight ratio of the tibialis and gastrocnemius muscles (Figure 3C). The sciatic transection surgery reduced the wet weight ratio in all rats, as compared with sham rats (F(3, 20) = 7.28; P < 0.01; Figure 3C). In the sham group, the mean wet weight ratio was 0.81 ± 0.05%. However, in the group of rats receiving suture only after sciatic nerve transection (i.e., Group 1), the mean wet weight ratio was 0.32 ± 0.04%. Furthermore, implantations of nerve conduits (i.e., Group 2; 0.38 ± 0.03%) failed to enhance the wet weight ratio, as compared with Group 1 (Figure 3B). However, implantations of PDLLA films (i.e., Group 3; 0.48 ± 0.05%) increased the wet weight ratio, as compared with Group 1 (Figure 3B).
Histological evaluation
Following 12 weeks of recovery from surgery, the rats were euthanized and a 1 cm-long segment of the sciatic nerve containing the anastomosis site was removed and examined. In the group of rats receiving suture only after sciatic nerve transection (i.e., Group 1; n = 2), the nerve fibers were twisted and displayed irregular arrangement (Figure 4A). However, in the group of rats receiving implantations of PDLLA films (Group 3; n = 2), the regenerated nerve fibers were relatively straight, and the aligned structure was complete (Figure 4B). In addition, little inflammation was observed.
Figure 4.
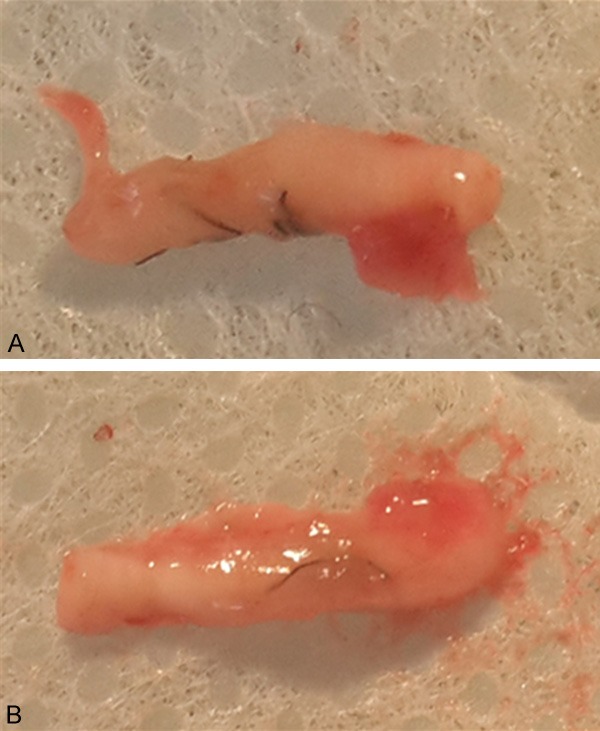
Histological evaluation of a segment of the sciatic nerve containing the anastomosis site following 12 weeks of recovery from surgery in the group of rats receiving suture only after sciatic nerve transection (A) or implantations of PDLLA films (B).
Discussion
In the present study, we demonstrated that implantation of a novel PDLLA film following sciatic nerve transection surgery promoted the regeneration of injured nerve tissues in rats. Specifically, rats with the implantations of either traditional nerve conduits or PDLLA films had no significant adhesion appearance between peripheral nerve tissues and the biomaterials. Furthermore, PDLLA film was clearly softened and closely attached with nerves after 12 weeks recovery from surgery. These results suggest that PDLLA films have better biocompatibility and biodegradability. Additionally, while implantations of PDLLA films were not able to increase the nerve conduction velocity, this therapeutic method increased the amplitude of action potentials following 8 weeks recovery from sciatic nerve transection surgery. These results indicate that PDLLA films may promote axon regeneration in vivo. Moreover, implantations of PDLLA films increased the tibialis and gastrocnemius muscle wet weight ratio, suggesting that PDLLA films can improve the nutritional status of the muscles innervated by the damaged nerves. Last but not the least, in rats with implantations of PDLLA films, the regenerated nerve fibers were relatively straight and the aligned structure was complete. In addition, little inflammation was observed. Taken together, our results suggest that PDLLA films promote the nerve regeneration in vivo.
The formation of adhesions with surrounding tissues and nerve scars are difficult complications after surgery [1,2]. The proliferating loose connective tissue between the nerve ends continuously grow into the epineurium, forming scar tissue [12]. The formation of scar tissue reduces the cross-sectional area and represents a major obstacle for axon regeneration. In order to prevent the adhesions between peripheral nerve and surrounding tissues and inhibit the formation of scar tissues, various surgical techniques, such as intravenous wrapping, muscle flaps, and free fat transplantation, as well as all kinds of biomaterials including a variety of biodegradable polymers, have been used in nerve regeneration [13-17]. Specifically, the collagen nerve conduits have been shown to have protective effect on the epineurium suture, improve the proliferation of connective tissue, and serve a similar protective role as sheath by itself [18]. Application of PLA film to the sciatic nerve in rats has been demonstrated to reduce the scar tissue formation [19]. In the present study, PDLLA films implantation resulted in the minimal formation of scar tissues, suggesting that PDLLA films can promote nerve regeneration by inhibiting the scar tissue formation process.
In recent years, PLA has become a hot topic in the field biomedical materials due to its non-toxicity, good biodegradability, and proven safety. The polymer has been widely used in abdominal surgery [20]. In addition, it has been shown that PLA film effectively reduces the proliferation and inflammation of loose connective tissue, and prevents peripheral nerve adhesion [21]. However, the degradation of PLA is relatively slow, and the material remains in the body for a long time. This is the main disadvantage that limits its application [21-24]. Meanwhile, non-permeable medical materials could hinder the nutrient and oxygen exchange between peripheral nerves and surrounding tissues, thus subsequently impairing the axon regeneration [25-27].
To overcome these disadvantages of PLA, a novel polylactic-DL-acid absorbable film (PDLLA film) was developed by Changchun Sino Biomaterials by using a phase inversion method. The novel anti-adhesion medical film has a porous structure. Our experimental results demonstrated that anastomosis coupled with a porous PDLLA film wrapping after sciatic nerve injury in rats resulted in diminished connective tissue adhesions, as compared with no wrapping or nerve conduit wrapping groups. Histological examination of nerve fibers also confirmed that better nerve fiber regeneration occurred in the PDLLA film group, as compared with the other two groups. Furthermore, functional examination of the sciatic nerve showed that sciatic nerve MNCV and CMAP recovered better in the PDLLA film implanted group. Finally, PDLLA film implanted group had higher muscle relative wet weight, which is an indirect index of axons and target organ function, suggesting that PDLLA film implantation can serve as a better therapeutic method for nerve regeneration.
The therapeutic effects of PDLLA films may also be ascribed to the good biodegradability. After 12 weeks recovery from surgery, implanted PDLLA films were substantially degraded, indicating good biodegradability of this type of biomaterials. PDLLA belongs to polylactic acid family of polymers [28]. Due to the irregular arrangement of the asymmetric carbon atoms in the polymer chain, PDLLA is a type of amorphous polymer with a Tm of 65°C [8,29,30]. The degradation and absorption rate of PDLLA is fast, generally around 3-6 months, which is superior to other PLA materials with slow degradation rates [8,29,30]. In addition, compared to the non-porous products, the porous PDLLA films maintain good mechanical properties and have a good balance between flexibility and controllability of degradation [8].
Furthermore, similar to other PLA films, PDLLA film has good barrier effect. It wraps on nerve anastomosis ends to form a concealed microenvironment. Therefore, PDLLA film can form a physically more resilient regeneration pipeline than epineurium. It prevents the proliferation of connective tissue ingrowth, inhibits the overflow of nerve growth factors, and creates a better micro-environment for nerve regeneration. Additionally, compared with other non-permeable materials, the presence of porous structure of PDLLA films allows smooth liquid flow across the film, which is beneficial to nerve regeneration. Thus, in the present study, the regenerated nerve fibers were relatively straight and the aligned structure was complete after the implantation of PDLLA films, as compared with direct suturing.
In summary, the novel absorbable PDLLA film provides superior nerve adhesion prevention, promotes nerve regeneration, and demonstrates good biodegradability characteristics. Therefore, this material appears to be very promising for clinical applications in the future.
Acknowledgements
This work was supported by the Science and Technology Research and Planning Project of Jilin Provincial Education Department (No. 440020031108).
Disclosure of conflict of interest
None.
References
- 1.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 3.Vleggeert-Lankamp CL, de Ruiter GC, Wolfs JF, Pego AP, van den Berg RJ, Feirabend HK, Malessy MJ, Lakke EA. Pores in synthetic nerve conduits are beneficial to regeneration. J Biomed Mater Res A. 2007;80:965–982. doi: 10.1002/jbm.a.30941. [DOI] [PubMed] [Google Scholar]
- 4.Aldini N, Fini M, Rocca M, Giavaresi G, Giardino R. Guided regeneration with resorbable conduits in experimental peripheral nerve injuries. Int Orthop. 2000;24:121–125. doi: 10.1007/s002640000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez FJ, Gomez N, Perego G, Navarro X. Highly permeable polylactide-caprolactone nerve guides enhance peripheral nerve regeneration through long gaps. Biomaterials. 1999;20:1489–1500. doi: 10.1016/s0142-9612(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 6.Stang F, Keilhoff G, Fansa H. Biocompatibility of Different Nerve Tubes. Materials. 2009;2:1480–1507. [Google Scholar]
- 7.Welch WC, Thomas KA, Cornwall GB, Gerszten PC, Toth JM, Nemoto EM, Turner AS. Use of polylactide resorbable film as an adhesion barrier. J Neurosurg. 2002;97:413–422. doi: 10.3171/spi.2002.97.4.0413. [DOI] [PubMed] [Google Scholar]
- 8.Ohya Y. Synthesis of nobel poly (lactic acid)-based biodegradable polymers and their application as biomaterials. Kobunshi Ronbunshu. 2002;59:484–498. [Google Scholar]
- 9.Omidi Y, Davaran S. Handbook of Applied Biopolymer Technology: Synthesis, Degradation and Applications. 2011. Impacts of Biodegradable Polymers: Towards Biomedical Applications; pp. 388–418. [Google Scholar]
- 10.Welch WC, Cornwall GB, Toth JM, Turner AS, Thomas KA, Gertszten PC, Nemoto EM. Use of polylactide resorbable film as an adhesion barrier. Orthopedics. 2002;25:S1121–S1130. doi: 10.3928/0147-7447-20021002-02. [DOI] [PubMed] [Google Scholar]
- 11.Ulery BD, Nair LS, Laurencin CT. Biomedical Applications of Biodegradable Polymers. J Polym Sci B Polym Phys. 2011;49:832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millesi H. Factors affecting the outcome of peripheral nerve surgery. Microsurgery. 2006;26:295–302. doi: 10.1002/micr.20242. [DOI] [PubMed] [Google Scholar]
- 13.Ohsumi H, Hirata H, Nagakura T, Tsujii M, Sugimoto T, Miyamoto K, Horiuchi T, Nagao M, Nakashima T, Uchida A. Enhancement of perineurial repair and inhibition of nerve adhesion by viscous injectable pure alginate sol. Plast Reconstr Surg. 2005;116:823–830. doi: 10.1097/01.prs.0000176893.44656.8e. [DOI] [PubMed] [Google Scholar]
- 14.Dam-Hieu P, Lacroix C, Said G, Devanz P, Liu S, Tadie M. Reduction of postoperative perineural adhesions by Hyaloglide gel: an experimental study in the rat sciatic nerve. Neurosurgery. 2005;56:425–433. doi: 10.1227/01.neu.0000156845.41626.e9. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda K, Yamauchi D, Osamura N, Hagiwara N, Tomita K. Hyaluronic acid prevents peripheral nerve adhesion. Br J Plast Surg. 2003;56:342–347. doi: 10.1016/s0007-1226(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 16.Merle M, Lallemand B, Lim A, Gantois G. Experimental and clinical evaluation of an absorbable biomaterial inducing an anti-adhesive barrier (Divide (R)) Eur J Orthop Surg Tr. 2008;18:255–263. [Google Scholar]
- 17.Xu J, Varitimidis SE, Fisher KJ, Tomaino MM, Sotereanos DG. The effect of wrapping scarred nerves with autogenous vein graft to treat recurrent chronic nerve compression. J Hand Surg Am. 2000;25:93–103. doi: 10.1053/jhsu.2000.jhsu025a0093. [DOI] [PubMed] [Google Scholar]
- 18.Kim PD, Hayes A, Amin F, Akelina Y, Hays AP, Rosenwasser MP. Collagen nerve protector in rat sciatic nerve repair: A morphometric and histological analysis. Microsurgery. 2010;30:392–396. doi: 10.1002/micr.20760. [DOI] [PubMed] [Google Scholar]
- 19.Okui N, Yamamoto M, Fukuhira Y, Kaneko H, Hirata H. A new nerve coaptation technique using a biodegradable honeycomb-patterned film. Microsurgery. 2012;32:466–474. doi: 10.1002/micr.21998. [DOI] [PubMed] [Google Scholar]
- 20.Bostman OM. Absorbable implants for the fixation of fractures. J Bone Joint Surg Am. 1991;73:148–153. [PubMed] [Google Scholar]
- 21.Scherman P, Kanje M, Dahlin LB. Local effects on triiodothyronine-treated polyglactin sutures on regeneration across peripheral nerve defects. Tissue Eng. 2004;10:455–464. doi: 10.1089/107632704323061816. [DOI] [PubMed] [Google Scholar]
- 22.Scherman P, Kanje M, Dahlin LB. Bridging short nerve defects by direct repair under tension, nerve grafts or longitudinal sutures. Restor Neurol Neurosci. 2004;22:65–72. [PubMed] [Google Scholar]
- 23.Fujiwara T, Matsuda K, Kubo T, Tomita K, Hattori R, Masuoka T, Yano K, Hosokawa K. Axonal supercharging technique using reverse end-to-side neurorrhaphy in peripheral nerve repair: an experimental study in the rat model. J Neurosurg. 2007;107:821–829. doi: 10.3171/JNS-07/10/0821. [DOI] [PubMed] [Google Scholar]
- 24.Turgut M, Uysal A, Pehlivan M, Oktem G, Yurtseven ME. Assessment of effects of pinealectomy and exogenous melatonin administration on rat sciatic nerve suture repair: an electrophysiological, electron microscopic, and immunohistochemical study. Acta Neurochir (Wien) 2005;147:67–77. doi: 10.1007/s00701-004-0426-x. [DOI] [PubMed] [Google Scholar]
- 25.Cemil B, Ture D, Cevirgen B, Kaymaz F, Kaymaz M. Comparison of collagen biomatrix and omentum effectiveness on peripheral nerve regeneration. Neurosurg Rev. 2009;32:355–362. doi: 10.1007/s10143-009-0193-5. [DOI] [PubMed] [Google Scholar]
- 26.Lundborg G. Ischemic nerve injury. Experimental studies on intraneural microvascular pathophysiology and nerve function in a limb subjected to temporary circulatory arrest. Scand J Plast Reconstr Surg Suppl. 1970;6:3–113. [PubMed] [Google Scholar]
- 27.Lundborg G. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. J Bone Joint Surg Am. 1975;57:938–948. [PubMed] [Google Scholar]
- 28.Sodergard A, Stolt M. Properties of lactic acid based polymers and their correlation with composition. Prog Polym Sci. 2002;27:1123–1163. [Google Scholar]
- 29.Zhao J, Liao W, Wang Y, Pan J, Liu F. Preparation and degradation characteristic study of bone repair composite of DL-polylactic acid/hydroxyapatite/ decalcifying bone matrix. Chin J Traumatol. 2002;5:369–373. [PubMed] [Google Scholar]
- 30.Xiang Y, Wang YL, Luo YF, Zhang BB, Xin J, Zheng DF. Molecular biocompatibility evaluation of poly (D, L-lactic acid)-modified biomaterials based on long serial analysis of gene expression. Colloids Surf B Biointerfaces. 2011;85:248–261. doi: 10.1016/j.colsurfb.2011.02.036. [DOI] [PubMed] [Google Scholar]


