Abstract
Purpose: Aberrant expression of CDK5 involved in epithelial-to-mesenchymal transition had been reported in various types of cancers, but its functions in nasopharyngeal carcinoma have not been fully clarified yet. The principal purpose of this research was to investigate the clinicopathological significance of CDK5 and its potential effect on NPC carcinogenesis. Methods: Pre-treated formalin-fixed paraffin-embedded biopsy samples of 393 patients between January 2011 and December 2013 were collected for tissue microarrays (TMAs). Immunohistochemistry was performed on sequential TMA sections stained with antibodies against CDK5, EGFR and P53. Results: The expression of CDK5 in NPC tissues was significantly higher than that in normal nasopharyngeal tissues. Among squamous carcinomas, the expression of CDK5 in undifferentiated tissues was noticeably increased compared with that in differentiated tissues. NPC patients in advanced T category showed a perceptibly higher level of CDK5 than those in early T category. The relative level of CDK5 in NPC sufferers with lymph node metastasis was obviously higher than that of patients without. Compared with patients in early TNM stages, the relative expression level of CDK5 of those in advanced TNM stages was notably up-regulated. Moreover, the CDK5 expression was positively correlated with EGFR and P53 expression. Nevertheless, no significant association was observed between CDK5 and gender, age or histological type. Conclusion: Overexpression of CDK5 might be considered as a warning signal for NPC. Consequently, CDK5 could serve as a potential target for diagnosis and gene therapy for NPC patients.
Keywords: Nasopharyngeal carcinoma, CDK5, EGFR, P53, immunohistochemistry
Introduction
Nasopharyngeal carcinoma, or NPC for short, is one of the most frequent head and neck cancers and claims higher morbidity in Asia than any other continents and even higher in China than any other countries in Asia. The International Agency for Research on Cancer (IARC) estimated that there were 86,691 incidences of NPC worldwide in 2012, and 38.3% of these cases took place in China [1,2]. Three subtypes of NPC are now recognized in the World Health Organization (WHO) classification: keratinizing squamous cell carcinoma (WHO I), non-keratinizing differentiated carcinoma (WHO II) and non-keratinizing undifferentiated carcinoma (WHO III). Despite the recent improvements in diagnostic technology and clinical strategies, distant metastasis remains an issue [3,4]. The majority of patients are initially diagnosed at advanced stages with distant metastasis, resulting in a low survival rate [5,6]. It still remains mysterious when it comes to the exact molecular and morphological changes responsible for its potential behavior of high aggressiveness. Since a growing number of methods for early diagnosis and more individualized therapeutic strategies are being introduced, it has become extremely necessary to explore novel biomarkers that can predict the carcinogenesis as effectively and efficiently as possible.
Cyclin-dependent kinase 5 (CDK5), a protein coding gene, is located in chromosome 7q36, and includes 12 exons. CDK5, i.e. PSSALRE, discovered by Meyerson et al [7] in the first place, is best known for its functions in neuronal development and postmitotic neuronal activities, which has been proved to be increasingly crucial in the process of tumor invasion and metastases. To date, aberrant expressions of CDK5 have been reported in various malignances, such as non-small cell lung cancer, gastric cancer, prostate cancer and so on [8-10]. However, as stated by the current reports, the data regarding CDK5 in NPC are still scarce. The epidermal growth factor receptor (EGFR), one of the members of the ErbB family of protein tyrosine kinase receptors, is encoded by the c-erbB-1 proto-oncogene and involved in the regulation and modulation of cell metabolism, development, migration and differentiation [11-14]. The P53 tumor suppressor gene is among the most attractive genes in scientific studies and gets mutated or deleted with a considerably sizable frequency in the majority of cancer types and these mutations or deletions are related to malignant transformation as well as tumor progression [11,15]. In this retrospective study, we aimed to elucidate the abnormal expression of CDK5 in NPC patients, and evaluate the diagnostic value of CDK5 in combination with EGFR and P53.
Materials and methods
Tissue samples
A total of 393 cases NPC tissues collected from January 2011 to December 2013 were identified as retrievable biopsy samples, either from primary sites or from metastatic nodes. Additionally, 54 cases of normal nasopharyngeal tissue samples were collected from individuals diagnosed with chronic nasopharyngitis or rhinopolyp, or from fresh autopsy. The study cohort included 142 females and 305 males, aged from 18 to 85 with a mean of 59.6. Furthermore, all 111 cases of NPC patients without distant metastasis were further restaged by the seventh edition of UICC Staging System for NPC. After pathological examination process of primary tumors along with regional lymph nodes, 6 patients were classified as stage I (5.4%), 22 as stage II (19.8%), 48 as stage III (43.2%) and 35 as stage IV (31.5%). All tissue samples preserved in paraffin blocks were assembled for tissue microarrays (TMAs). The clinicopathological features of these NPC patients were obtained from medical records and summarized in Table 1. The current study was approved by The Ethical Committee of First Affiliated Hospital of Guangxi Medical University, China, and written informed consents had been obtained from all involved patients.
Table 1.
Relationship between CDK5 expression and clinicopathological features
| Parameters | Total (n) | Expression of CDK5 n (%) | X2 | P | |
|---|---|---|---|---|---|
|
| |||||
| Negative | Positive | ||||
| Tissue | |||||
| Normal tissue | 54 | 36 (66.7%) | 18 (33.3%) | -6.217 | <0.001 |
| NPC | 393 | 99 (25.2%) | 294 (74.8%) | ||
| Histological type | |||||
| Adenocarcinoma | 4 | 0 | 4 (100%) | -1.165 | 0.244 |
| Squamous carcinoma | 389 | 99 (25.4%) | 290 (74.6%) | ||
| Squamous differentiation | |||||
| Differentiated | 250 | 76 (30.4%) | 174 (69.6%) | -3.002 | 0.003 |
| Undifferentiated | 139 | 23 (16.5%) | 116 (83.5%) | ||
| Gender | |||||
| Female | 121 | 28 (23.1%) | 93 (76.9%) | -0.624 | 0.533 |
| Male | 272 | 71 (26.1%) | 201 (73.9%) | ||
| Age | |||||
| ≤50 | 208 | 56 (26.9%) | 152 (73.1%) | -0.838 | 0.402 |
| >50 | 185 | 43 (23.2%) | 142 (76.8%) | ||
| T category | |||||
| T1 | 30 | 17 (56.7%) | 13 (43.3%) | H=22.069a | <0.001 |
| T2 | 32 | 12 (37.5%) | 20 (62.5%) | ||
| T3 | 27 | 2 (7.4%) | 25 (92.6%) | ||
| T4 | 22 | 2 (9.1%) | 20 (90.9%) | ||
| N category | |||||
| N0 | 6 | 4 (66.7%) | 2 (33.3%) | H=14.189a | 0.003 |
| N1 | 22 | 12 (54.5%) | 10 (45.5%) | ||
| N2 | 62 | 14 (22.6%) | 48 (77.4%) | ||
| N3 | 21 | 3 (14.3%) | 18 (85.7%) | ||
| TNM stage | |||||
| I | 6 | 4 (66.7%) | 2 (33.3%) | H=17.750a | <0.001 |
| II | 22 | 12 (54.5%) | 10 (45.5%) | ||
| III | 48 | 14 (29.2%) | 34 (70.8%) | ||
| IV | 35 | 3 (8.6%) | 32 (91.4%) | ||
Kruskal-Wallis H test was performed to analyze the difference.
Chi-square test was used in the rest of statistics.
Immunohistochemistry
The immunohistochemical staining method was performed on the formalin-fixed, paraffin embedded, 4-lm-thick tissue sections for expression studies of CDK5. In brief, deparaffinization of all sections was conducted through a range of xylene baths, and rehydration was performed with a series of alcohol solutions with graded concentrations. As to immune-staining, the primary antibodies used are as follows: mouse monoclonal anti-CDK5 (Abcam, Clone ID: EP715Y); mouse monoclonal anti-EGFR (Beijing Jinqiao Biological Co. LTD.); mouse monoclonal anti-P53 (Beijing Jinqiao Biological Co. LTD). A standard avidin-biotin immunoperoxidase complexes detection system was performed as previously described [16-19], according to the guideline of the manufacturer. After the immunodetection, all samples were reviewed and diagnosed by two pathologists (GC and YD), who discussed each case until they would reach an agreement. Immunoreactivities for CDK5 expression were defined by the presence of cytoplasm staining and it would be considered as CDK5-positive if 25% of the tumor cells showed immunoreactivities. The number of EGFR and P53 was calculated with the formula (number of positive cells/total number of the cells ×100%) by counting at least 10 random representative fields distant from necrotic areas at high magnification(40×40) and the results were noted as negative staining (-), weak staining (+), moderate staining (++) and strong staining (+++) [15].
Statistical analysis
Statistical Package for the Social Sciences 20.0 (SPSS20.0) was used for statistical analyses. Categorical variables were presented in frequency and percentage. Chi-square test was selected to compare the differences of CDK5 expression in two corresponding groups of different clinicopathological features. Concerning the clinicopathological parameters with more than two groups, Kruskal-Wallis H test was used for analyses. The correlations between the biomarkers were assessed by the Spearman rank correlation coefficient. Moreover, the receiver operating characteristic (ROC) analysis was conducted to find out the diagnostic values of CDK5, EGFR and P53. Among all statistics, P values are two sided, and it was considered statistically significant when P<0.05.
Results
The expression of CDK5 in NPC tissues
The images presented in Figure 1 were representative ones of immunohistochemical staining of CDK5 in 393 cases of NPC tissues and 54 cases of normal nasopharyngeal tissues. CDK5-positive staining was predominantly observed in the cytoplasm of the NPC cells, but there was weaker or even no expression of CDK5 in the normal nasopharyngeal tissues. By statistical analysis, the expression of CDK5 in NPC tissues (74.8%, 294/393) was significantly higher than that in normal nasopharyngeal tissues (33.3%, 18/54, P<0.001, Figure 2A). In addition, ROC curve was performed to prove its diagnostic value in nasopharyngeal tissues, and the AUC of CDK5 was 0.707 (95% CI: 0.630-0.784, P<0.001).
Figure 1.
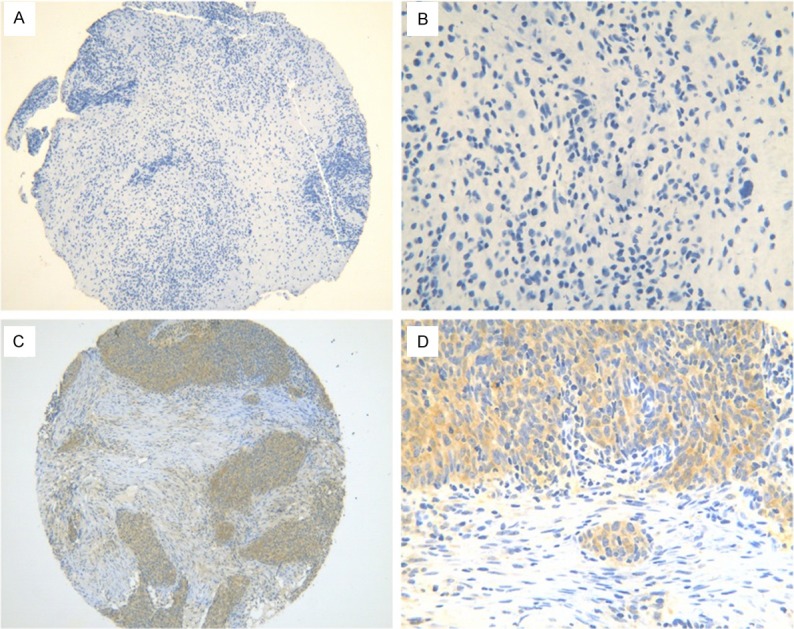
The representative photomicrographs of staining in nasopharyngeal tissues. No positive staining of CDK5 was found in normal nasopharyngeal tissues (A, ×100; B, ×400). Brown CDK5 immunostaining was observed in t NPC cells (C, ×100; D, ×400).
Figure 2.
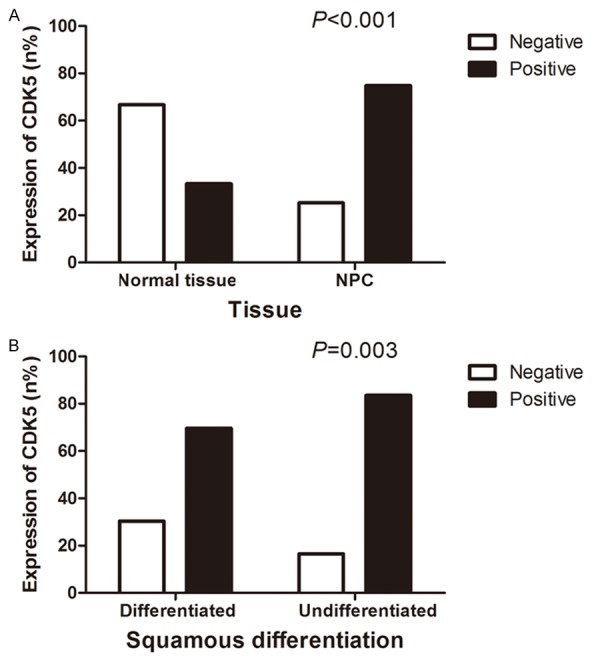
The expression of CDK5 in nasopharyngeal tissues. The CDK5 expression in NPC tissues (74.8%, 294/393) was significantly increased compared with that in normal nasopharyngeal tissues (33.3%, 18/54, P<0.001) (A). As for the correlation between CDK5 expression and squamous carcinoma differentiating degree, the expression of CDK5 in the undifferentiated tissues (83.5%, 116/139) was significantly higher than that in differentiated tissues (69.6%, 174/250, P=0.003) (B).
The entire cohort of NPC cases included both adenocarcinoma and squamous carcinoma, but as to the difference of CDK5 expression, there emerged no statistical significance (P=0.244). With respect to the association between CDK5 expression and differentiation degree of squamous cell carcinoma, the expression of CDK5 in the undifferentiated tissues (83.5%, 116/139) was significantly increased compared with that in differentiated tissues (69.6%, 174/250, P=0.003, Figure 2B). Due to the limited sample scale of adenocarcinoma, which included only 4 cases, and the lack of statistical significance, the further subgroup analysis for adenocarcinoma was omitted.
Relationship between CDK5 expression and clinicopathological characteristics
Several potential features in clinical practice were selected to investigate the relationship between the aberrant expression of CDK5 and clinical outcomes for NPC patients. As is illustrated in Table 1, CDK5 expression did not vary significantly with gender or age. The CDK5-positive rate was remarkably increased with the aggravation of tumor infiltration degree (H=22.069, P<0.001). NPC patients in T3 and T4 (91.8%, 45/49) showed a perceptibly higher level of CDK5 than those in T1 and T2 (53.2%, 33/62, P<0.001, Figure 3A). The development of lymphatic metastasis stage was correlated with the positive rate of CDK5 (H=14.189, P=0.003), and with regard to N category, a considerably higher level of CDK5 expression emerged among patients in N2 and N3 (79.5%, 66/83) than that in N0 and N1 (42.9%, 12/28, P=0.03, Figure 3B). Meanwhile, the relative level of CDK5 in NPC patients with lymph node metastasis (72.4%, 76/105) was obviously higher than that of patients without (33.3%, 2/6, P=0.043). Among NPC in more advanced clinical stages, CDK5 demonstrated stronger expression (H=17.750, P<0.001). Compared with patients in early stages (I and II, 42.9%, 12/28), the relative expression level of CDK5 of those in advanced stages (III and IV, 79.5%, 66/83, P<0.001) was notably up-regulated (Figure 3C). ROC curve showed an AUC of 0.693 (95% CI: 0.595-0.791, P<0.001) to predict the status of tumor infiltration. The AUC of ROC curve was 0.683 (95% CI: 0.562-0.804, P=0.004) to evaluate the clinical TNM stages of NPC. Moreover, it was inferred by the spearman analysis that CDK5 was correlated with tumor infiltration (r=0.429, P<0.001), lymph node metastasis (r=0.333, P<0.001), clinical TNM stage (r=0.398, P<0.001), squamous cell carcinoma differentiated (r=-0.152, P=0.003), EGFR (r=0.258, P=0.002) and P53 expression (r=0.441, P<0.001).
Figure 3.
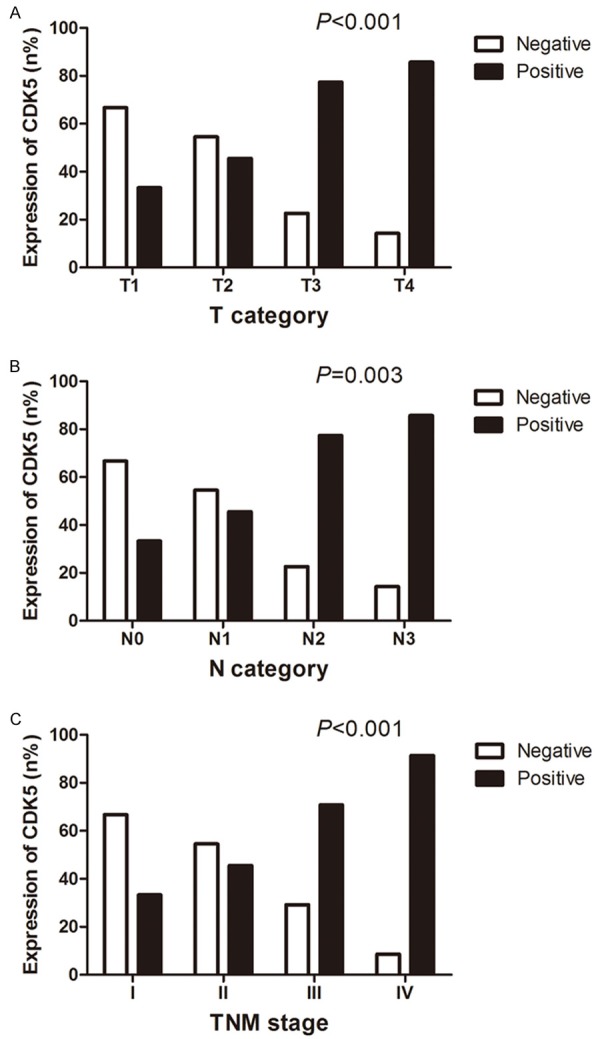
Relationship between CDK5 expression and clinicopathological parameters. As is illustrated, NPC patients in T3 and T4 (91.8%, 45/49) expressed a noticeably higher level of CDK5 than those in T1 and T2 (53.2%, 33/62, P<0.001) (A). A considerably higher level of NPC expression regarding N category emerged among patients in N2 and N3 (79.5%, 66/83) than that in N0 and N1 (42.9%, 12/28, P=0.03) (B). Meantime, NPC patients in more advanced clinical stages demonstrated relatively stronger CDK5 expression (III and IV, 79.5%, 66/83, P<0.001) when compared with patients in early stages (I and II, 42.9%, 12/28) (C).
Relationships between CDK5 expression and other biochemical indicators
The positive ratio of CDK5 expression was 74.2% (72/97) in the positive group of EGFR, significantly higher than that in EGFR-negative group (42.9%, 6/14, P=0.017). Comparing four groups sequentially, patients with high EGFR expression exhibited significantly stronger positive rates of CDK5 than those with low EGFR expression (H=15.093, P=0.002, Figure 4A) and CDK5 expression was significantly higher in the P53-positive group (73.7%, 73/99) than that in the P53-negative group (41.71%, 5/12, P=0.022). The positive rate of CDK5 was consistent with the trend of P53 and experienced a rise in relatively high-level group of P53 (H=13.263, P=0.004, Figure 4B, Table 2). In addition, Spearman analysis showed a positive correlation between the expressions of EGFR and P53 (r=0.780, P<0.001).
Figure 4.
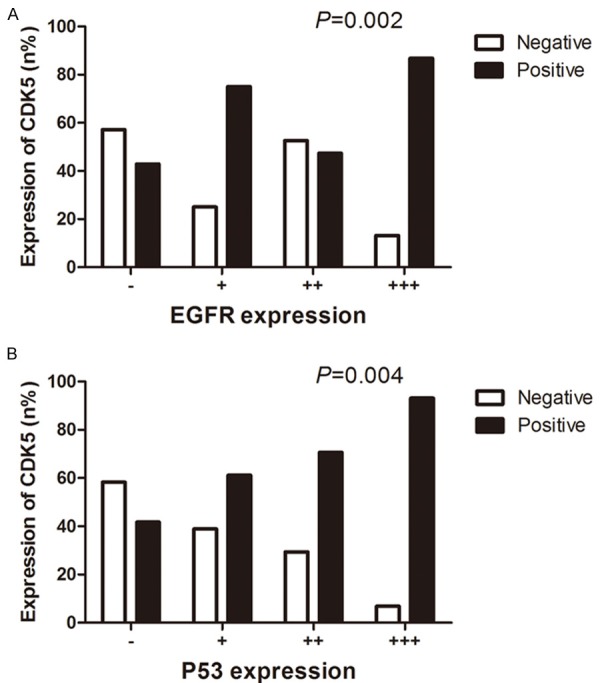
The relationships between CDK5 expression and other biochemical indicators. Patients expressing high levels of EGFR presented significantly higher positive rates of CDK5 than those with low expression levels of EGFR (P=0.002) (A). It was obvious that the CDK5-positive rate was consistent with the inclination of P53 and experienced an upturn in relatively high-level group of P53 (P=0.004) (B).
Table 2.
The relationship between CDK5 expression and other biochemical indicators
| Parameters | Total (n) | Expression of CDK5 n (%) | H | P | |
|---|---|---|---|---|---|
|
| |||||
| Negative | Positive | ||||
| EGFR | |||||
| - | 14 | 8 (57.1%) | 6 (42.9%) | 15.093a | 0.002 |
| + | 40 | 10 (25.0%) | 30 (75.0%) | ||
| ++ | 19 | 10 (52.6%) | 9 (47.4%) | ||
| +++ | 38 | 5 (13.2%) | 33 (86.8%) | ||
| P53 | |||||
| - | 12 | 7 (58.3%) | 5 (41.7%) | 13.263a | 0.004 |
| + | 36 | 14 (38.9%) | 22 (61.1%) | ||
| ++ | 34 | 10 (29.4%) | 24 (70.6%) | ||
| +++ | 29 | 2 (6.9%) | 27 (93.1%) | ||
Kruskal-Wallis H test was performed to analyze the difference.
Discussion
CDK5 is considered as a member of the CDK family of serine/threonine kinases and activated by its upstream regulators p35 or p39 [8]. Mao et al [20] illustrated that the induction of senescent phenotype by p35 required the activation of CDK5, and that CDK5 was mobilized in the process of cellular senescence, which might provide insights into the cancer cell proliferation. Thus, we hypothesized that CDK5 might play an important role in carcinogenesis and development of malignancies. Moreover, CDK5 expression profiles in various classes of tumors in vitro and in vivo had been investigated previously [8-10], but no publications were available with regard to the expression or the possible mechanisms of CDK5 in nasopharyngeal carcinoma. Therefore, the purpose of the current research was to scrutinize the expression condition of CDK5 in NPC patients and to demonstrate the relationship between CDK5 and the prognosis of NPC.
With respect to existing literature, results by Alaine et al [21] identified a possible role of CDK5 in mediating apoptosis in human glioblastoma multiforme cells, suggesting that CDK5 might function as an oncogenic factor in NPC to some degree. Furthermore, CDK5 was found positive in 66 (69.5%) of the 95 lung carcinoma tissue specimens by Liu et al [8], whereas no or weak expression of CDK5 in non-cancer lung tissues. According to our results, an increased expression of CDK5 was observed in NPC tissues, but there was no noticeable expression of CDK5 in the control group with tissues of benign nasopharyngeal diseases. Aforementioned data indicated the role of CDK5 as a tumor promoter in NPC, consistent with our initial expectations. Additionally, Huang et al. found that the major pathological type of NPC in southern China is non-keratinizing undifferentiated carcinoma [22]. The sample of current study also came from southern China, and demonstrated an overexpression of CDK5 in undifferentiated group, which backed the theory that CDK5 might be a risk factor for Chinese ethnics.
As for clinicopathological characteristics, in spite of no obvious correlation between CDK5 expression and gender, age, or histological type, we still found significant variances of CDK5 expression between different statuses of tumor infiltration, lymph node metastasis, as well as clinical TNM stage. Upon the study by Liu et al [8], positive CDK5/p35 expression was related to the degrees of differentiation, lymph node metastasis, pathological stage, and survival time in patients with non-small-cell lung cancer. The features showed in the previous report by Liu et al [8], were consistent with the current study and further supported our hypothesis.
EGFR expresses in 88 to 100 percent of head and neck squamous cell carcinomas, and is crucial in tumor cell growing, repairing and surviving [23]. With regard to our cohort, the overexpression of CDK5 in NPC was consistently associated with a high accumulation of EGFR. That is, in the more actively proliferating NPC cells, the positive expression of CDK5 showed stronger expression, which indicated that CDK5 was closely related to the proliferation of NPC cells. Furthermore, P53 is widely accepted as a tumor suppressive gene. Previous in vivo researches uncovered that wild type P53 activated the promoter of EGFR [24]. It was common for a Malaysian research team to observe P53 high-intensity staining in their series of NPC tissue samples when using immunohistochemistry [25]. In the current research, high-level P53 expression was found in the positive group of CDK5. The carcinogenesis and deterioration of malignancies usually result from the disequilibrium between proto-oncogenes and anti-oncogenes. Thus, we suppose CDK5 as a tumor promoter, which is likely to locate on a crucial node among the complex tumor mediation network for NPC patents.
To summarize, the current research confirmed a relatively higher level of CDK5 in NPC tissues and its associations with various clinicopathological characteristics. Aforementioned data revealed that the overexpression of CDK5 might be a novel molecular alteration involved in NPC progression and that CDK5 might serve as a valuable biomarker to diagnose NPC and to monitor its clinical courses. Future prospective studies are needed to identify the clinical significance of CDK5 and its potential mechanisms in tumor invasiveness and metastasis are warranted.
Acknowledgements
This study was supported by the Fund of Guangxi Zhuang Autonomous Region University Student Innovative Plan (No. 201410598007), China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Xin Zhang and Tengfei Zhong contributed equally to this paper.
Disclosure of conflict of interest
None.
References
- 1.Yuan T, Zhang H, Liu B, Zhang Q, Liang Y, Zheng R, Deng J, Zhang X. Expression of MTA1 in nasopharyngeal carcinoma and its correlation with prognosis. Med Oncol. 2014;31:330. doi: 10.1007/s12032-014-0330-z. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, Sun Y, Lin AH, Liu MZ, Ma J. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Zeng L, Tian YM, Sun XM, Chen CY, Han F, Xiao WW, Deng XW, Lu TX. Late toxicities after intensity-modulated radiotherapy for nasopharyngeal carcinoma: patient and treatment-related risk factors. Br J Cancer. 2014;110:49–54. doi: 10.1038/bjc.2013.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG, Deng XW, Lu TX, Cui NJ, Zhao C. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117:1874–1883. doi: 10.1002/cncr.25754. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Shi M, Hsia Y, Luo S, Zhao L, Xu M, Xiao F, Fu X, Li J, Zhou B, Long X. Failure patterns and survival in patients with nasopharyngeal carcinoma treated with intensity modulated radiation in Northwest China: a pilot study. Radiat Oncol. 2012;7:2. doi: 10.1186/1748-717X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human cdc2-related protein kinases. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JL, Wang XY, Huang BX, Zhu F, Zhang RG, Wu G. Expression of CDK5/p35 in resected patients with non-small cell lung cancer: relation to prognosis. Med Oncol. 2011;28:673–678. doi: 10.1007/s12032-010-9510-7. [DOI] [PubMed] [Google Scholar]
- 9.Cao L, Zhou J, Zhang J, Wu S, Yang X, Zhao X, Li H, Luo M, Yu Q, Lin G, Lin H, Xie J, Li P, Hu X, Zheng C, Bu G, Zhang Y, Xu H, Yang Y, Huang C, Zhang J. Cyclin dependent kinase 5 decreases in gastric cancer and its nuclear accumulation suppresses gastric tumorigenesis. Clin Cancer Res. 2015;21:1419–1428. doi: 10.1158/1078-0432.CCR-14-1950. [DOI] [PubMed] [Google Scholar]
- 10.Wissing MD, Dadon T, Kim E, Piontek KB, Shim JS, Kaelber NS, Liu JO, Kachhap SK, Nelkin BD. Small-molecule screening of PC3 prostate cancer cells identifies tilorone dihydrochloride to selectively inhibit cell growth based on cyclin-dependent kinase 5 expression. Oncol Rep. 2014;32:419–424. doi: 10.3892/or.2014.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Wu SK, Wang Y, Fan ZX, Li CR, Feng M, Xu P, Wang WD, Lang JY. p53, MDM2, eIF4E and EGFR expression in nasopharyngeal carcinoma and their correlation with clinicopathological characteristics and prognosis: A retrospective study. Oncol Lett. 2015;9:113–118. doi: 10.3892/ol.2014.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, He R, Rong M, Dang Y, Chen G. Synergistic effect of MiR-146a mimic and cetuximab on hepatocellular carcinoma cells. Biomed Res Int. 2014;2014:384121. doi: 10.1155/2014/384121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Umelo IA, Lv S, Teugels E, Fostier K, Kronenberger P, Dewaele A, Sadones J, Geers C, De Greve J. miR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS One. 2013;8:e60317. doi: 10.1371/journal.pone.0060317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen G, Noor A, Kronenberger P, Teugels E, Umelo IA, De Greve J. Synergistic effect of afatinib with su11274 in non-small cell lung cancer cells resistant to gefitinib or erlotinib. PLoS One. 2013;8:e59708. doi: 10.1371/journal.pone.0059708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang YQ, Zhong TF, Dang YW, Zou LS, Yang L, Yang X, Chen G. Overexpression and clinicopathological contribution of DcR3 in bladder urothelial carcinoma tissues. Asian Pac J Cancer Prev. 2014;15:9137–9142. doi: 10.7314/apjcp.2014.15.21.9137. [DOI] [PubMed] [Google Scholar]
- 16.Chen G, Luo D. Expression of decoy receptor 3 in liver tissue microarrays. Natl Med J India. 2008;21:275–278. [PubMed] [Google Scholar]
- 17.Chen G, Rong M, Luo D. TNFRSF6B neutralization antibody inhibits proliferation and induces apoptosis in hepatocellular carcinoma cell. Pathol Res Pract. 2010;206:631–641. doi: 10.1016/j.prp.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Yang M, Chen G, Dang Y, Luo D. Significance of decoy receptor 3 in sera of hepatocellular carcinoma patients. Ups J Med Sci. 2010;115:232–237. doi: 10.3109/03009734.2010.516410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S, Chen G, Dang Y, Chen LH. Overexpression of DcR3 and its significance on tumor cell differentiation and proliferation in glioma. ScientificWorldJournal. 2014;2014:605236. doi: 10.1155/2014/605236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao D, Hinds PW. p35 is required for CDK5 activation in cellular senescence. J Biol Chem. 2010;285:14671–14680. doi: 10.1074/jbc.M109.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catania A, Urban S, Yan E, Hao C, Barron G, Allalunis-Turner J. Expression and localization of cyclin-dependent kinase 5 in apoptotic human glioma cells. Neuro Oncol. 2001;3:89–98. doi: 10.1093/neuonc/3.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang TR, Zhang SW, Chen WQ, Deng W, Zhang CY, Zhou XJ, Zhai RH. Trends in nasopharyngeal carcinoma mortality in China, 1973-2005. Asian Pac J Cancer Prev. 2012;13:2495–2502. doi: 10.7314/apjcp.2012.13.6.2495. [DOI] [PubMed] [Google Scholar]
- 23.Berg M, Soreide K. EGFR and downstream genetic alterations in KRAS/BRAF and PI3K/AKT pathways in colorectal cancer: implications for targeted therapy. Discov Med. 2012;14:207–214. [PubMed] [Google Scholar]
- 24.Esteve A, Lehman T, Jiang W, Weinstein IB, Harris CC, Ruol A, Peracchia A, Montesano R, Hollstein M. Correlation of p53 mutations with epidermal growth factor receptor overexpression and absence of mdm2 amplification in human esophageal carcinomas. Mol Carcinog. 1993;8:306–311. doi: 10.1002/mc.2940080414. [DOI] [PubMed] [Google Scholar]
- 25.Hoe SL, Lee ES, Khoo AS, Peh SC. p53 and nasopharyngeal carcinoma: a Malaysian study. Pathology. 2009;41:561–565. doi: 10.1080/00313020903071504. [DOI] [PubMed] [Google Scholar]


