Abstract
Neurotrophic factor decreased in the early stage of diabetic retinal nerve cells. Neurons damage brain derived neurotrophic factor (BDNF) and receptor TrkB expression reduced. Erythropoietin (EPO) plays an important role in protecting early diabetic retinopathy. The rats were euthanized at 24 h after EPO vitreous injection and the retina was separated. HE staining was applied to observe the pathological tissue morphology. Immunohistochemistry, immunofluorescence, and Western blot were used to detect BDNF, TrkB, extracellular signal-regulated kinase (ERK), and glial fibrillary acidic portein (GFAP) expression. Retinal structure was clear in group C, while the retinal thickness and RGCs number decreased in group B at 24 w. Retinal thickness in group E was greater than in group B but lower than in group C. GFAP and ERK expression increased in both group B and E, whereas the latter was significantly lower than the former. TrkB protein level was in group E > B > C at 4 w, while it was in group C > group E > group B at 24 w. BDNF expression in group B was higher than in group C at 4 w, whereas it was opposite at 24 w. BDNF expression increased in group E at 4 w, and it was similar in group E compared with group C at 24 w. EPO vitreous injection can increase BDNF and TrkB expression, while reduce GFAP and ERK expression in diabetes rat retina. It could protect Müller cells through BDNF/TrkB pathway to play a role of nerve nutrition.
Keywords: EPO, BDNF, GFAP
Introduction
Diabetic retinopathy (DR) is a common complication of diabetes. It has become a hot issue in ophthalmology clinical and basic research [1,2]. Studies have shown that diabetic patients appeared abnormal retinal glial cells (Müller cells) and retinal ganglion cells (RGCs) before retinal microvascular lesions occur [3,4], suggesting that diabetic retinopathy is neural degenerative process. RGCs damage and cell hydroxy-ischemia may block axoplasmic transport in ganglion cell axon, interrupt neurotrophic factors supply, and cause apoptosis [5,6]. In the early phase of diabetes, neurotrophic factor expression decreased, neurons damage brain derived neurotrophic factor (BDNF) and receptor TrkB expression reduced in the retina nerve cells [5,7], indicating that diabetic retinal neural degenerative disease may be associated with lack of neurotrophic factors. Müller cells have multiple physiological functions. It can release neurotrophic factors and plays an important role in maintaining retina steady. Müller cells reactive gliosis is the common character after retinal damage, as it plays an important role in protecting retina [7,8]. Brain derived neurotrophic factor (BDNF) is important in neuron survival. Existing studies suggested that erythropoietin (EPO) has nerve protective effect in retinal disease by combining with EpoR mediated by the EPO/EPO receptor (EpoR) to activate multiple signaling pathways. EPO protection role in the early stage of diabetic retina is closely related to the MAPK/ERK1/2 signaling pathway [8,9]. Studies found that EPO protection is associated with the interaction between glial cells and neurotrophic factors. Whether EPO plays a role of neurotrophic effect on Müller cells in diabetic rat retina remains to be further investigated. This study investigated the role of EPO on diabetic rat retinal pathological changes and the tyrosine kinase receptor B (TrkB), BDNF, extracellular signal-regulated kinase (ERK), glial fibrillary acidic protein (GFAP) expression in diabetic rat model to observe EPO protection on Müller cells under high glucose state.
Materials and methods
Animals and grouping
8 weeks male Wistar rats weighed 180~220 g were provided by experimental animal center of Weifang Medical College (animal certification number: SCXK 2006-0002). The animals were feed in SPF laboratory. The rats were randomly divided into control group (C), model group (B), and EPO group (E) with 20 in each group.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of our hospital.
Drugs and reagents
Streptozotocin (provided by Sigma) was dissolved in citrate buffer. Trypsin (serva1:250) was provided by Amresco. BDNF and primary antibody for TrkB, ERK, BDNF and GFAP were purchased from Sigma. β-actin primary antibody and secondary antibody were got from Biovision.
Modeling
Streptozotocin was adopted to establish the diabetic rat model. After fasting for 12 h, the rats in the model group received 60 mg/kg 1% streptozotocin solution intraperitoneal injection. The rats in the control received equal volume of citrate buffer. The diabetic model was confirmed when the fasting blood-glucose ≥ 16.7 mmol/L after 72 h.
Drug administration
EPO was injected to the vitreous cavity at 4 week/24 week at 8 U/eye, while rats in group C and B received equal volume of normal saline.
Index detection
General condition of the rats were observed daily including mental state, drinking, diet, defecate and urine. The rats were euthanized at 24 h after injection and the retina was separated. HE staining was applied to observe the pathological tissue morphology. Immunohistochemistry, immunofluorescence, and Western blot were used to detect BDNF, TrkB, ERK, and GFAP expression. Rat retina tissue paraffin section was used to test TrkB and ERK expression after dewaxing, and immunohistochemistry (Ultra Vision Detection System three-step method). Immunofluorescence was adopted to determine retinal GFAP expression: retinal paraffin section was observed after washing and incubating with GFAP antibody (1:2000). Western blot was applied to detect GFAP and BDNF protein expression: total protein of the retina was separated by denaturing SDS-polyacrylamide gel electrophoresis and analyzed.
Statistical analysis
All statistical analyses were performed using SPSS17.0 software. Numerical data were presented as means and standard deviation (X̅±S). Differences between multiple groups were analyzed using one-way ANOVA or LSD-t test. P < 0.05 was considered as significant difference.
Results
EPO effect on pathologic morphology of the diabetic rats’ retinal tissue
Retinal structure was clear in group C with normal and in alignment cells (Figure 1A, 1D). The retinal cell morphology did not change obviously after 4 w in model group, while the retinal thickness and RGCs number decreased at 24 w. Meanwhile, it also presented retinal nerve fiber layer edema, glial cell proliferation, outer nuclear layer and inner nuclear layer cell arrangement disorder (Figure 1B, 1E). Retinal thickness in EPO group was greater than in modeling group (Figure 1C, 1F).
Figure 1.
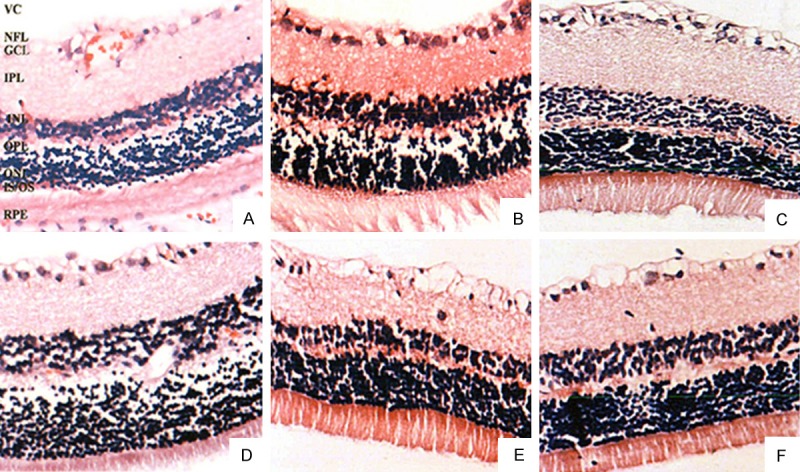
EPO effect on pathologic morphology of the diabetic rats’ retinal tissue (× 400). VC, vitreous cavity; NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, photoreceptor-inner segment; OS, photoreceptor-outer segment.
EPO effect on GFAP expression in diabetic rats’ retinal tissue
Immunofluorescence showed that GFAP mainly expressed in the ganglion cell layer, nerve fiber layer and inner plexiform layer in the control, while little in the outer nuclear layer (Figure 2A, 2D). GFAP fluorescence intensity increased in the 4 w of the diabetic rats. GFAP fluorescence intensity in nerve fiber layer upregulated at 24 w following the process of the disease. Inner nuclear layer surrounds the Müller cells, ganglion cell layer surrounds the RGCs, and outer nuclear layer exists in the synapse of the Müller cells with filiform trend (Figure 2B, 2E). GFAP fluorescence intensity in EPO group was weaker than that of the model group but stronger than in the control (Figure 2C, 2F). Western blot showed that GFAP expression increased significantly with the longer duration of diabetes (P < 0.05) (Figure 2G). GFAP expression in EPO group was lower than the diabetic group but higher than the control (P < 0.05). EPO can obviously reduce GFAP expression level (Figure 2H, 2I).
Figure 2.

EPO effect on GFAP expression in diabetic rats’ retinal tissue.
EPO effect on ERK and TrkB expression in diabetic rats’ retinal tissue
Immunohistochemistry results showed that TrkB protein mainly exists in the nerve fiber layer, ganglion cell layer, inner nuclear layer, inner/outer plexiform layer, pigment epithelium layer, and photoreceptor inner section. Brown product distributed in the bulge of the neuron cell body (Figure 3A). TrkB protein expression in diabetic rats was higher than the control group at 4 w, whereas it was lower than the control at 24 w. It mainly expressed in the ganglion cell layer, and a little in the other parts (Figure 3B, 3D). EPO group rats showed higher TrkB protein expression than that of diabetic group at 4 w and 24 w, and lower level than the control at 24 w. It suggested that TrkB protein upregulated in the early stage of diabetic retina, and reduced following longer duration. EPO can increase TrkB protein expression (Figure 3C, 3E). ERK expression was weak in the control and mainly existed in the ganglion cell layer (Figure 3F). ERK protein increased in diabetic rats at 4 w and 24 w and mainly expressed in the inner nuclear layer and ganglion cell layer significantly (Figure 3G, 3I). ERK protein expression in EPO group was lower than the diabetic group and higher than the control (Figure 3H, 3J), indicating that EPO can downregulate ERK expression.
Figure 3.

EPO effect on ERK and TrkB expression in diabetic rats’ retinal tissue (× 400).
EPO effect on BDNF expression in diabetic rats’ retinal tissue
BDNF overexpressed in the model group at 4 w, and it decreased obviously at 24 w (P < 0.05). BDNF expression upregulated in the EPO group at 4 w, and it was similar with control at 24 w. It showed that EPO vitreous injection can increase BDNF level in the retina (Figure 4).
Figure 4.

EPO effect on BDNF expression in diabetic rats’ retinal tissue.
Discussion
Currently, multiple studies focused on investigating EPO role in DR. Clinical trial showed that [10], EPO intraocular injection can relieve refractory macular edema in DR and improve patients’ eyesight level. In vivo animal experiments indicated that [11,12], early EPO vitreous cavity or intraperitoneal injection can decrease retinal nerve cells apoptosis, improve the glial cell electrophysiologic function, and protect blood retinal barrier in STZ established type 1 diabetic rat model. Intraocular EPO application security has been verified in rabbits, rats, and human. This study discussed EPO effect on retinal pathological changes in diabetic rats and relationship with TrkB, BDNF, ERK and GFAP expression to observe EPO protection on Müller cells and indirect neurotrophic factor effect under high glucose state through establishing diabetic rat model.
Müller cells are the major retinal glial cells on retina. Their bodies run through all layers of the retina and make up of retinal neurons for material exchange. Müller cells have different ion channels for regulating a variety of physiological functions to maintain the normal function of neurons. Injury or disease can lead Müller cells gliosis. Müller cells glial activity increased and GFAP expression upregulated in the early stage of diabetes. GFAP mainly expressed in the Müller cells and astrocytes of the rat retina. The compensatory response of astrocytes in injury was reactive gliosis. However, excessive gliosis may lead to glial scar, which is not conducive to neurons repair, resulting in tractional retinopathy [13,14]. Müller cells characteristic changes (hyperplasia, swelling, etc.) and GFAP overexpression appeared before the vascular lesions in the process of retinopathy. Apoptosis related signaling molecules can be detected in diabetic retina. DR nerve apoptosis is related to the lack of neurotrophic factor. Neurotrophic factors supplement can protect DR neurons. Studies have found that EPO can induce BDNF expression in the brain astrocytes and promote neurons regeneration [15-17]. Our results revealed that retinal structure was clear in group C under microscope, while the retinal thickness and RGCs number decreased in group B. Retinal thickness in group E was greater than in group B but lower than in group C. GFAP immunofluorescence intensity and ERK expression in group C were weak. They increased in both group B and E at 24 w, whereas the latter was significantly lower than the former. TrkB protein level was in group E > B > C at 4 w, while it was in group C > group E > group B at 24 w. BDNF expression in group B was higher than in group C at 4 w, whereas it was opposite at 24 w. BDNF expression increased in group E at 4 w, and it was similar in group E compared with group C at 24 w. The results demonstrated that EPO can upregulate BDNF and TrkB expression, while downregulate retina gliosis marker GFAP and ERK expression in the Müller cells of the diabetic rat retina. EPO indirect trophic factors may be involved in the protection effect of EPO on retinal neurons under high glucose condition. In the retina, neurotrophic factors mainly come from Müller cells. BDNF is an important neurotrophic factor in the nervous system that can promote nerve regeneration and maintain normal neuronal function. BDNF may play a role through binding with receptor P75 and TrkB on nerve cells and activating various signaling pathways [18-20]. BDNF overexpressed in the early stage in this study, which may be associated with that BDNF expression is affected by retinal metabolism. BNDF can respond quickly to diabetes stimulation and overexpressed in retina to prevent damage. BDNF expression reduced at 24 w in the retina of diabetic rats, and Müller cell function declined. EPO can improve Müller cells function and upregulate BDNF expression. Exogenous EPO can increase BDNF and TrkB protein, reduce GFAP level in the retina, and regulate retinal neurons apoptosis through BDNF/TrkB pathway under high glucose condition. In this study, TrkB protein level increased in the early stage of diabetes. TrkB level decreased following the extension of the disease. EPO can increase TrkB expression. Early TrkB overexpression in diabetic rats might be a protective effect to high glucose condition. Along with the extension of the course, TrkB downregulation may be associated with decompensation to chronic high glucose injury.
To sum up, vitreous injection of EPO can upregulate BDNF and TrkB expression in the retina of diabetic rats, reduce GFAP and ERK expression. EPO play an indirect neurotrophic factor role to protect Müller cells through BDNF/TrkB pathway.
Disclosure of conflict of interest
None.
References
- 1.Liu X, Zhu B, Zou H, Hu D, Gu Q, Liu K, Xu X. Carbamylated erythropoietin mediates retinal neuroprotection in streptozotocin-induced early-stage diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2015 doi: 10.1007/s00417-015-2969-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Song Q, Zhang Y, Wu Y, Zhou F, Qu Y. Association of erythropoietin gene polymorphisms with retinopathy in a Chinese cohort with type 2 diabetes mellitus. Clin Experiment Ophthalmol. 2015 doi: 10.1111/ceo.12505. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Cancarini A, Costagliola C, Dell’omo R, Romano M, Morescalchi F, Agnifili L, Ruggeri G, Semeraro F. Effect of intravitreal bevacizumab on serum, aqueous, and vitreous humor levels of erythropoietin in patients with proliferative diabetic retinopathy. Minerva Endocrinol. 2014;39:305–311. [PubMed] [Google Scholar]
- 4.Gu L, Xu H, Wang F, Xu G, Sinha D, Wang J, Xu JY, Tian H, Gao F, Li W, Lu L, Zhang J, Xu GT. Erythropoietin exerts a neuroprotective function against glutamate neurotoxicity in experimental diabetic retina. Invest Ophthalmol Vis Sci. 2014;55:8208–8222. doi: 10.1167/iovs.14-14435. [DOI] [PubMed] [Google Scholar]
- 5.Semeraro F, Cancarini A, Morescalchi F, Romano MR, dell’Omo R, Ruggeri G, Agnifili L, Costagliola C. Serum and intraocular concentrations of erythropoietin and vascular endothelial growth factor in patients with type 2 diabetes and proliferative retinopathy. Diabetes Metab. 2014;40:445–451. doi: 10.1016/j.diabet.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Gholamhossein Y, Behrouz H, Asghar Z. Diabetic retinopathy risk factors: plasma erythropoietin as a risk factor for proliferative diabetic retinopathy. Korean J Ophthalmol. 2014;28:373–378. doi: 10.3341/kjo.2014.28.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Deng Y, Gu H, Ren X, Li N, Lim A, Snellingen T, Liu X, Wang N, Liu N. Candidate gene association study for diabetic retinopathy in Chinese patients with type 2 diabetes. Mol Vis. 2014;20:200–214. [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsuhashi J, Morikawa S, Shimizu K, Ezaki T, Yasuda Y, Hori S. Intravitreal injection of erythropoietin protects against retinal vascular regression at the early stage of diabetic retinopathy in streptozotocin-induced diabetic rats. Exp Eye Res. 2013;106:64–73. doi: 10.1016/j.exer.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Loukovaara S, Robciuc A, Holopainen JM, Lehti K, Pessi T, Liinamaa J, Kukkonen KT, Jauhiainen M, Koli K, Keski-Oja J, Immonen I. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013;91:531–539. doi: 10.1111/j.1755-3768.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- 10.DeNiro M, Al-Mohanna FA. Erythropoietin and anemia: biological markers that cannot be ignored in diabetic retinopathy. Med Hypotheses. 2012;78:555–556. doi: 10.1016/j.mehy.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Bikbova G, Oshitari T, Baba T, Yamamoto S. Neurotrophic factors for retinal ganglion cell neuropathy - with a special reference to diabetic neuropathy in the retina. Curr Diabetes Rev. 2014;10:166–176. doi: 10.2174/1573399810666140508121927. [DOI] [PubMed] [Google Scholar]
- 12.Abu El-Asrar AM, Nawaz MI, Siddiquei MM, Al-Kharashi AS, Kangave D, Mohammad G. High-mobility group box-1 induces decreased brain-derived neurotrophic factor-mediated neuroprotection in the diabetic retina. Mediators Inflamm. 2013;2013:863036. doi: 10.1155/2013/863036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu El-Asrar AM, Mohammad G, De Hertogh G, Nawaz MI, Van Den Eynde K, Siddiquei MM, Struyf S, Opdenakker G, Geboes K. Neurotrophins and neurotrophin receptors in proliferative diabetic retinopathy. PLoS One. 2013;8:e65472. doi: 10.1371/journal.pone.0065472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ola MS, Ahmed MM, Abuohashish HM, Al-Rejaie SS, Alhomida AS. Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem Res. 2013;38:1572–1579. doi: 10.1007/s11064-013-1058-4. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Tao L, Fu X, Zhao Y, Xu X. BDNF protects retinal neurons from hyperglycemia through the TrkB/ERK/MAPK pathway. Mol Med Rep. 2013;7:1773–1778. doi: 10.3892/mmr.2013.1433. [DOI] [PubMed] [Google Scholar]
- 16.Si YF, Wang J, Guan J, Zhou L, Sheng Y, Zhao J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br J Pharmacol. 2013;169:619–631. doi: 10.1111/bph.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ola MS, Nawaz MI, El-Asrar AA, Abouammoh M, Alhomida AS. Reduced levels of brain derived neurotrophic factor (BDNF) in the serum of diabetic retinopathy patients and in the retina of diabetic rats. Cell Mol Neurobiol. 2013;33:359–367. doi: 10.1007/s10571-012-9901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Y, Wang R, Xu J, Sun J, Xu T, Gu Q, Wu X. Hydrogen-rich saline prevents early neurovascular dysfunction resulting from inhibition of oxidative stress in STZ-diabetic rats. Curr Eye Res. 2013;38:396–404. doi: 10.3109/02713683.2012.748919. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Gu Z, Shen B, Xu G, Zhou T, Jiang J, Xing J, Liu S, Li M, Tan W, Feng G, Sang A, Li L. Roles of Wnt/beta-catenin signaling in retinal neuron-like differentiation of bone marrow mesenchymal stem cells from nonobese diabetic mice. J Mol Neurosci. 2013;49:250–261. doi: 10.1007/s12031-012-9917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong Y, Chang ZP, Ren RT, Wei SH, Zhou HF, Chen XF, Hou BK, Jin X, Zhang MN. Protective Effects of Adeno-associated Virus Mediated Brain-derived Neurotrophic Factor Expression on Retinal Ganglion Cells in Diabetic Rats. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-011-9779-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


