Abstract
Aim: To investigate the potential role of CXCR3 expression on prostate cancer cell proliferation and invasion and to illustrate its mechanism. Methods: Human PC-3 cells were transfected with siRNA-CXCR3A and siRNA-CXCR3B plasmids respectively. The mRNA expressions of CXCR3A and CXCR3B in PC-3 cells from each group were analyzed using RT-PCR. Besides, cell proliferation ability and cell invasion ability of PC-3 cells in each group were analyzed using MTT assay and Matrige assay respectively. Additionally, expressions of CXCR3 downstream proteins were detected using Western blotting. Results: mRNA level of CXCR3A was decreased while CXCR3B mRNA level was increased in PC-3 cells (P<0.05). Compared with the controls, down-regulation of CXCR3A but up-regulation of CXCR3B significantly inhibited PC-3 cell proliferation and cell invasion ability (P<0.05). Besides, aberrant CXCR3 expression significantly increased expressions of phospholipase C (PLCβ), matrix metallo proteinase (MMP-1), and MMP-3 except MMP-7 in PC-3 cells (P<0.05). Conclusion: The data presented in our study suggested that aberrant CXCR3 expression may play crucial roles in suppressing PC metastasis via inhibiting cell proliferation and invasion ability through the PCLβ signaling pathway.
Keywords: Prostate cancer, PC-3 cell line, cell proliferation, cell invasion, prognosis
Introduction
Prostate cancer (PC) is a worldwide life threatening disorder in male, which accounts for 27% of incident cases in male from year to year [1]. The main treatments methods on PC including surgery, radiotherapy, low temperature operation, chemotherapy, and endocrine therapy [2,3]. However, the mortality and morbidity for PC still remain high due to its hard detection because of the complicate pathologic stage and easy metastasis [4,5]. Therefore, to explore several key targets for the diagnosis in early stage and treatment of PC in clinical will be necessary.
Chemokines are some soluble proteins that bind with homology G protein receptors, and then activate the subunit PLCβ of G protein and PI3K for starting the signal pathway in bodies [6]. Recent evidences have demonstrated that chemokines play pivotal roles in cancer development, progression and metastasis. For example, overexpression of CXCL13 is correlated to breast cancer and may be a diagnostic marker in this malignancy [7]. Also, CCL5 can be secreted by CD4+ T cells in gastric cancer and resulted in cancer progression [8], and Obermajer et al. reported that productions of CXCL12 and CXCR4 could regulate the accumulation of human ovarian cancer [9,10].
There are four types of chemokines based on the position of N-cysteine residues, such as CXC, CC, CX3C, and C type [11]. CXC chemokine play crucial roles in repairing inflammatory response, formation of blood vessel, and migration inflammatory nidus to leukocytes through combining with receptors of neutrophils, lymphocytes, endothelial cells and epithelial cells [12,13]. Papers have referred that CXCR3 was the common receptor for chemokines including CXCL4, CXCR9, and CXCR10, which produced by tumor cells, epithelial cells, and fibroblasts [14,15]. On the other hand, increasing researches have reported that CXCR3 played key roles in cancer metastasis and progression. For instance, Walser et al. proved that CXCR3 performed an inhibition role in lung cancer metastasis in a murine model of metastatic breast cancer [16]. As is known, chemokine CXCR3 has three variants including CXCR3A, CXCR3B, and CXCR3alt, which could mediate suppressing tumor vessels formation [17]. Furuya and his colleagues analyzed the expressions of variants of CXCR3 in ovarian cancer cells and proved their different roles in tumor metastasis and development [18]. CXCR3A has been reported to be associated with tumor cell proliferation [19], while CXCR3B is correlated with inhibiting cell migration and inducing cell apoptosis [20]. Besides, clinical evidence showed that abnormal expression of CXCR3 could regulate the PC cell migration and invasion [21]. Despite several articles have reported the association between CXCR3 expression and tumor migration, the role and mechanism of CXCR3 in PC cell proliferation and metastasis still remain incomplete described.
In this present study, we specific down-regulating the CXCR3 expression in PC-3 cell line based on siRNA slicing method. Comprehensive experimental methods were used to analyze the effects of variants CXCR3A and CXCR3B on PC-3 cell proliferation, invasion and migration abilities. This study aimed to investigate the role of CXCR3 and its potential mechanism in PC progression and metastasis.
Materials and methods
Cell culture and cell proliferation
Human prostate cancer PC-3 cell line (purchased from Invitrogen, USA) was cultured in F12K medium mixed with 10% fetal bovine serum (FBS). siRNA plasmids of CXCR3A and CXCR3B (Invitrogen, USA) were mixed with serum-free medium. PC-3 cells were transfected into 6-well plates and cultured at 37°C in an atmosphere of 5% CO2. After being cultured for 4 h, PC-3 cells (1×105 cells/well) were mixed with 500 ng of CXCR3A and CXCR3B plasmids respectively, and then cultured at 37°C for 48 h. PC-3 cells transfected without siRNA plasmid or with disorder sequence siRNA plasmid were considered as the controls.
qRT-PCR
PC-3 cells in different groups collected at 48 h were grinded in liquid nitrogen and then washed with PBS buffer (PH 7.4) for 3 times. Total RNA from PC-3 cells was extracted using Trizol extraction reagent (Invitrogen, USA) as previously described [22]. Then, RNase-free Dnase I (Promega, Biotech) was added into mixtures to remove DNA. The concentration and purity of extracted RNA was detected using SMA4000 UV-VIS (Merinton, Shanghai, China) at 260 nm. The purified RNA of 0.5 μg/μL was used for cDNA synthesis with the PrimerScript 1st Strand cDNA Synthesis Kit (Invitrogen, USA). Primers used for targets amplification were as follows: CXCR3: sense, 5’-TTCATGCCACCCAGCTCTAC-3’; antisense, 5’-TGAGGTCTCAGACCAGGATGA-3’; CXCR3B sense: 5’-GACAGTTATAGGAGGAGCTGCTC-3’; antisense: 5’-CAGTGTCAGCACCAGCAGC-3’. Expression was detected using Applied Biosystems TaqMan Gene Expression Assay. The total reaction system of 20 μL volume containing 1 μL cDNA from the above PCR, 10 μL SYBR Premix EX Taq, 1 μL each of the primers (10 μM), and 7 μL ddH2O. PCR reaction was carried out at 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Melting curve analysis of amplification products was performed at the end of each PCR to confirm that only one product was amplified and detected. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control.
MTT assay
PC-3 cells transfected with different siRNA vectors at logarithmic stage were cultured in DMEM medium supplemented with 10% FBS. Cells were adjusted to 5×103 cells for injection into the 96-well plates. After being cultured for 24 h, cells were centrifuged at 12,000 rpm, and then supernatant was removed. Followed with addition into 20 μL MTT and then cultured for 4 h. Finally, 150 μL dimethylsulfoxide (DMSO) was used to mix with the cells for 10 min. Absorbance of cells in each well was observed at 570 nm under an absorption spectrophotometer (Olympus, Japan). All experiments were conducted independently for 3 times.
Cell invasion assay
After being transfected for 48 h, PC-3 cells in each group were culture in serum-free F12K medium (Invitrogen, USA) supplemented with 0.2% bull serum albumin (BSA, Sigma, USA) for 24 h. The upper layer was enveloped with serum-free F12K medium mixed with 0.2% BSA while the lower layer was enveloped by F12K medium supplemented with 10% FBS. Transwell treated with 50 mg/L Matrigel (BD, USA) was put into the 24-well plates and then cultured in DMEM medium supplemented with 10% FBS for 48 h while PC-3 cells were cultivated with serum-free DMEM medium in Transwell. PC-3 cells in control group were cultivated in Transwell that treated without Matrigel with the same condition. After being cultured for 48 h, Transwell in each group was washed with PBS buffer to remove cells on upper layer, followed with fixed in ice-cold alcohol. After that, membrane was stained with 0.1% crystal violet for 30 min, and then decolorated with 33% acetic acid. The absorbance of eluents was observed at OD 570 nm with a microplate reader (Biotech, USA). All experiments were conducted independently for 3 times.
Western blotting analysis
PC-3 cells cultured at 48 h in each group were lapped in radioimmunoprecipitation assay (RIPA, Sangon Biotech) lysate containing phenylmethanesufonyl fluoride (PMSF), and then were centrifuged at 12,000 rpm for 5 min at 4°C. Supernatant was collected to measure the concentrations of proteins using bicinchoninic acid (BCA) protein assay kit (Pierce, Rochford, IL).
After that, a total of 30μg protein per cell lysate was subjected to a 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto a polyvinylidencefluoride (PVDF) membrane (Mippore). The membrane was blocked in Tris buffered saline Tween (TBST) mixed with 5% non-fat milk for 1 h, and subsequently incubated with rabbit anti-human antibodies (Caspase-3, Bcl-XL, Cytc, 1:100 dilution, Invitrogen, USA) overnight at 4°C, followed with incubated with hoseradish peroxidase labeled goat anti-rat secondary antibody (1:1000 dilution) at room temperature for 1 h. After that, PVDF was washed with 1×TBST buffer for 10 min with 3 times. Finally, detection was performed using the development of X-ray after chromogenic substrate with an enhanced CEL (chemiluminescence) method. In addition, ß-actin (Sigma, USA) served as the internal control.
Statistical analysis
All data were expressed as mean ± standard error of mean (SEM). Independent sample t-test was used to calculate the difference between two groups using the graph prism 5.0 software (GraphPad Prism, San Diego, CA). Post-hoc Tukey-test was used to calculate the difference among groups. The P<0.05 was defined as statistically significant.
Results
mRNA expression of CXCR3 in PC-3 cell line
The mRNA expression of CXCR3A or CXCR3B was analyzed using RT-PCR analysis (Figure 1). There was no significant difference of CXCR3A mRNA level between Blank and Control group, but when CXCR3A level was significantly down-regulated by siRNA-CXCR3A in CXCR3A group, CXCR3B level would significantly increase in CXCR3B group compared with the two control groups (P<0.05) (Figure 1A). Otherwise, when CXCR3B level was significantly down-regulated by siRNA-CXCR3B, the mRNA level of CXCR3A would significantly increase compared with the negative or positive controls (P<0.05) (Figure 1B). Interestingly, the results performed that down-regulation of CXCR3A level resulted in a high expression of CXCR3B level, and in reverse order from that stated, indicating that there was a negative regulation correlation between CXCR3A and CXCR3B, who were the different two transcription products of the same gene.
Figure 1.
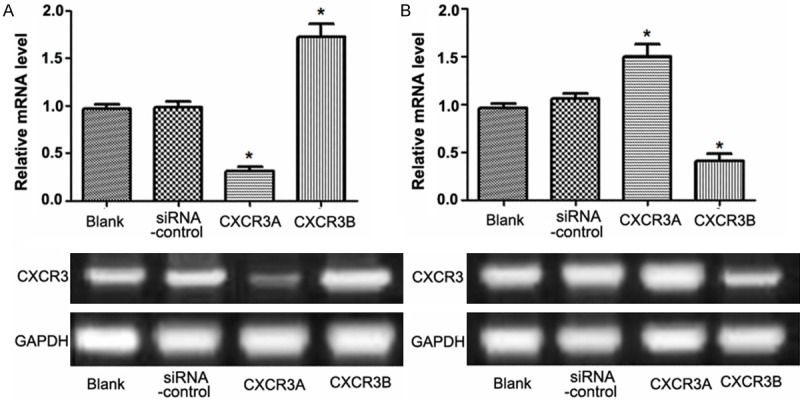
RT-PCR analysis of CXCR3 expression in PC-3 cell line. A: Expression of CXCR3A and CXCR3B in PC-3 cells when CXCR3A was down-regulated by siRNA-CXCR3A; B: Expression of CXCR3A and CXCR3B in PC-3 cells when CXCR3B was down-regulated by siRNA-CXCR3B. *: P<0.05.
MTT assay
In order to analyze the effect of CXCR3A and CXCR3B expressions on PC-3 cells, MTT was used to detect the cell proliferation ability at 24 h (Figure 2). The results showed that down-regulation of CXCR3A could well inhibit cell proliferation ability of PC-3 cells, while down-regulation of CXCR3B could enhance the cell proliferation ability of PC-3 cells (Figure 2).
Figure 2.
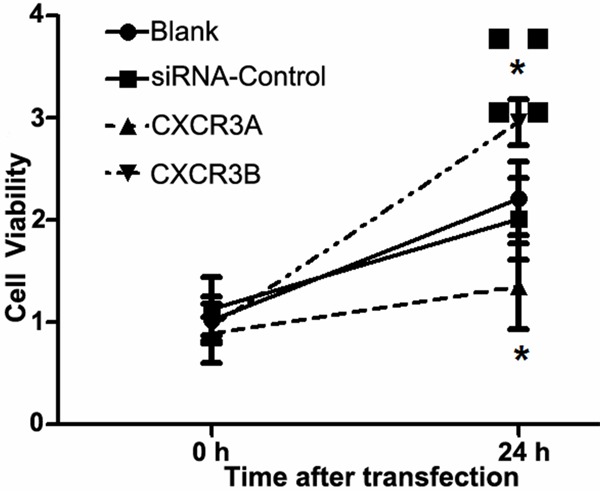
MTT assay on cell proliferation ability. The cell ability of PC-3 cells at 0 h was considered as relative 1, cell proliferation ability was analyzed at 24 h. *: P<0.05.
Cell invasion assay
Matrigel method was used to detect the cell invasion ability of PC-3 cells in four groups (Figure 3). There were about 11-12 invasion cells both in Blank and in Control groups. However, number of invasion cells in siRNA-CXCR3A group was 5.8, but invasion cells in siRNA-CXCR3B group was about 18.3, suggesting that down-regulation of CXCR3A and up-regulation of CXCR3B could inhibit PC-3 cell invasion ability.
Figure 3.
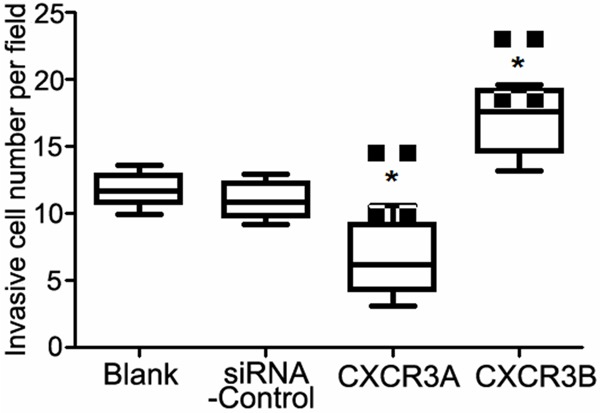
Invasion analysis of PC-3 cells in four groups. *: compared with the Blank group, P<0.05.
Western blotting analysis
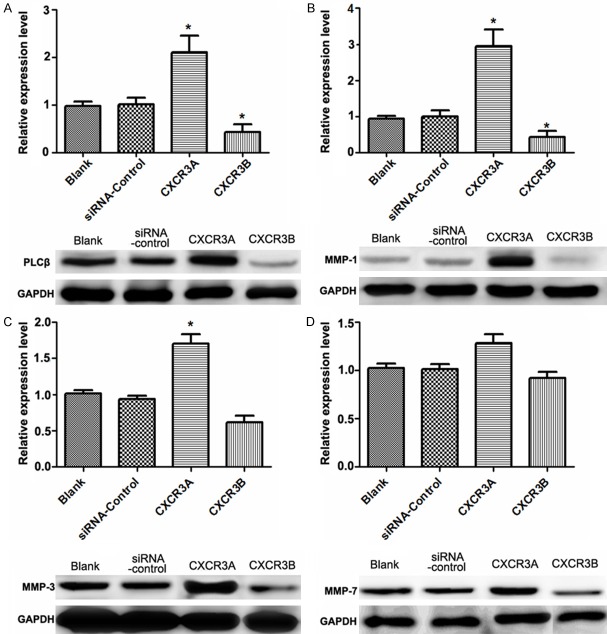
In order to analyze the effect of CXCR3 on downstream proteins expression, western blotting analysis was used to detect the expressions of downstream proteins (Figure 4). When CXCR3A level was down-regulated, the expressions of phospholipase C (PLCβ), matrix metallo proteinase (MMP-1), and MMP-3 were significantly increased compared with the control group (Figure 4A-C). Otherwise, expressions of PLCβ, MMP-1, and MMP-3 were all significantly decreased when CXCR3B was down-regulated. However, there was no obvious change of MMP-7 expression in the two conditions (Figure 4D).
Figure 4.
Western blotting analysis of CXCR3 downstream proteins expressions. A: Relative expression of PLCβ in four groups; B: Relative expression of MMP-1 in four groups; C: Relative expression of MMP-3 in four groups; D: Relative expression of MMP-7 in four groups. *: P<0.05.
Discussion
PC is a worldwide life threatening urinary system disorder in male, which has high morbidity and mortality due to its easy bone metastasis and hard diagnosis [1]. Chemokine CXCR3 has been reported to play key roles in endothelial cells growth and migration [19,20]. In this study, we specifically down-regulating the expression of CXCR3A or CXCR3B with gene silencing method to investigate the potential role and mechanism of CXCR3 in PC metastasis and migration based on the model of PC-3 cells line. Our data showed that down-regulation of CXCR3A induced up-regulation of CXCR3B in PC-3 cells (P<0.05). Besides, down-regulation of CXCR3A and up-regulation of CXCR3B could inhibit PC-3 cell proliferation and suppress cell invasion ability. Moreover, down-regulation of CXCR3A induced the increasing expressions of MMP-1, MMP-3, and PLCβ except MMP-7.
CXCR3 (a pertussis toxin) is a seven transmembrane domain-spanning G protein-couple receptor, that can bind the proinflammatory and CXC chemokines, and several pathways, including MAPK, PI3K signaling pathways [23]. Andreas have proved that intestinal myofibroblasts expressed CXCR3 variants with different signaling properties, and several chemokines such as CXCL9 and CXCL10 could bind with CXCR3 may be useful for inflammatory disease alternative therapy [24]. CXCR3A is expressed on TH1 T cells and cytotoxic CD8+ T cells, which could mediate cell migration while CXCR3B has been proved to be associated with inducing cell apoptosis in endothelial cells [25]. Coincidence with former evidences, in this study, down-regulation of CXCR3A will cause the up-regulation of CXCR3B in PC-3 cells and in reverse order from that stated, indicating that there was a negative regulation correlation between CXCR3A and CXCR3B expression.
Meanwhile, our data presented that down-regulation of CXCR3A and up-regulation of CXCR3B inhibited PC-3 cell proliferation and invasion ability, implying that CXCR3 may correlate to PC-3 cell proliferation and invasion. Role of CXCR3 in prostate cancer has not been fully discussed, however, previous papers have demonstrated that overexpression of CXCR3 was expressed in breast cancer and mediated cellular functions, and was associated with poor survival and promoted metastasis in breast cancer model [26,27]. Gene silencing of CXCR3B promoted cell proliferation of breast tumor cells [27]. Besides, gene silencing of CXCR3 in melanoma B16F10 cells suppressed the development of lymph node metastasis, suggesting that CXCR3 was correlated to melanoma metastasis [28]. Additionally, Well and his colleagues proved that mRNA level of CXCR3A was increased while mRNA level of CXCR3B was decreased in PC cells, and abnormal expression of CXCR3 isoform regulated PC cell migration and invasion [21]. Based on our results, we speculated that mRNA expression pattern of CXCE3A and CXCR3B leading to abnormal expression of CXCR3, and then resulting in inhibiting cell proliferation and cell invasion.
Additionally, our results performed that down-regulation of CXCR3A and up-regulation of CXCR3B brought about the increased expressions of PLCβ, MMP-1, and MMP-3 except MMP-7 in PC-3 cells, indicating that aberrant CXCR3 expression may affect PC-3 cells via the downstream signaling pathway. PLCβ is a member of phospholipases family enzyme that hydrolyze phospholipids into fatty acids and other lipophilic molecules [29], while MMP-1, MMP-3, and MMP-7 belong to MMP family proteins that are involved in the breakdown of extracellular matrix in normal physiological processes, such as reproduction and tissue remodeling, as well as in disease processes [30]. It has been said that activated PLCβ play pivotal roles in tumor cell proliferation, growth, and differentiation through signaling transduction, such as gastric cancer, colon cancer, and carcinoma of urinary bladder [31,32]. The two isoforms of CXCR3 appear to activate different downstream signaling pathways. Smit and his colleagues have demonstrated that CXCR3A and CXCR3B could activate PLC-dependent pathway [33]. Therefore, down-regulation of CXCR3A and up-regulation of CXCR3B may promote PC-3 cell metastasis through activating PLCβ signal. On the other hand, Laragione said that activated CXCR3 directly regulated MMP-1 and MMP-3 activation [34]. Zinzindohoue said that MMP-1 and MMP-3 expressions were correlated to colorectal cancer metastasis and invasion [35]. Thus, we speculated that aberrant expression of CXCR3 may result in PC metastasis through increasing MMP-1 and MMP-3 expresions. Previous paper showed that MMP-7 overexpression was found in PC tissue [36], Interestingly, our results performed that there was no correlation between aberrant CXCR3 expression and MMP-7 expression in PC-3 cells, indicating that there may be no association between CXCR3 in PC metastasis and MMP-7 expression.
In conclusion, our study suggests that CXCR3 play key roles in PC cell proliferation and invasion. Aberrant CXCR3 expression inhibited PC cell proliferation and invasion. However, down-regulation of CXCR3A and up-regulation of CXCR3B increased expressions of MMP-1 and MMP3, and activated PLCβ signaling pathway in PC-3 cells. Our study may provide basis for the potential exploration of CXCR3 in suppressing PC metastasis and progression in clinical. However, further experimental studies are still needed to seek the underlying mechanism of CXCR3 in PC.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Pei X, Chou JF, Schechter M, Kollmeier M, Cox B, Yamada Y, Fidaleo A, Sperling D, Happersett L. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases–free survival outcomes. Eur Urol. 2011;60:1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barekzi N, Laroussi M. Effects of low temperature plasmas on cancer cells. Plasma Processes and Polymers. 2013;10:1039–1050. [Google Scholar]
- 4.De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F. Abiraterone and increased survival in metastatic prostate cancer. New England J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panse J, Friedrichs K, Marx A, Hildebrandt Y, Luetkens T, Bartels K, Horn C, Stahl T, Cao Y, Milde-Langosch K. Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. Br J cancer. 2008;99:930–938. doi: 10.1038/sj.bjc.6604621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugasawa H, Ichikura T, Kinoshita M, Ono S, Majima T, Tsujimoto H, Chochi K, Hiroi S, Takayama E, Saitoh D. Gastric cancer cells exploit CD4+ cell-derived CCL5 for their growth and prevention of CD8+ cell-involved tumor elimination. Int J Cancer. 2008;122:2535–2541. doi: 10.1002/ijc.23401. [DOI] [PubMed] [Google Scholar]
- 9.Bedognetti D, Wang E, Marincola FM. Meta-analysis and metagenes: CXCL-13-driven signature as a robust marker of intratumoral immune response and predictor of breast cancer chemotherapeutic outcome. Oncoimmunology. 2014;3:e28727. doi: 10.4161/onci.28727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE2-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–7470. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilbanks A, Zondlo SC, Murphy K, Mak S, Soler D, Langdon P, Andrew DP, Wu L, Briskin M. Expression cloning of the STRL33/BONZO/TYMSTR ligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol. 2001;166:5145–5154. doi: 10.4049/jimmunol.166.8.5145. [DOI] [PubMed] [Google Scholar]
- 12.Grbic DM, Degagné É, Langlois C, Dupuis AA, Gendron FP. Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol. 2008;180:2659–2668. doi: 10.4049/jimmunol.180.4.2659. [DOI] [PubMed] [Google Scholar]
- 13.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Ann Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 14.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller A, Meiser A, McDonagh EM, Fox JM, Petit SJ, Xanthou G, Williams TJ, Pease JE. CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J Leukoc Biol. 2008;83:875–882. doi: 10.1189/jlb.1006645. [DOI] [PubMed] [Google Scholar]
- 16.Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66:7701–7707. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 17.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp Cell Res. 2011;317:685–690. doi: 10.1016/j.yexcr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuya M, Yoneyama T, Miyagi E, Tanaka R, Nagahama K, Miyagi Y, Nagashima Y, Hirahara F, Inayama Y, Aoki I. Differential expression patterns of CXCR3 variants and corresponding CXC chemokines in clear cell ovarian cancers and endometriosis. Gynecol Oncol. 2011;122:648–655. doi: 10.1016/j.ygyno.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 19.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98:617–625. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Q, Dhir R, Wells A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Mol Cancer. 2012;11:3. doi: 10.1186/1476-4598-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27:126–139. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. e193. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouroumalis A, Nibbs RJ, Aptel H, Wright KL, Kolios G, Ward SG. The chemokines CXCL9, CXCL10, and CXCL11 differentially stimulate Gαi-independent signaling and actin respon- ses in human intestinal myofibroblasts. J Immunol. 2005;175:5403–5411. doi: 10.4049/jimmunol.175.8.5403. [DOI] [PubMed] [Google Scholar]
- 25.García-López MÁ, Sánchez-Madrid F, Rodríguez-Frade JM, Mellado M, Acevedo A, García MI, Albar JP, Martínez-A C, Marazuela M. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab Invest. 2001;81:409–418. doi: 10.1038/labinvest.3780248. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, Lipsky M, Li Y, Holt D, Fulton A. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther. 2009;8:490–498. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]
- 27.Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Res. 2006;66:9509–9518. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]
- 28.Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 29.Katan M. Families of phosphoinositide-specific phospholipase C: structure and function. Biochim Biophys Acta. 1998;1436:5–17. doi: 10.1016/s0005-2760(98)00125-8. [DOI] [PubMed] [Google Scholar]
- 30.Woessner J, Parks W, Mecham R. The matrix metalloproteinase family. Matrix Metalloproteinases. 1998:1–14. [Google Scholar]
- 31.Ou L, Guo Y, Luo C, Wu X, Zhao Y, Cai X. RNA interference suppressing PLCE1 gene expression decreases invasive power of human bladder cancer T24 cell line. Cancer Genet Cytogenet. 2010;200:110–119. doi: 10.1016/j.cancergencyto.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Park JB, Lee CS, Jang JH, Ghim J, Kim YJ, You S, Hwang D, Suh PG, Ryu SH. Phospholipase signalling networks in cancer. Nat Rev Cancer. 2012;12:782–792. doi: 10.1038/nrc3379. [DOI] [PubMed] [Google Scholar]
- 33.Smit MJ, Verdijk P, van der Raaij-Helmer EM, Navis M, Hensbergen PJ, Leurs R, Tensen CP. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi-and phospholipase C–dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood. 2003;102:1959–1965. doi: 10.1182/blood-2002-12-3945. [DOI] [PubMed] [Google Scholar]
- 34.Laragione T, Brenner M, Sherry B, Gulko PS. CXCL10 and its receptor CXCR3 regulate synovial fibroblast invasion in rheumatoid arthritis. Arthritis Rheum. 2011;63:3274–3283. doi: 10.1002/art.30573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zinzindohoué F, Lecomte T, Ferraz JM, Houllier AM, Cugnenc PH, Berger A, Blons H, Laurent-Puig P. Prognostic significance of MMP-1 and MMP-3 functional promoter polymorphisms in colorectal cancer. Clin Cancer Res. 2005;11:594–599. [PubMed] [Google Scholar]
- 36.Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]