Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver tumor. Due to the asymptomatic nature of early HCC and lack of effective screening strategies, 80% of patients present with advanced HCC at the time of diagnosis. Novel molecular marker identification will be valuable for effective diagnosis and treatment. In this study we reported HCLS1-associated protein X-1 (HAX-1) is overexpression in HCC in human HCC sample. Furthermore, we provided evidence that HAX-1 expression positively correlated with that of Ki67 in patient sample. Statistic analysis indicated that HAX-1 expression level significantly correlated with clinic outcome of HCC. Cell based assay revealed that knockdown of HAX-1 inhibits cell proliferation. This result suggests that HAX-1 can be a novel molecular marker for HCC.
Keywords: Human hepatocellular carcinoma (HCC), HAX-1, cell proliferation
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver tumor and the fifth most common malignancies worldwide and the third leading cause of cancer-related death in the world [1,2]. HCC is a highly aggressive disease and resistant to systematic therapies and the 5-year postoperative survival rate is 30%-40% [3,4]. Most cases of HCC are secondary to either a virus infection or cirrhosis. Chronic infections with hepatitis B or C virus and aflatoxin B1 are responsible for most HCC cases [5-7].
The standard treatments for early stage HCC are surgical resection and liver transplantation. However, due to the asymptomatic nature of early HCC and lack of effective screening strategies, 80% of patients present with advanced HCC at the time of diagnosis [8]. Therefore, identification and investigation of new genes and signaling pathway involved in HCC will be valuable for effective diagnosis and treatment. Significant progress has been made towards better understanding of molecular basis of HCC. Several major cellular signaling pathways have been implicated in HCC, such as Wnt, VEGF and Hippo [4,9]. Yes-associated protein (YAP), downstream effector of Hippo pathway, was recently linked to hepatocarcinogenesis in a mouse HCC model. Mechanistic studies indicated that elevated YAP activity promotes adult hepatocytes into cells bearing progenitor characteristics [10].
HCLS1-associated protein X-1 (HAX-1) was originally identified as a protein interacting with HS-1 (hematopoietic lineage cell-specific protein-1), a Src kinase substrate, and was suggested to be involved in B cell signal transduction [11]. HAX-1 interacts with a variety of structurally unrelated proteins, suggesting its involvement in intracellular signaling and in cytoskeletal control and shuttling of various intracellular molecules [11-13]. The human HAX1 gene is located within the epidermal differentiation complex on chromosome 1 (1q21). HAX-1 is ubiquitously expressed in human and murine tissues and is reported to be localized in mitochondria as well as the nuclear membrane and endoplasmic reticulum (ER). The HAX-1 protein has multiple biological functions, such as anti-apoptosis, cell migration and endocytosis. HAX-1 is involved in invasion, metastasis and genesis of various types of tumors [14]. Emerging evidence indicated that HAX-1 expression was elevated in several solid tumors, such as oral squamous cell carcinoma, lung cancer and breast cancer. These data suggest that deregulation of HAX-1 expression plays a key role in oncogenesis.
Previous studies have revealed that up-regulation of HAX-1 was observed in HCC [15]. However, the relationship between HAX-1 and clinicopathological character of HCC is still unknown. In the present study, we investigated HAX-1 function in HCC and the potential mechanism of HAX-1 involvement in HCC.
Materials and methods
Patients and tissue samples
HCC tumor samples were obtained from patients undergoing hepatic surgical resection without preoperative chemotherapy at the Affiliated Hospital of Nantong University between the years of 2002 and 2005. This study was approved by the ethics committee of Nantong University. All the patients gave an informed consent about the usage of tissues for research purposes.
The 100 HCC cases include 84 males and 16 females, ranging from 35 to 84 years, with a median age of 59.5 years. The clinical features of the patients, including age, gender, histological grade, tumor size, metastasis and Ki-67 are shown in Table 1. Resected specimens were classified according to the International Union Against Cancer TNM classification system [16]. Histological grades were classified to well (grade I; n = 30), moderately (grade II; n = 52), and poorly differentiated tumors (grade III; n = 18). Details are shown in Table 1. For histological examination, all tumor and surrounding nontumorous tissue portions were fixed in formalin and embedded in paraffin. Fresh samples were frozen in liquid nitrogen immediately after surgical removal and maintained at -80°C.
Table 1.
HAX-1 and Ki-67 expression and clinicopathologic characterization in HCC samples
| Criteria | HAX-1 expression | Ki67 expression | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Low (< 0.46) | High (≥ 0.46) | P valuea | Low (< 33.5) | High (≥ 33.5) | P valuea | |
| Age (y) | ||||||
| ≤ 40 | 15 | 13 | 0.957 | 13 | 15 | 0.656 |
| > 40 | 39 | 33 | 37 | 35 | ||
| Gender | ||||||
| Male | 50 | 34 | 0.011* | 45 | 39 | 0.102 |
| Female | 4 | 12 | 5 | 11 | ||
| Histological grade | ||||||
| Well | 22 | 8 | 0.008* | 19 | 11 | 0.001* |
| Mod | 27 | 25 | 29 | 23 | ||
| Poor | 5 | 13 | 2 | 16 | ||
| Tumor size (cm) | ||||||
| ≤ 5 | 38 | 18 | 0.001* | 32 | 25 | 0.183 |
| > 5 | 16 | 28 | 18 | 25 | ||
| HBsAg | ||||||
| Negative | 5 | 6 | 0.875 | 6 | 4 | 0.751 |
| Positive | 47 | 42 | 45 | 45 | ||
| Cirrhosis | ||||||
| Negative | 33 | 18 | 0.028* | 29 | 22 | 0.161 |
| Positive | 21 | 28 | 21 | 28 | ||
| Serum AFP level (ng/mL) | ||||||
| Low (< 40) | 28 | 26 | 0.641 | 32 | 22 | 0.045* |
| High (≥ 40) | 26 | 20 | 18 | 28 | ||
Abbreviations: HBsAg = hepatitis B surface antigen; AFP = alpha fetoprotein. NOTE. Statistical analyses were performed by the Pearson χ2 test.
P < 0.05 is considered significant.
Immunohistochemistry methods
Specific experimental methods can be found in the study by Ke et al [17].
Antibodies
The antibodies used for immunohistochemistry included: anti-HAX-1 rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Ki-67 mouse monoclonal antibody (Zymed Laboratories, San Francisco, CA), and a nonspecific immunoglobulin IgG (Sigma Chemical Co., St. Louis, MO, USA). The antibodies for western blot were anti-HAX-1 (Santa Cruz Biotechnology), anti–PCNA (Santa Cruz Biotechnology), anti–β-actin (Santa Cruz Biotechnology), and anti-GAPDH (Santa Cruz Biotechnology), Antibodies against YAP, c-Myc, cyclinD1, p16 and p21 were purchased from Santa Cruz Biotechnology.
Evaluation of the results of immunohistochemical staining
Two observers (Y.X.w and X.H) independently evaluated the immunostaining results, similar results were obtained in these samples. For assessment of HAX-1 and Ki-67, at least five fields were randomly chosen and cytoplasmic (nuclear) staining was examined under microscope with high magnification. More than 500 cancer cells were counted to determine the mean value, which represented the percentage of immunostained cells relative to the total number of cancer cells [17].
Cell culture and cell cycle analysis
HCC cell lines (HUH7, Hep3B, and SMMC-7721) and normal liver cell lines [16] were obtained from the Shanghai Institute of Cell Biology, Academic Sinica, and were cultured in RPMI 1640 (GibCo BRL, Grand Island, NY, USA) supplemented or Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS), penicillin 100 U/ml, and streptomycin 100 μg/mL at 37°C in a humidified chamber containing 5% CO2 [17].
Western blot analysis
Specific experimental methods can be found in the study by Ke et al [17].
siRNA and transfection
Small interference RNAs (siRNA) were chemically synthesized (GenePharma Co. Ltd). The synthesized oligonucleotides for RNA interference (RNAi) HAX-1 targeted the sequence: 5’-CCGAATTCCCATGAGCCTCTTTCATCTCTTCC-3’. Nonspecific scrambled siRNA with a sequence of 5’-AAGGTACCCCGGGACCGGAACCAACG-3’ was used as a negative control. The transfections of HAX-1 siRNA were performed with Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. SMMC-7721 cells that were not treated served as mock-treated cells.
Cell proliferation assay
Cell viability was measured using the Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) assay following the manufacturer’s instructions. In brief, cells were seeded on a 96-well cluster plate (Corning Inc, Corning, NY, USA) at a concentration of 2 × 104/well in a volume of 100 μl and grown overnight. After adding CCK-8 reagents to each well under different treatments, the cells were incubated for 2 h at 37°C. Each experiment was performed in triplicate and repeated at least three times.
Statistical analysis
Statistical analysis was performed using the StatView 5.0 software package (SAS Institute Inc.). The association between HAX-1 and Ki-67 expression and clinicopathological variables was analyzed using χ2 test. The correlation between Ki-67 and HAX-1 were studied using the Pearson rank correlation test. For analysis of survival data, Kaplan-Meier curves were constructed, and the log-rank test was performed. Multivariate analysis was performed using Cox’s proportional hazards model, with P < 0.05 considered statistically significant. The results are expressed as the mean ± SE.
Results
HAX-1 expression level is elevated in HCC
Previous studies indicated that HAX-1 expression is upregulated in different cancer, such as breast cancer, lung cancer and melanoma [18]. HAX-1 also has been reported to interact with hepatitis C virus core protein, leading to the p53-dependent caspase-7 activation and hepatocyte growth inhibition [15]. However, HAX-1 expression and function in HCC is unknown. To bring insight into this question, we first analyzed the protein expression level of HAX-1 in human HCC tissue by immunohistochemistry. A total of 100 samples from patients with HCC were used in this study. The demographic and clinicopathologic features of the patients in this study were listed in Table 1. Representative images of HAX-1 expression in HCC and adjacent normal tissue were shown in Figure 1. Our results indicated that HAX-1 was highly expressed in HCC tumor tissue compared to the normal tissue (Figure 1A, 1B). HAX-1 immunoreactivity was mainly limited to the cytoplasm (Figure 1B). The stained HCC samples were grouped into low (scores 0-4) and high (scores 6-12) expression according to the Remmele Scale [19]. The results of the immunohistochemical analysis of HAX-1 expression in HCC are summarized in Table 1. Statistical significance for HAX-1 expression was assessed with χ2 test. The high levels of expression, significantly exceeding the level of normal control, were observed. Expression of HAX-1 was significantly correlated with histological grade (P = 0.008), gender (P = 0.011), tumor size (P = 0.001), and cirrhosis (P = 0.028), but there was no direct relationship between HAX-1 expression and other prognostic factors (Table 1).
Figure 1.
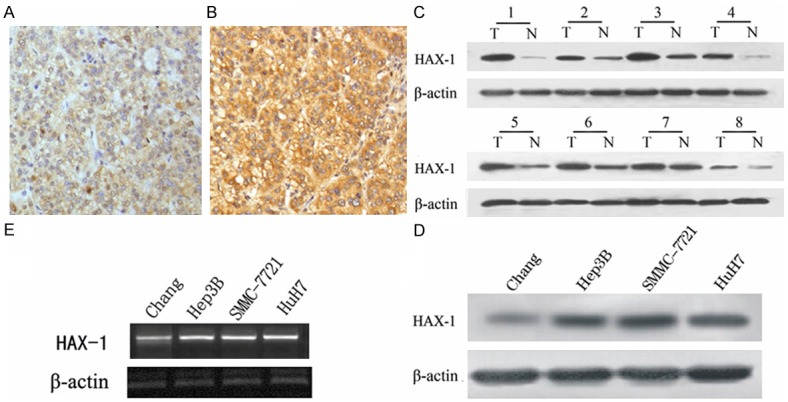
HAX-1 expression is elevated in HCC tissue. Paraffin-embedded tissue sections were stained with HAX-1 antibody and counterstained with hematoxylin. A. HAX-1 staining in adjacent normal tissues (×400). B. HAX-1 staining in HCC tissue (×400). C. Expression of HAX-1 in 8 representative paired samples of HCC tissue (T) and adjacent normal tissues (N). D. HAX-1 expression in cell line. E. HAX-1 expression at mRNA level in different cell lines. β-actin was used as a loading control. The same experiment was repeated at least 3 times.
To verify the results obtained from IHC staining, we examined HAX-1 expression in both normal (adjacent to tumor) and tumor tissue using western blot. This type of samples enables matched pair analysis. In agreement with our IHC data, western blot results also indicated a high HAX-1 expression in HCC tissue compared to normal tissue (Figure 1C).
We next compared HAX-1 expression levels in three human hepatocarcinoma cell lines (Hep-3B, HuH7 and SMMC-7721) and one normal liver cell lines Chang at both protein and mRNA levels. The endogenous HAX-1 in these three tumor cell lines was higher than that of the normal liver cell lines (Figure 1D and 1E). Collectively, our results showed that HAX-1 is highly expressed in HCC tissue.
HAX-1 expressions correlates with that of Ki67
We examined Ki67 expression in both normal and HCC tissue. The representative staining images were shown in Figure 2A and 2B. To better understand the clinicopathological significance of HAX-1 expression in HCC, we evaluated the association of HAX-1 and Ki-67 expression with clinicopathological variables. For statistical analysis of HAX-1 and Ki-67 expression, the carcinoma specimens were divided into either high or low expression group, according to the staining of HAX-1 and Ki-67 positive cells base on a mean value of 46% and 33.5% for HAX-1 and Ki-67, respectively. We observed that in most specimens HAX-1 staining showed a similar pattern as that of Ki67. So we further analyzed the correlation between HAX-1 and Ki-67. Our data suggested that the expression levels of HAX-1 directly correlated with those of Ki-67 in HCC tumor tissue base on Pearson’s correlation analysis (P < 0.01) (Figure 2C).
Figure 2.
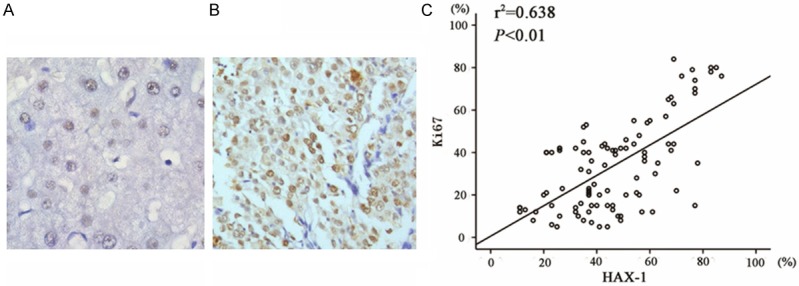
HAX-1 expression correlates with Ki67 expression in HCC tissue. A. Ki-67 staining was shown in normal human hepatic tissue (×400). B. Ki-67 staining in HCC (400x). C. Scatter plot of Ki-67 versus HAX-1 with regression line showing a positive correlation using the Spearman’s correlation coefficient.
HAX-1 overexpression indicates poor clinic outcome
With the available survival information for the patients in this study, we analyzed the relationship between HAX-1 expression level and clinic outcome. Among these patients, 50% (23 out of 46) of patients with high HAX-1 expression and 50% (27 out of 54) with low HAX-1 expression survived the cancer (Table 2). When all variables were compared separately with survival status, HAX-1 (P = 0.027), Ki-67 (P = 0.001), Cirrhosis (P = 0.022) and tumor size (P = 0.032) significantly influenced survival (Table 2). In univariate analysis, the Kaplan-Meier survival curves showed that high HAX-1 expression correlated with poor survival with statistical significance (Figure 3A). The Cox proportional hazards regression model proved that HAX-1 (P < 0.05), Ki-67 (P < 0 .05), Gender (P < 0.05), HBsAg (P < 0.05) and tumor size (P < 0.05) were independent prognostic factors (Table 3).
Table 2.
Survival status and clinicopathologic parameters in 100 HCC specimens
| Total | Survival status | P | ||
|---|---|---|---|---|
|
|
||||
| Alive | Dead | |||
| Age (years) | ||||
| ≤ 45 | 28 | 14 | 14 | 0.058 |
| > 45 | 72 | 46 | 26 | |
| Gender | ||||
| Male | 84 | 47 | 37 | 0.058 |
| Female | 16 | 13 | 3 | |
| Histological grade | ||||
| Well | 30 | 18 | 12 | 0.203 |
| Mod | 52 | 28 | 24 | |
| Poor | 28 | 14 | 4 | |
| Tumor size (cm) | ||||
| ≤ 5 | 57 | 29 | 28 | 0.032* |
| > 5 | 43 | 31 | 12 | |
| HBsAg | ||||
| Negative | 10 | 5 | 5 | 0.496 |
| Positive | 90 | 55 | 35 | |
| Cirrhosis | ||||
| Negative | 51 | 25 | 26 | 0.022* |
| Positive | 49 | 35 | 14 | |
| AFP (ng/mL) | ||||
| ≤ 50 | 54 | 31 | 23 | 0.566 |
| > 50 | 46 | 29 | 17 | |
| HAX-1-1 | ||||
| Low expression | 54 | 27 | 27 | 0.027* |
| High expression | 46 | 23 | 13 | |
| Ki67 | ||||
| Low expression | 52 | 23 | 29 | 0.001* |
| High expression | 48 | 37 | 11 | |
Abbreviations: HBsAg = hepatitis B surface antigen; AFP = alpha fetoprotein. NOTE. Statistical analyses were performed by the Pearson χ2 test.
P < 0.05 is considered significant.
Figure 3.
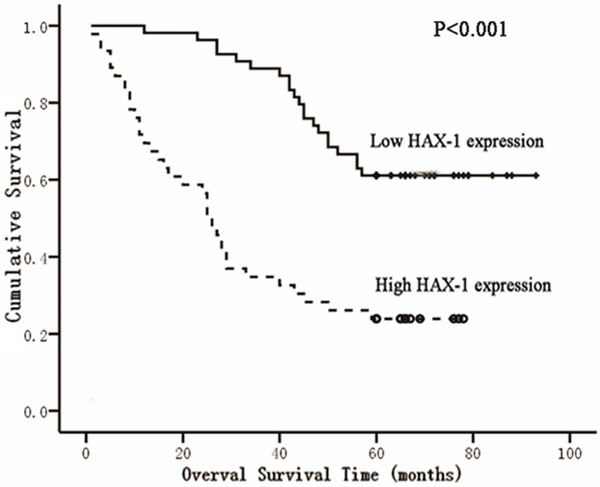
HAX-1 overexpression indicates poor clinic outcome. A. Kaplan-Meier survival curves for low HAX-1 expression versus high HAX-1 expression in 100 patients of HCC showed a highly significant separation (P < 0.001, log rank test).
Table 3.
Contribution of various potential prognostic factors to survival by Cox regression analysis in 100 HCC specimens
| Hazard ratio | 95% Confidence interval | Pa | |
|---|---|---|---|
| Age (year) | 1.326 | 0.700-2.509 | 0.386 |
| Gender | 3.682 | 1.583-8.563 | 0.002* |
| Tumor grade | 1.282 | 0.862-1.908 | 0.220 |
| Tumor size | 2.004 | 1.085-3.703 | 0.026* |
| Serum AFP level | 0.927 | 0.477-1.805 | 0.824 |
| HBsAg | 5.390 | 1.323-21.949 | 0.019* |
| Cirrhosis | 0.780 | 0.415-1.466 | 0.441 |
| HAX-1 | 0.227 | 0.115-0.449 | 0.000* |
| Ki67 | 1.029 | 1.007-1.053 | 0.012* |
Statistical analyses were performed by the Cox regression analysis.
P < 0.05 is considered significant.
HAX-1 knockdown inhibits cell proliferation in SMMC-7721 cell
To further investigate the effect of HAX-1 on cell proliferation, we used chemically synthesized siRNA to knock down HAX-1 expression in SMMC-7721 cells. The knockdown efficiency of siRNA was assessed 48 hours after transfection by western blot. The results showed that the HAX-1 siRNA effectively decreased the expression of HAX-1 in SMMC-7721 cells (Figure 4A). Using CCK-8 assay, we found that cell proliferation rate of SMMC-7721 cells treated with siRNA exhibited a significant decrease compared with the control siRNA or mock-treated cells (Figure 4B). Additional molecular markers involved in cell proliferation of HCC were examined [20-24]. We found that expression levels of c-myc, cyclinD1 and YAP were decreased with the depletion of HAX-1 (Figure 4A). On the other hand, p16 and p21 expression were elevated (Figure 4A).
Figure 4.
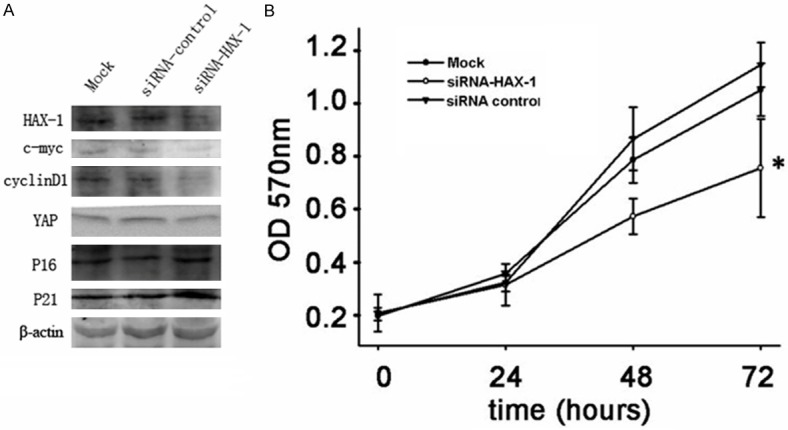
HAX-1 knockdown inhibits cell proliferation in SMMC-7721 cell. A. SMMC-7721 cells were transfected with HAX-1 siRNA or control siRNA. 48 hrs after transfection, cell lysis were collected and indicated protein levels were examined by western blot. β-Actin was used as a loading control. B. Cell proliferation was measured using the CCK-8 assay. Cell Counting Kit-8 reagents were added to the medium and incubated for additional 2 hours before absorbance was measured. The data are means ± SEM (P < 0.05).
Taken together, these results suggested that HAX-1 knockdown inhibit cell proliferation by regulating expression of cell cycle related genes.
Discussion
HCC is one of the most deadly human carcinomas. Despite of extensive research, the prognosis for HCC patients remains extremely poor because of the frequent failure of conventional treatment strategies [25-27]. Therefore, new molecules for diagnosis and therapeutic targets will be valuable to improve the clinic outcome of HCC patients.
In the present study, we first examined HAX-1 protein expression in paired clinical HCC tissues and HCC cell lines using both immunochemical staining and western blot. Our results suggested that HAX-1 was overexpressed in the majority of HCC samples and three HCC cell lines. HAX-1 was mainly located in the cytoplasm of tumor cells. In addition to this, we found a direct correlation between HAX-1 expression and Ki-67 in HCC tumor tissue. We also evaluated the correlation between HAX-1 and clinicopathological features. Our data indicated that high HAX-1 expression was significantly correlated with histological grade, cirrhosis, tumor size, and poor clinic outcome. This data suggest that HAX-1 may be associated with the development and progression of HCC.
Based on the above observation, we proposed that HAX-1 may be involved in cell proliferation of HCC. To explore this possibility, we examined the expression of HAX-1 during cell-cycle progression in SMMC-7721 cell. We found that HAX-1 expression is increased when the cell re-enter into cell cycle from G1 phase arrest. This result indicated that HAX-1 may contribute to hepatocarcinogenesis by regulating cell proliferation of HCC. Consistent with this notion, we further found that HAX-1 knockdown in SMMC-7721 inhibited cell proliferation. Mechanistic studies indicated that HAX-1 knockdown decreased c-Myc, cyclin D1 and YAP expression, while increased p21 and p16 expression. This could the potential molecular basis of HAX-1 overexpression in HCC. Interestingly, HAX-1 has been reported to be overexpressed in different tumor [18,28]. Further studies are needed to reveal whether HAX-1 regulates cell proliferation in other tumor.
In conclusion, our study suggests that HAX-1 expression was elevated in HCC samples and high expression of HAX-1 was associated with histological grade, tumor size, cirrhosis as well as poor clinic outcome. Mechanically, HAX-1 overexpression promoted cell proliferation. Our results provide evidence that HAX-1 may serve as a novel prognostic biomarker and a new molecular therapy target for HCC.
Acknowledgements
Everyone who has contributed to the work has been listed.
Disclosure of conflict of interest
None.
References
- 1.Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32:225–237. doi: 10.1016/s0168-8278(00)80428-6. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J. Clin. Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 7.Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas MB, Abbruzzese JL. Opportunities for targeted therapies in hepatocellular carcinoma. J. Clin. Oncol. 2005;23:8093–8108. doi: 10.1200/JCO.2004.00.1537. [DOI] [PubMed] [Google Scholar]
- 9.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 10.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y, Demoliere C, Kitamura D, Takeshita H, Deuschle U, Watanabe T. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J Immunol. 1997;158:2736–2744. [PubMed] [Google Scholar]
- 12.Kawaguchi Y, Nakajima K, Igarashi M, Morita T, Tanaka M, Suzuki M, Yokoyama A, Matsuda G, Kato K, Kanamori M, Hirai K. Interaction of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP) with HS1-associated protein X-1: implication of cytoplasmic function of EBNA-LP. J Virol. 2000;74:10104–10111. doi: 10.1128/jvi.74.21.10104-10111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radhika V, Onesime D, Ha JH, Dhanase-karan N. Galpha13 stimulates cell migration through cortactin-interacting protein Hax-1. J Biol Chem. 2004;279:49406–49413. doi: 10.1074/jbc.M408836200. [DOI] [PubMed] [Google Scholar]
- 14.Ramsay AG, Keppler MD, Jazayeri M, Thomas GJ, Parsons M, Violette S, Weinreb P, Hart IR, Marshall JF. HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin alphavbeta 6. Cancer Res. 2007;67:5275–5284. doi: 10.1158/0008-5472.CAN-07-0318. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee A, Saito K, Meyer K, Banerjee S, Ait-Goughoulte M, Ray RB, Ray R. Hepatitis C virus core protein and cellular protein HAX-1 promote 5-fluorouracil-mediated hepatocyte growth inhibition. J Virol. 2009;83:9663–9671. doi: 10.1128/JVI.00872-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kee KM, Wang JH, Lee CM, Chen CL, Changchien CS, Hu TH, Cheng YF, Hsu HC, Wang CC, Chen TY, Lin CY, Lu SN. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer. 2007;120:2650–2655. doi: 10.1002/ijc.22616. [DOI] [PubMed] [Google Scholar]
- 17.Ke Q, Ji J, Cheng C, Zhang Y, Lu M, Wang Y, Zhang L, Li P, Cui X, Chen L, He S, Shen A. Expression and prognostic role of Spy1 as a novel cell cycle protein in hepatocellular carcinoma. Exp Mol Pathol. 2009;87:167–172. doi: 10.1016/j.yexmp.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Trebinska A, Rembiszewska A, Ciosek K, Ptaszynski K, Rowinski S, Kupryjanczyk J, Siedlecki JA, Grzybowska EA. HAX-1 overexpression, splicing and cellular localization in tumors. BMC Cancer. 2010;10:76. doi: 10.1186/1471-2407-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woelfle U, Cloos J, Sauter G, Riethdorf L, Janicke F, van Diest P, Brakenhoff R, Pantel K. Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res. 2003;63:5679–5684. [PubMed] [Google Scholar]
- 20.Behne T, Copur MS. Biomarkers for hepatocellular carcinoma. Int J Hepatol. 2012;2012:859076. doi: 10.1155/2012/859076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deane NG, Parker MA, Aramandla R, Diehl L, Lee WJ, Washington MK, Nanney LB, Shyr Y, Beauchamp RD. Hepatocellular carcinoma results from chronic cyclin D1 overexpression in transgenic mice. Cancer Res. 2001;61:5389–5395. [PubMed] [Google Scholar]
- 23.Joo M, Kang YK, Kim MR, Lee HK, Jang JJ. Cyclin D1 overexpression in hepatocellular carcinoma. Liver. 2001;21:89–95. doi: 10.1034/j.1600-0676.2001.021002089.x. [DOI] [PubMed] [Google Scholar]
- 24.Kao JT, Chuah SK, Huang CC, Chen CL, Wang CC, Hung CH, Chen CH, Wang JH, Lu SN, Lee CM, Changchien CS, Hu TH. P21/WAF1 is an independent survival prognostic factor for patients with hepatocellular carcinoma after resection. Liver Int. 2007;27:772–781. doi: 10.1111/j.1478-3231.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 25.Minguez B, Lachenmayer A. Diagnostic and prognostic molecular markers in hepatocellular carcinoma. Dis Markers. 2011;31:181–190. doi: 10.3233/DMA-2011-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer. 2007;43:979–992. doi: 10.1016/j.ejca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–392. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei XJ, Li SY, Yu B, Chen G, Du JF, Cai HY. Expression of HAX-1 in human colorectal cancer and its clinical significance. Tumour Biol. 2014;35:1411–1415. doi: 10.1007/s13277-013-1194-0. [DOI] [PubMed] [Google Scholar]


