Abstract
Objectives: Regulatory B cells (Bregs) have recently been implicated in the pathogenesis of autoimmune diseases. However, their role in the pathogenesis of dilated cardiomyopathy (DCM) remains uncertain. We examined the levels of circulating interleukin 10 (IL-10)-secreting Bregs in patients with DCM. Methods: The frequency of CD19+IL-10+-, CD19+CD5+-, CD19+CD5+CD1d+-, and CD19+CD5+CD1d+IL-10+-B cells in peripheral blood from DCM patients, heart failure (HF) control patients, and healthy controls (HCs) were analyzed by flow cytometry. Results: DCM and HF control patients showed significantly elevated heart rate, increased left ventricular end-diastolic and left ventricular end-systolic diameters, higher levels of serum brain natriuretic peptide and reduced left ventricular ejection fraction, compared to subjects in the HC group. DCM patients showed significantly elevated levels of circulating CD19+IL-10+-, CD19+CD5+-, CD19+CD5+CD1d+-, and CD19+CD5+CD1d+IL-10+-B cells than HF control and HC patients (all P < 0.05). Conclusions: DCM patients exhibited elevated peripheral blood IL-10-secreting B cell levels, suggesting that IL-10-secreting B cells may play an important role in the pathogenesis of DCM.
Keywords: IL-10-secreting B cells, regulatory B cells, dilated cardiomyopathy
Introduction
Dilated cardiomyopathy (DCM) is a condition of the heart characterized by systolic dysfunction and ventricular dilation that may progress into terminal heart failure and, eventually, heart transplantation [1,2]. Myocyte injury and myocardial fibrosis, occurring as a result of overactive immune responses after viral heart infection, are critical factors in the progression of DCM [3]. Nevertheless, the underlying mechanisms responsible for the development of DCM are not completely understood.
Regulatory B cells (Bregs) are a small subset of B cells that have important roles as suppressors of inflammation and other autoimmune responses [4,5]. Various phenotypic variants of Bregs have been identified that express different cell markers, with emerging evidence indicating that a subset of Bregs secrete interleukin-10 (IL-10), a cytokine that plays a key role in the suppression of autoimmune responses [6-8]. Bregs were first defined as a subset of B cells expressing the CD5 and CD1d cell markers [9]. Subsequent studies showed that CD5+CD1d+ B cells were associated with the suppression of inflammation and autoimmune disease through IL-10 secretion [10]. We previously showed that the level of IL-10-producing B cells was increased in a mice model of CVB3-induced acute viral myocarditis (AVMC) [11]. Regardless of the close relationship between AVMC and DCM, little is known about the function of Bregs in DCM.
In our present study we analyzed the percentage of circulating CD19+IL-10+-, CD19+CD5+-, CD19+CD5+CD1d+-, and CD19+CD5+CD1d+IL-10+-B cells in patients with DCM. Our results may provide new insights into the role of Breg cells in DCM.
Materials and methods
Patients and controls
The study, conducted from December 2012 to December 2014, included 90 individuals: 30 patients with primary DCM exhibiting different stages of heart failure (DCM group); 30 patients presenting heart failure due to causes other than DCM, such as ischemic heart disease and valvular heart disease (HF control group); and 30 gender-, age-, and ethnicity-matched healthy individuals (HC group).
DCM was diagnosed in patients in accordance with World Health Organization criteria and guidelines [12]. HF controls were included in the study in an attempt to diminish the potential confounding effects caused by the etiological heterogeneity of heart failure. A diagnosis of DCM was discarded in HC individuals by a normal echocardiogram. Potential subjects presenting inflammatory or autoimmune disease, diabetes mellitus, or other underlying acute or chronic disease, were receiving anti-inflammatory or immunosuppressive medications, or had recently been treated with antibiotics were excluded from the cohort.
The Guangxi Medical University Ethics Committee (Guangxi, China) approved the experimental protocol. Each subject provided written informed consent to participate in the study. Clinical characteristics of the study subjects are summarized in Table 1.
Table 1.
Clinical characteristics of DCM patients and control individuals
| Characteristics | HC N = 30 | HF N = 30 | DCM N = 30 |
|---|---|---|---|
| Age (years) | 41.9 ± 6.4 | 43.6 ± 7.2 | 40.2 ± 6.1 |
| Male/female (n) | 19/11 | 20/10 | 19/11 |
| Heart rate (beats/min) | 70.9 ± 9.5** | 83.6 ± 7.7 | 85.6 ± 8.6 |
| Functional class (NYHA) | |||
| I | 30 | 0 | 0 |
| II | 0 | 3 | 4 |
| III | 0 | 19 | 20 |
| IV | 0 | 8 | 6 |
| LVEF (%) | 63.1 ± 6.0** | 37.4 ± 10.5 | 36.2 ± 10.1 |
| LVEDD (mm) | 45.3 ± 7.1** | 59.8 ± 13.1 | 61.6 ± 11.7 |
| LVESD (mm) | 34.5 ± 7.3** | 52.7 ± 11.8 | 53.1 ± 10.5 |
| BNP (pg/ml) | 54.7 ± 18.2** | 2362.9 ± 2234.3 | 2686.5 ± 2164.7 |
DCM, dilated cardiomyopathy group; HF, heart failure control group; HC, healthy control group; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; BNP, brain natriuretic peptide. Class I: no cardiac symptoms even with activity; Class II: cardiac symptoms lead to some difficulties with usual activity; Class III: cardiac symptoms make patient unable to perform usual physical activity; Class IV: cardiac symptoms present even at rest. Data expressed as mean ± SD;
P < 0.01.
Laboratory tests
Venous blood was drawn from all study participants on the morning following enrollment into the study. The levels of serum brain natriuretic peptide (BNP) were determined.
Flow cytometry analysis
Peripheral blood (500 µl) from individuals in each group was drawn in 50 U/ml heparin and incubated in the presence of 25 ng/ml phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis, MO, USA), 1 µg/ml ionomycin (Sigma-Aldrich), and 1 µl/106 cells GolgiPlugTM (BD Biosciences, San Diego, CA, USA) at 37°C in a 5% CO2 air atmosphere for 5 h. Samples were treated with Lysing buffer (to lyse red cells) and cells were harvested and stained with FITC-anti-CD19 (BD Biosciences), PerCP-Cy5.5-anti-CD5 (eBioscience, San Diego, CA, USA), and APC-anti-CD1d (eBioscience). After treatment with a fixation solution and permeabilization solution (BD Biosciences), intracellular IL-10 cytokine was stained by adding PE-anti-IL-10 (BD Biosciences). Stained cells were analyzed on a FACSCalibur flow cytometer (BD Bioscience). Data was acquired and analyzed using FlowJo 7.6 (Tree Star Inc., San Carlos, CA, USA).
Statistical analyses
Data were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was performed to compare mean values from different samples. Differences between sample means for which P < 0.05 were deemed to be statistically significant. Statistical analyses were performed using SPSS 17.0 software (IBM, USA).
Results
Clinical characteristics of study participants
DCM and HF control patients presented significantly elevated heart rate, increased left ventricular end-diastolic and left ventricular end-systolic diameters (LVEDD and LVESD, respectively), higher levels of serum BNP, and reduced left ventricular ejection fraction (LVEF), compared to subjects in the HC group (Table 1; Figures 1 and 2).
Figure 1.
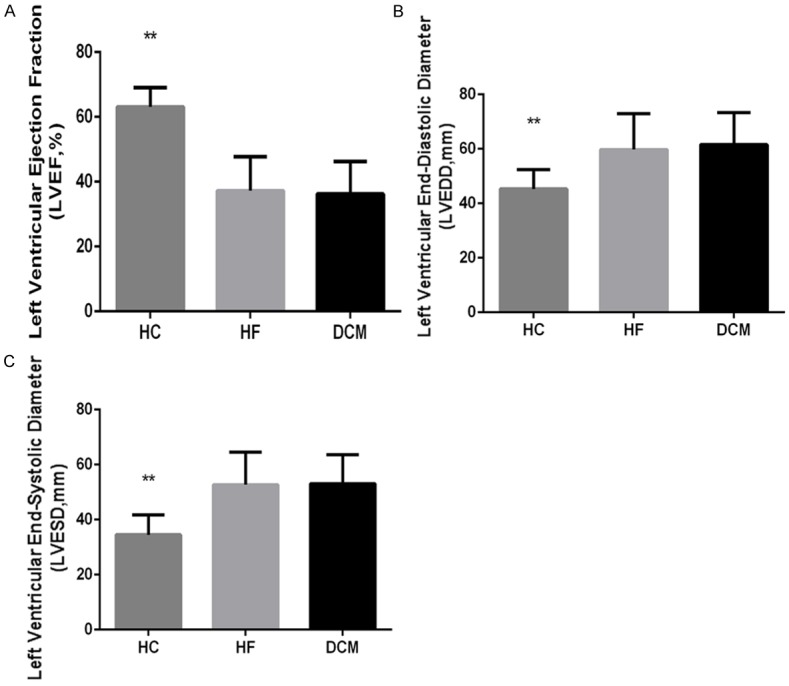
Echocardiographic variables of DCM patients and controls. Echocardiographic variables were measured in DCM and HF control patients and in HC individuals. A. Left ventricular ejection fraction (LVEF). B. Left ventricular end-diastolic diameter (LVEDD). C. Left ventricular end-systolic diameter (LVESD). Data are presented as mean values for each group, with error bars indicating the SD; ** indicates P < 0.01.
Figure 2.
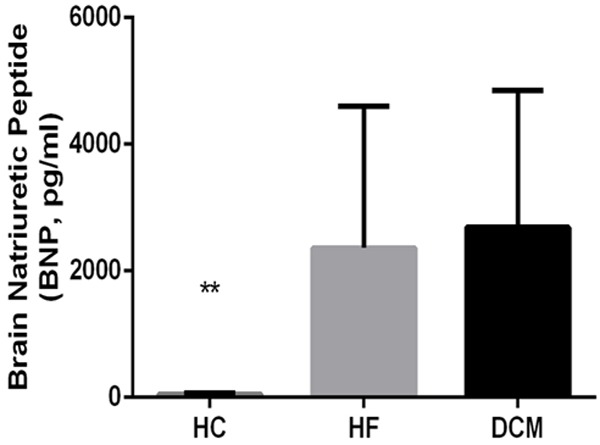
Serum brain natriuretic peptide (BNP) in DCM patients and controls. Brain natriuretic peptide (BNP) was measured in serum from individuals in the DCM, HF control, and HC groups. Data are presented as mean values for each group, with error bars indicating the SD; ** indicates P < 0.01.
Frequencies of circulating Breg subtypes in DCM patients and controls
We sorted and determined the frequency of CD19+IL-10+-, CD19+CD5+-, CD19+CD5+CD1d+-, and CD19+CD5+CD1d+IL-10+-B cells in peripheral blood from DC, HF control, and HC individuals by flow cytometry analysis (Figure 3). The frequency of CD19+IL-10+ B cells was higher in peripheral blood from DCM patients (5.19 ± 2.64%) than in blood from HF controls (1.31 ± 0.63%) and HC individuals (2.25 ± 0.87%) (Figure 4A). Similarly, the frequency of CD19+CD5+ B cells was significantly higher in DCM patients than in HF control and HC individuals (7.40 ± 4.06%, 2.16 ± 1.23%, and 2.30 ± 0.94%, respectively; P < 0.01; Figure 4B). Compared to HF control patients and HC individuals, DCM patients showed a higher percentage of CD19+CD5+CD1d+ B cells (1.15 ± 0.52%, 1.47 ± 0.63%, and 4.02 ± 2.41%, respectively; P<0.05) and CD19+CD5+CD1d+IL-10+ B cells (1.91 ± 1.13%, 1.57 ± 0.59%, and 4.89 ± 1.66%, respectively; P < 0.01) in peripheral blood (Figure 4C and 4D). Consistently, DCM patients showed significantly higher frequencies of each Breg subtype than HF patients and HC individuals. The differences in Breg frequencies between HF control patients and HC individuals were not significant (P > 0.05).
Figure 3.
Fluorescence-activated cell sorting (FACS) of Bregs in peripheral blood from DCM patients and controls. CD19+IL-10+-, CD19+CD5+-, CD19+CD5+CD1d+-, and CD19+CD5+CD1d+IL-10+-B cells were sorted and counted in peripheral blood from DCM, HF control, and HC subjects by FACS.
Figure 4.
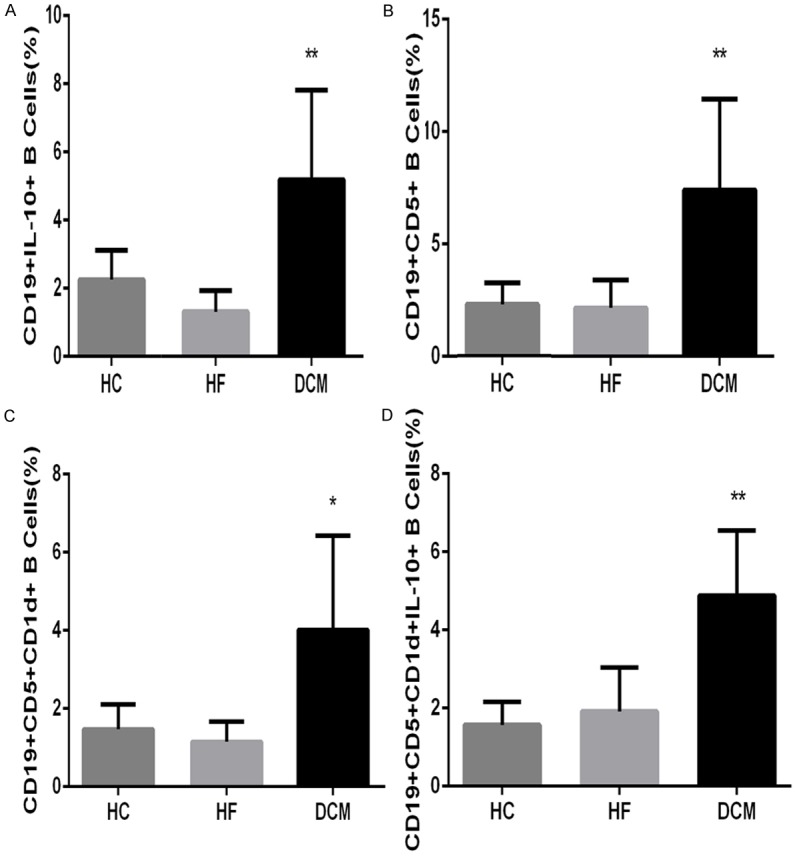
Graphic representation of Bregs FACS data from DCM patients and controls. CD19+IL-10+- (A), CD19+CD5+- (B), CD19+CD5+CD1d+- (C), and CD19+CD5+CD1d+IL-10+-B cell (D) frequencies in DCM patients, HF controls, and HC subjects. Data are presented as mean frequencies for each group, with error bars indicating the SD. * indicates P < 0.05; ** indicates P < 0.01.
Discussion
IL-10-secreting B cells play an important role in maintaining immune homeostasis [9,13,14]. However, the role of IL-10-secreting B cells in the progression of DCM has not been investigated. In this study, we compared the frequencies of different circulating Breg subtypes in DCM patients, HF controls, and healthy subjects. We determined that the frequency of circulating Bregs, especially those producing IL-10, was dramatically higher in DCM patients than in individuals from the control groups.
Bregs are important suppressors of inflammation and other autoimmune responses, producing regulatory cytokines, such as IL-10, [6-8,13,15] TNF-α, lymphotoxin, IL-6, and IL-17 [16,17] The rare IL-10-secreting B cells are distinguished from other Bregs by their capacity to produce anti-inflammatory cytokine IL-10 [14,18]. IL-10-secreting B cell development and function have not been completely elucidated; however, B cell antigen receptor- and toll-like receptor-related signals appear to be involved [19,20]. Emerging evidence indicates that CD5+CD1d+ B cell subsets are rich in IL-10+ B cells, and that the regulation of CD5+CD1d+ B cells function depends on IL-10 [10,21]. CD19+CD5+CD1dhiIL-10+ B cells play a critical role in the maintenance of immune homeostasis [22].
Increased levels of IL-10-secreting B cells were shown to regulate the immune responses in patients with human immunodeficiency virus infection, chronic hepatitis B virus infection, systemic lupus erythematosus, immune thrombocytopenia, and experimental autoimmune encephalomyelitis, suggesting that these cells were involved in the immunological response elicited after viral infections and in T cell-mediated autoimmune diseases [5,13,23-26]. On the other hand, the level of IL-10-secreting B cells was reduced in rheumatoid arthritis patients [27]. Clinical trials and animal experiments studying this B cell subset have provided inconsistent results, suggesting that IL-10-producing Bregs may be elevated in some but reduced in other autoimmune diseases.
A number of patients with viral myocarditis (VMC) may gradually progress to DCM with systolic dysfunction and ventricular dilation. An increasing number of studies suggest that myocardial tissue injury can occur not only by viral proliferation, but also as a result of inflammation and other autoimmune responses [28,29]. Some researchers perceive VMC and DCM as different stages of the same disease. Our unpublished data indicated that the level of IL-10-producing B cells was elevated in CVB3-induced AVMC mice. However, it was not clear whether a similar increase in IL-10-producing B cells would be observed in DCM patients.
Yu et al. [30] have demonstrated that the levels of B cell subsets secreting IL-1β, IL-6, IL-10, IL-17, and TNF-α were all elevated in DCM patients; however, only TNF-α-producing B cell frequencies were significantly higher in DCM patients than in healthy controls. Even though our present study is similar to the one above, important differences can be identified between the two. Our data showed that the frequencies of CD19+IL-10+-, CD19+CD5+-, CD19+CD5+CD1d+-, and CD19+CD5+CD1d+IL10+-B cells were significantly higher in DCM patients than in HF control and HC individuals. However, no statistically significant differences in the levels of Breg subsets between HC and HF control patients, including those with ischemic heart disease and valvular heart disease, were found (Figure 4).
Our study presents a number of limitations, including a small sample size and the unmet challenge of eliminating the confounding effects of heart failure etiological heterogeneity, in spite of the inclusion of the HF control group. As part of future efforts, it will be important to study bigger patient cohorts, including DCM patients with normal cardiac function. Further prospective studies should also explore the relationship between B cells and myocardial fibrosis in order to elucidate the pathophysiological function of B cells in patients with DCM.
Conclusion
The results of the present study show, for the first time, that the levels of IL-10-secreting Bregs are significantly increased in DCM patients, suggesting that this Breg subset may participate in the pathogenesis of DCM. However, how B cells differentiate into IL-10-secreting Bregs and what functions these cells play in DCM progression remain to be further investigated.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (grant No. 81450055). We thank Dr. Jiao Lan for technical assistances.
Disclosure of conflict of interest
None.
References
- 1.Luk A, Ahn E, Soor GS, Butany J. Dilated cardiomyopathy: a review. J Clin Pathol. 2009;62:219–225. doi: 10.1136/jcp.2008.060731. [DOI] [PubMed] [Google Scholar]
- 2.Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet. 2010;375:752–762. doi: 10.1016/S0140-6736(09)62023-7. [DOI] [PubMed] [Google Scholar]
- 3.Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann MK, Ray A, Basu S, Karp CL, Dittel BN. Pathogenic and regulatory roles for B cells in experimental autoimmune encephalomyelitis. Autoimmunity. 2012;45:388–399. doi: 10.3109/08916934.2012.665523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, Kennedy PT, Brunetto M, Lampertico P, Mauri C, Maini MK. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauri C, Ehrenstein MR. The “short” history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, Sato S, Tedder TF, Fujimoto M. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4809. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronet C, Hauyon-La Torre Y, Revaz-Breton M, Mastelic B, Tacchini-Cottier F, Louis J, Launois P. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 2010;184:886–894. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- 9.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Jeong YI, Hong SH, Cho SH, Lee WJ, Lee SE. Induction of IL-10-producing CD1dhighCD5+ regulatory B cells following Babesia microti-infection. PLoS One. 2012;7:e46553. doi: 10.1371/journal.pone.0046553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cen Z, Guo Y, Kong Q, Zhou Q, Wu W. IL-10-producing B cells involved in the pathogenesis of Coxsackie virus B3-induced acute viral myocarditis. Int J Clin Exp Pathol. 2015;8:830–835. [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 13.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 15.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, Musette P. IL-10 produced by activated human B cells regulates CD4 (+) T-cell activation in vitro. Eur J Immunol. 2010;40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, Smith CH. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010;67:452–461. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez-Tello A, Halwani R, Li R, Nadigel J, Bar-Or A, Mazer BD, Eidelman DH, Al-Muhsen S, Hamid Q. IL-17A and IL-17F expression in B lymphocytes. Int Arch Allergy Immunol. 2012;157:406–416. doi: 10.1159/000329527. [DOI] [PubMed] [Google Scholar]
- 18.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259:259–272. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. Regulatory B cells control T-cell autoimmunity through IL-21-de- pendent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 22.Xing C, Ma N, Xiao H, Wang X, Zheng M, Han G, Chen G, Hou C, Shen B, Li Y, Wang R. Critical role for thymic CD19+CD5+CD1dhiIL-10+ regulatory B cells in immune homeostasis. J Leukoc Biol. 2015;97:547–556. doi: 10.1189/jlb.3A0414-213RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Zhan W, Kim CJ, Clayton K, Zhao H, Lee E, Cao JC, Ziegler B, Gregor A, Yue FY, Huibner S, MacParland S, Schwartz J, Song HH, Benko E, Gyenes G, Kovacs C, Kaul R, Ostrowski M. IL-10-producing B cells are induced early in HIV-1 infection and suppress HIV-1-specific T cell responses. PLoS One. 2014;9:e89236. doi: 10.1371/journal.pone.0089236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Yang J, Chu Y, Wang J, Guan M, Zhu X, Xue Y, Zou H. T follicular helper cells mediate expansion of regulatory B cells via IL-21 in Lupus-prone MRL/lpr mice. PLoS One. 2013;8:e62855. doi: 10.1371/journal.pone.0062855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hua F, Ji L, Zhan Y, Li F, Zou S, Chen L, Gao S, Li Y, Chen H, Cheng Y. Aberrant frequency of IL-10-producing B cells and its association with Treg/Th17 in adult primary immune thrombocytopenia patients. Biomed Res Int. 2014;2014:571302. doi: 10.1155/2014/571302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang MH, Couto MC, Duarte CC, Gall V, White P, Naides S, Schumacher HR Jr, Hwang AS, Holers VM, Deane KD, Gupta S, Ho IC, Finckh A, Kopec J, Choi CB, Sayre EC. An Internet-based technique for the identification of persons with symptoms of inflammatory polyarthritis of less than 12 weeks. Clin Rheumatol. 2015;34:465–470. doi: 10.1007/s10067-014-2796-7. [DOI] [PubMed] [Google Scholar]
- 28.Reddy J, Massilamany C, Buskiewicz I, Huber SA. Autoimmunity in viral myocarditis. Curr Opin Rheumatol. 2013;25:502–508. doi: 10.1097/BOR.0b013e3283620036. [DOI] [PubMed] [Google Scholar]
- 29.Yajima T, Knowlton KU. Viral myocarditis: from the perspective of the virus. Circulation. 2009;119:2615–2624. doi: 10.1161/CIRCULATIONAHA.108.766022. [DOI] [PubMed] [Google Scholar]
- 30.Yu M, Wen S, Wang M, Liang W, Li HH, Long Q, Guo HP, Liao YH, Yuan J. TNF-alpha-secreting B cells contribute to myocardial fibrosis in dilated cardiomyopathy. J Clin Immunol. 2013;33:1002–1008. doi: 10.1007/s10875-013-9889-y. [DOI] [PubMed] [Google Scholar]



