Abstract
Flotillin-2 (Flot-2) is an important component of cellular membrane, which involves in various cellular processes and recent studies have revealed that Flot-2 played important roles in cancer progression. The expression and prognostic impact of Flot-2 in oral squamous cell carcinoma (OSCC) have not been well studied. So, a tissue microarray (TMA) based on immunohistochemical analysis of surgical resection of tumor tissues of 78 cases of OSCC patients and 27 cases of adjacent non-cancerous squamous epithelium tissues was conducted. This study focused on detecting Flot-2 expression and analyzing its prognostic impact on OSCC. The result showed that the positive percentage of Flot-2 expression in OSCC (74.4%, 58/78) was significantly higher than that in adjacent non-cancerous squamous epithelium tissues (25.9%, 7/27) (P<0.001). Additionally, the positive expression of Flot-2 in OSCC patients with a history of alcohol consumption was significantly higher than those nonusers (P=0.027). Both univariate and multivariate survival analysis indicated that increased expression Flot-2 protein was significantly correlated inversely with overall survival rates in OSCC patients (P=0.046, P=0.002). Taken together, positive expression of Flot-2 protein may be an independent biomarker for poor prognosis in OSCC.
Keywords: Oral squamous cell carcinoma (OSCC), flotillin-2 (flot-2), prognosis, biomarker, immunohistochemistry
Introduction
Oral cancer is one of the 10 most common cancers in the world. Its high mortality rate and the disfigurement that survivors may suffer gives rise to a considerable global public health burden. More than 90% of the malignant oral neoplasm’s patients are oral squamous cell carcinomas (OSCC). Despite the currently available therapeutic strategies which include the excision of malignant tissue and combination of radiotherapy and chemotherapy, the five-year survival rate is only 53% [1]. Increased mortality rate could be attributed to late diagnosis and lack of specific biomarkers to predict tumor progression and prognosis [2] Therefore, new discoveries of biomarkers for determining the risks of occurrence, progression and metastasis and approaches for therapeutic treatment of OSCC are the most significant important problem in reduction of mortality.
Flotillin-1 (Flot-1) and Flottillin-2 (Flot-2) are ubiquitous and highly conserved proteins. They were initially discovered in 1997 as being associated with specific caveolin-independent cholesterol- and glycosphingolipid-enriched membrane microdomains. They have been shown to be associated with, for example, various signaling pathways, such as EGFR signaling [3,4], cadherin signaling [5], cell adhesion [6], membrane trafficking and anon regeneration [7-9]. Recent findings have revealed that the expression of Flotillins is frequently up-regulated in various types of human cancers and it is associated with poor patient survival prognosis and high risk of metastasis formation.
Increased expression of Flot-2 is detected in several types of human cancer and links with poor survival. For example, over-expression of Flot-2 is associated with human melanoma progression and lymph node metastasis [10,11]; higher expression of Flot-2 also be detected in metastatic NPC cells [12]; consistently, positive percentage of Flot-2 expression in NPC patients with lymph node metastasis is significantly higher than those without lymph node metastasis [13]. Also, high expression of Flot-2 protein is associated with poor outcomes in patients with several solid tumors, and these studies provide a basis for the consideration of Flot-2 as a potential marker for tumors [14,15].
Although Flot-2 has been discovered for decades, its potential clinical significance in OSCC is largely unknown. In the present study, we found that the expression of Flot-2 was up-regulated in surgical specimens of OSCC. Moreover, over-expression of Flot-2 was associated with alcohol consumption, the known risk factor for OSCC. Multivariate analysis revealed that Flot-2 might be an independent biomarker for the prediction of OSCC prognosis. Taken together, our data will facilitate an understanding of OSCC carcinogenesis and mining biomarkers for the diagnosis and treatment of this disease.
Materials and methods
Tissue microarrays (TMA) and clinical data
In this study, these OSCC patients were submitted to surgical treatment at the Department of Thoracic Surgery at the Second Xiangya Hospital of Central South University (Changsha, China) from Apl. 2007 to Dec. 2013. All tumor samples and non-cancerous squamous epithelium tissues were obtained from Department of pathology, the Second Xiangya Hospital of Central South University. These patients had been submitted to routine staging and definitive surgical resection of part of tongue and systematic neck lymph node dissection. All patients had a confirmed histological diagnosis of OSCC according to WHO histological classification of the oral cancer. The staging classification of the current analysis was carried out based on the criteria of the 7th edition of the AJCC/UICC TNM staging system of oral cancer. No patients had been previously treated with chemotherapy and radiotherapy at the time of original operation. Complete clinical record and follow-up data were available for all patients. Written informed consent was obtained from these patients, and this study was approved by the Ethics Review Committee of the Second Xiangya Hospital of Central South University. Patient characteristics were detailed in Table 1. In this study, we used the high-throughput OSCC TMAs containing 78 cases of OSCC, 27 cases of non-cancerous squamous epithelium tissues.
Table 1.
78 cases of oral squamous cell carcinoma (OSCC) patients feature
| Patients characteristics | No. of patients (%) |
|---|---|
| Age (years) | |
| <50 | 29 (37.2) |
| ≥50 | 49 (62.8) |
| Gender | |
| Male | 63 (80.8) |
| Female | 15 (19.2) |
| Cigarette | |
| Yes | 47 (60.3) |
| No | 31 (39.7) |
| Alcohol | |
| Yes | 40 (51.3) |
| No | 38 (48.7) |
| Areca nut | |
| Yes | 33 (42.3) |
| No | 45 (57.7) |
| Pathological grades | |
| Well | 57 (73.1) |
| Moderate | 13 (16.7) |
| Poor | 8 (10.3) |
| Clinical stages | |
| StageI | 22 (28.2) |
| StageII | 19 (24.4) |
| StageIII | 17 (21.8) |
| StageIV | 20 (25.6) |
| Lymph node status | |
| N0 | 50 (64.1) |
| N1/N2/N3 | 28 (35.9) |
| Survival status | |
| Alive | 55 (70.5) |
| Death | 23 (29.5) |
Construction of TMAs and validation of the arrayed specimens
Representative areas of OSCC and non-cancerous control squamous epithelium were marked on each hematoxylin-eosin (H&E) slide and tissue paraffin block, and the marked areas of tissue paraffin blocks were sampled for the TMAs. The TMAs were assembled using the tissue-arraying instrument (Beecher Instruments, Silver Springs, MD, USA) as described by Fan et al [16]. Briefly the instrument was used to create holes in a recipient paraffin block with defined array coordinates. A solid stylet was used to transfer the tissue cores into the recipient block. Two 0.6-mm-diameter tissue cores were taken from each OSCC and the adjacent non-cancerous squamous epithelium tissues. All specimens were distributed in 3 regular-sized paraffin receptive blocks, each containing 100 spots. A serial of 5-µm-thick sections were obtained using a microtome and one slide from each recipient block was stained with H&E and evaluated under the light microscope. The remaining slides were covered with thin paraffin and stored at 4°C before IHC. In this study all TMAs specimens diagnosed as 78 cases of OSCC and 27 cases of non-cancerous control squamous epithelium tissues were valid for IHC.
IHC and scores
The IHC staining for samples on the TMAs was carried out using ready-to-use Envision TM+ Dual Link System-HRP methods (Dako; Carpintrria, CA). Briefly, each TMA section was deparaffinized and rehydrated, and high-temperature antigen retrieval was achieved by heating the samples in 0.01 M citrate buffer in a domestic microwave oven at full power (750 Watts) for 30 minutes, then the samples were immersed into methanol containing 0.3% H2O2 to inactivate endogenous peroxidase at 37°C for 30 minutes. To eliminate nonspecific staining, the slides were incubated with appropriate preimmune serum for 30 minutes at room temperature. After incubation with a 1:500 dilution of primary antibody to Flot-2 protein (Rabbit polyclonal antibody, Catalog: #S0051, Epitomics, Inc.) at 4°C overnight, slides were rinsed with phosphate-buffered saline (PBS) and incubated with a labeled polymer-HRP was added according to the manufacturer’s instructions and incubated 30 minutes. Color reaction was developed by using 3, 3’-diaminobenzidine tetrachloride (DAB) chromogen solution. All slides were counterstained with hematoxylin. Positive control slides were included in every experiment in addition to the internal positive controls. The specificity of the antibody was determined with matched IgG isotype antibody as a negative control.
Immunohistochemical staining of TMA sections were scored independently by QW and SF who were blinded to the clinicopathological data, at 200× magnification light microscopy. The evaluation was based on the staining intensity and extent of staining. Staining intensity for Flot-2 was scored as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). Staining extent was scored as 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%), and 4 (76-100%), depending on the percentage of positive-stained cells. Staining positivity was determined by the formula: overall scores=percentage score × intensity score. The overall score of ≤1 was defined as negative (0), of ≥2 and ≤4 as weak positive (1+), of ≥6 and ≤8 as moderate positive (2+), and of ≥9 as strong positive (3+). Agreement between the two evaluators was 95%, and all scoring discrepancies were resolved through discussion between the two evaluators. In present analysis, weak positive, moderate positive and strong positive were combined as positive to suit the paired statistical analysis.
Statistical analysis
All statistical analyses were performed using SPSS 19.0. The chi-square test was used to analyze the relationship between the expression of Flot-2 protein and the clinicopathological characteristics and prognostic factors in OSCC. Kaplan-Meier analysis was performed for overall survival curves and statistical significance was assessed using the log-rank test. Overall survival was defined as the time from the treatment initiation (diagnosis) to the data of death. To identify whether expression of Flot-2 protein is an independent prognostic factor of overall survival for OSCC, the multivariate analysis using the Cox proportional hazard regression model was performed. All P values were based on the two-sided statistical analysis and P-value less than 0.05 was considered to be statistically significant.
Results
Expression of Flot-2 protein significantly increased in OSCC tissues
We first determined the expression and cellular localization of Flot-2 in OSCC and adjacent non-cancerous squamous epithelium tissues by IHC. Strong positive staining of Flot-2 (Figure 1A) was located on cell membranes of OSCC and weak staining was found in adjacent non-cancerous squamous epithelium control tissue (Figure 1C). Tissue sections stained with matched IgG isotype antibody as negative control showed no positive staining of Flot-2 in OSCC cells (Figure 1D). Next, we enumerate the expression of Flot-2 in OSCC and non-cancerous squamous epithelium control tissues. The positive percentage of Flot-2 expression in OSCC and non-cancerous squamous epithelium control tissue were 74.4% (58/78) and 25.9% (7/27). There was significantly higher positive expression of Flot-2 in OSCC compared with the adjacent non-cancerous squamous epithelium control tissues (P<0.001) (Figure 2).
Figure 1.
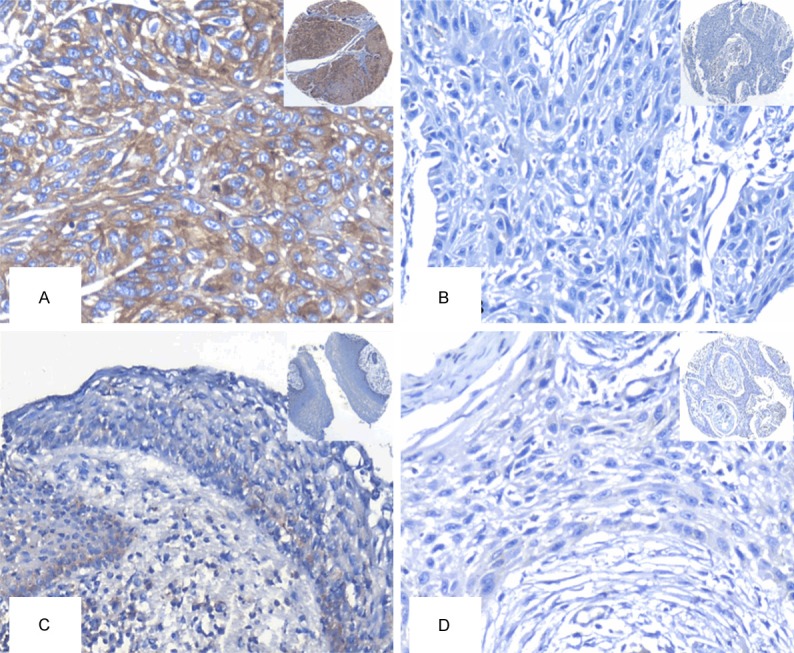
Expression of Flot-2 protein in OSCC cells and the control of non-cancerous squamous epithelium tissues were detected by IHC using specific antibody as described in the section of materials and methods. Strong positive expression of Flot-2 protein was found at cell membranes of OSCC cells (A, 20×, IHC, DAB staining). Negative staining of Flot-2 were showed in OSCC cells and adjacent non-cancerous squamous epithelium tissue (B, 20×, IHC, DAB staining). Weak staining of Flot-2 were showed in adjacent non-cancerous squamous epithelium tissue (C, 20×, IHC, DAB staining). Negative control showed no Flot-2 staining in OSCC cells (D, 20×, IHC, DAB staining).
Figure 2.
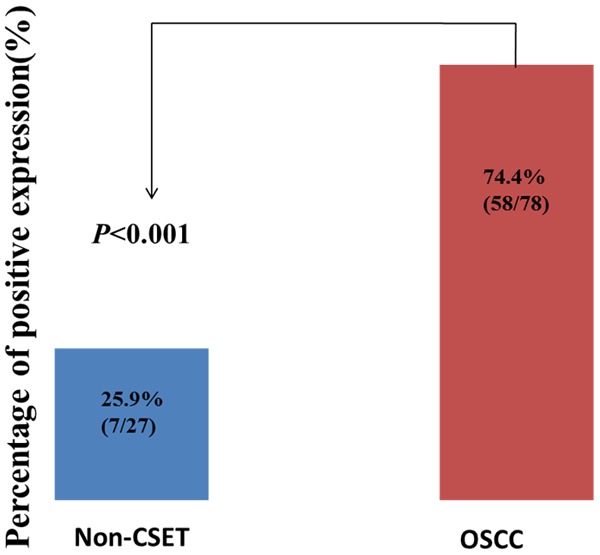
Expression of Flot-2 proteins in OSCC compared to the control of non-cancerous squamous epithelium tissues. Results showed that there was significant difference between the groups which were statistically evaluated by chi-square test.
Association between expression of Flot-2 protein and OSCC clinical pathological features
We further investigated the associations between expression of Flot-2 and various clinicopathological characteristics of OSCC patients by univariate chi-square test, which included age, gender, clinical stages, lymph node metastasis (LNM) status, pathological differentiation, living habits, such as tobacco using, alcohol consumption and areca nuts chewing. Date shown in Table 2 indicated a strong positive correlation between positive expression of Flot-2 and alcohol consumption of OSCC patients, patients who drinking frequently showed significantly higher positive percentage of Flot-2 expression than those with nonusers (P=0.027). However, there was no association between positive expression of Flot-2 protein in OSCC and other clinicopathological features such as age, gender, LNM status, clinical stages, pathological differentiation, tobacco consumption and areca nuts chewing.
Table 2.
Analysis of the association between expression of Flot-2 protein and clinicopathological features of oral squamous cell carcinoma (OSCC) (n=78)
| Flot-2 | |||
|---|---|---|---|
|
|
|||
| Clinicopathological features (n) | Positive (%) | Negative (%) | P-value |
| Age | |||
| <50 years (n=29) | 21 (72.4) | 8 (27.6) | |
| ≥50 years (n=49) | 37 (75.5) | 12 (24.5) | 0.762 |
| Gender | |||
| Male (n=63) | 49 (77.8) | 14 (22.2) | |
| Female (n=15) | 9 (60.0) | 6 (40.0) | 0.156 |
| Cigarette | |||
| Yes (n=47) | 37 (78.7) | 10 (21.3) | |
| No (n=31) | 21 (67.7) | 10 (32.3) | 0.277 |
| Alcohol | |||
| Yes (n=40) | 34 (85.0) | 6 (15.0) | |
| No (n=38) | 24 (63.2) | 14 (36.8) | 0.027* |
| Areca nut | |||
| Yes (n=33) | 26 (78.8) | 7 (21.2) | |
| No (n=45) | 32 (71.1) | 13 (28.9) | 0.443 |
| Pathological grades | |||
| Well (n=57) | 43 (75.4) | 14 (24.6) | |
| Moderate (n=13) | 9 (69.2) | 4 (30.8) | |
| Poor (n=8) | 6 (75.0) | 2 (25.0) | 0.898 |
| Clinical stages | |||
| StageI-II (n=42) | 31 (73.8) | 11 (26.2) | |
| StageIII-IV (n=36) | 27 (75.0) | 9 (25.0) | 0.904 |
| Lymph node status | |||
| LNM (n=28) | 21 (75.0) | 7 (25.0) | |
| No LNM (n=50) | 37 (74.0) | 13 (26.0) | 0.923 |
| Survival status | |||
| Alive (n=55) | 37 (67.3) | 18 (32.7) | |
| Death (n=23) | 21 (91.3) | 2 (8.7) | 0.027* |
Chi-square test, statistically significant difference (P<0.05).
Abbreviations: LNM, lymph node metastasis.
Impact of expression of Flot-2 protein on the prognosis of patients with OSCC
To further examine the impact of expression of Flot-2 protein on the survival status of OSCC patients, we employed the Kaplan-Meier analysis to plot the survival curve of all 78 OSCC patients, and statistical significance was assessed using the log-rank test. At the time of analysis, the number of OSCC patients’ specific deaths was 23 (29.5%).
Figure 3 illustrated the Kaplan-Meier survival plots for OSCC patients with different expression of Flot-2 protein (Figure 3A). Univariate survival (log-rank test) analysis showed that the overall survival rates for OSCC patients with negative Flot-2 were significantly higher than these with positive Flot-2 expression (P=0.046). We also plotted the survival curves for OSCC patients with conventional prognosis parameters, including clinical stages, lymph nodal status. As shown in Figure 3B, 3C, lower clinical stages (stage I and II) or absence of lymph node metastasis had a positive impact on the overall survival rates of OSCC. The OSCC patients with advanced stage OSCC (stage III and IV) and lymph node metastasis had lower overall survival that patients with early stage OSCC (stage I and II) and without lymph node metastasis (P=0.012, Figure 3B, and P=0.029, Figure 3C respectively).
Figure 3.
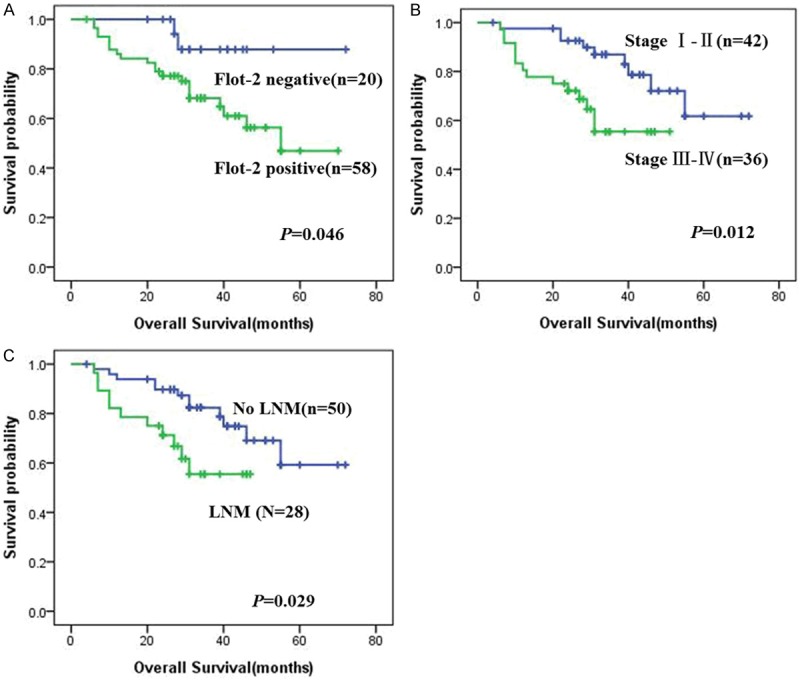
Kaplan-Meier curves for overall survival of OSCC patients with expression of Flot-2 and different clinicopathological characteristics. Kaplan-Meier analysis to plot the overall survival curves of all 78 OSCC patients with expression of Flot-2 and different clinicopathological characteristics and statistical significance was assessed using the log-rank test. A: OSCC patients with positive expression of Flot-2 protein showed worse overall survival rate compared with patients with Flot-2 negative staining (P=0.046, two sided). B: OSCC patients with clinical stage III-IV were significantly related to poor prognosis compared with patients with clinical stage I-II (P=0.012, two sided). C: OSCC patients with lymph node metastasis showed poor prognosis compared with patients without lymph node metastasis (P=0.029, two sided).
Besides univariate analysis, multivariate Cox proportional hazard regression analysis was also carried out to further investigate whether the expression of Flot-2 protein was the independent prognostic factors for OSCC, and these results were revealed in Table 3. During the multivariate analysis of the expression of Flot-2 in 78 cases of OSCC, which included clinical stages, lymph node metastasis status, pathological differentiation, treatment strategy, age, gender, tobacco using, alcohol consumption and areca nuts chewing. We have found that the positive expression of Flot-2 protein might serve as an independent poor prognostic factor for OSCC (P=0.002), as well as clinical stages, tobacco using, alcohol consumption and areca nuts chewing (P=0.024, P=0.048, P=0.024, P=0.022, respectively). Again, no impact was detected with age, gender, pathological differentiation and treatment strategy of OSCC (P>0.05 for all).
Table 3.
Summary of multivariate statistical analysis of Flot-2 protein expression for overall survival in 78 cases of OSCC
| Parameter | B | S.E. | Wald | Sig. | Exp (B) | 95.0% CI for Exp (B) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower | Upper | ||||||
| Age | -0.362 | 0.478 | 0.573 | 0.449 | 0.697 | 0.273 | 1.776 |
| Gender | -0.129 | 0.811 | 0.025 | 0.874 | 0.879 | 0.180 | 4.307 |
| Cigarette | 1.576 | 0.797 | 3.913 | 0.048* | 4.834 | 1.015 | 23.030 |
| Alcohol | -1.442 | 0.641 | 5.068 | 0.024* | 0.236 | 0.067 | 0.830 |
| Areca nut | -1.274 | 0.557 | 5.234 | 0.022* | 0.280 | 0.094 | 0.833 |
| Pathological grades | 0.192 | 0.349 | 0.302 | 0.582 | 1.211 | 0.611 | 2.400 |
| Clinical stages | 0.608 | 0.269 | 5.095 | 0.024* | 1.837 | 1.083 | 3.116 |
| LNM status | -0.142 | 0.676 | 0.044 | 0.833 | 0.867 | 0.231 | 3.260 |
| Treatment strategy | 0.616 | 0.515 | 1.435 | 0.231 | 1.852 | 0.676 | 5.077 |
| Flot-2 expression | 0.769 | 0.251 | 9.390 | 0.002* | 2.157 | 1.319 | 3.527 |
Abbreviations: LNM, lymph node metastasis; B, Beta coefficient (B); Exp (B), the odds ratio; CI, confidence interval. Note: multivariate analysis of Cox proportional hazard regression,
P<0.05.
Discussion
The pathogenesis of OSCC is multifactorial. The major risk factors of OSCC are tobacco smoking and alcohol consumption, with a population attributable risk of 74% [17,18]. Other important risk factors include betel quid chewing, infection with human papilloma virus (HPV) or human immunodeficiency virus (HIV) and so on [19,20]. Alcohol consumption is a known risk factor for OSCC. In our present study, Alcohol consumption was significantly associated with the expression of Flot-2, OSCC patients with a history of alcohol consumption showed a higher Flot-2 expression compared with those nonusers. This suggests that one of the pathogenic mechanisms of OSCC may be the synthesis of Flot-2 expression by epithelial cells in response to alcohol challenge.
In our study, the Flot-2 staining was found at the cell membrane, this is in accordance with the previously described localization of this protein in NPC [13]. In addition, we found that only weak positive expression of Flot-2 protein in the squamous epithelial layer of non-cancerous squamous epithelium control tissue. Interestedly, the positive expression of Flot-2 was significantly increased in OSCC compared with the adjacent non-cancerous squamous epithelium control tissue, which is also similar with the previously reported evidence that was found between NPC and normal nasopharyngeal mucosa epithelia. Hence, Flot-2 might play an important role in promoting the development and progression of OSCC. However, only this evidence was not enough to draw a strong conclusion; hence further investigation will be needed to clearly demonstrate the specific mechanism of Flot-2 in OSCC.
Although the prognosis in OSCC is mainly determined by the stage of the tumor at presentation, which is determined according to the TNM-staging system: tumor size (T), regional lymph node metastasis (N), and distant metastasis (M) [20,21], biomarkers do not only play overwhelmingly important role in establishing an accurate diagnosis, but also providing prognostic data for OSCC [22,23]. Therefore, new discoveries of biomarkers for determining the risks of occurrence, progression and approaches for therapeutic treatment of OSCC are of extreme importance for the development of therapeutic strategies to improve outcome and survival for OSCC patients.
Flotillins are clearly involved in many cellular processes, however, the molecular mechanisms that underlie their different functions in OSCC remain poorly understood. Also, there is not any report about expression of Flot-2 and clinicopathological association in OSCC so far. Flot-2 is a target gene of p63 and p73, member of the p53 transcription factor family [24] and which has been proposed as a prognostic marker linked to poor prognosis in several human tumors, such as breast cancer [14], gastric cancer [15], non-small cell lung cancer (NSCLC) [25], cervical carcinoma [26] and so on. Similar results were found in our present study. OSCC patients with positive Flot-2 had an obvious shorter overall survival time than those with negative Flot-2. Furthermore, multivariate analysis proved that the positive expression of Flot-2 was an independent factor for poor prognosis in OSCC patients, just like other known risk factors for OSCC, such as tobacco using, alcohol consumption and areca nut chewing. So, increased expression of Flot-2 might act as a novel biomarker to predict poor prognosis for OSCC patients.
Taken together, the positive expression of Flot-2 was identified as an independent prognostic marker for OSCC patients. In addition, increased expression of Flot-2 protein in OSCC as compared to normal oral mucosa provides further evidence for its role in genesis and progression of OSCC. Further investigations regarding the interaction of Flot-2 with other potential genes or environmental risk factors may be shed light on the important role of this gene in OSCC.
Acknowledgements
The work was supported by grants from The National Natural Sciences Foundations of China (No: 81472773).
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Chin D, Boyle GM, Williams RM, Ferguson K, Pandeya N, Pedley J, Campbell CM, Theile DR, Parsons PG, Coman WB. Novel markers for poor prognosis in head and neck cancer. Int J Cancer. 2005;113:789–797. doi: 10.1002/ijc.20608. [DOI] [PubMed] [Google Scholar]
- 3.Neumann-Giesen C, Fernow I, Amaddii M, Tikkanen R. Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J Cell Sci. 2007;120:395–406. doi: 10.1242/jcs.03336. [DOI] [PubMed] [Google Scholar]
- 4.Amaddii M, Meister M, Banning A, Tomasovic A, Mooz J, Rajalingam K, Tikkanen R. Flotillin-1/reggie-2 protein plays dual role in activation of receptor-tyrosine kinase/mitogen-activated protein kinase signaling. J Biol Chem. 2012;287:7265–7278. doi: 10.1074/jbc.M111.287599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solis GP, Schrock Y, Hulsbusch N, Wiechers M, Plattner H, Stuermer CA. Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking. Mol Biol Cell. 2012;23:1812–1825. doi: 10.1091/mbc.E11-12-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodin S, Planchon D, Rios Morris E, Comunale F, Gauthier-Rouviere C. Flotillins in intercellular adhesion-from cellular physiology to human diseases. J Cell Sci. 2014;127:5139–5147. doi: 10.1242/jcs.159764. [DOI] [PubMed] [Google Scholar]
- 7.Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- 8.Schulte T, Paschke KA, Laessing U, Lottspeich F, Stuermer CA. Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development. 1997;124:577–587. doi: 10.1242/dev.124.2.577. [DOI] [PubMed] [Google Scholar]
- 9.Stuermer CA. How reggies regulate regeneration and axon growth. Cell Tissue Res. 2012;349:71–77. doi: 10.1007/s00441-012-1343-6. [DOI] [PubMed] [Google Scholar]
- 10.Hazarika P, McCarty MF, Prieto VG, George S, Babu D, Koul D, Bar-Eli M, Duvic M. Up-regulation of Flotillin-2 is associated with melanoma progression and modulates expression of the thrombin receptor protease activated receptor 1. Cancer Res. 2004;64:7361–7369. doi: 10.1158/0008-5472.CAN-04-0823. [DOI] [PubMed] [Google Scholar]
- 11.Doherty SD, Prieto VG, George S, Hazarika P, Duvic M. High flotillin-2 expression is associated with lymph node metastasis and Breslow depth in melanoma. Melanoma Res. 2006;16:461–463. doi: 10.1097/01.cmr.0000222592.75858.20. [DOI] [PubMed] [Google Scholar]
- 12.Yang XY, Ren CP, Wang L, Li H, Jiang CJ, Zhang HB, Zhao M, Yao KT. Identification of differentially expressed genes in metastatic and non-metastatic nasopharyngeal carcinoma cells by suppression subtractive hybridization. Cell Oncol. 2005;27:215–223. doi: 10.1155/2005/108490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen Q, Li J, Wang W, Xie G, Xu L, Luo J, Chu S, She L, Li D, Huang D, Fan S. Increased expression of flotillin-2 protein as a novel biomarker for lymph node metastasis in nasopharyngeal carcinoma. PLoS One. 2014;9:e101676. doi: 10.1371/journal.pone.0101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Yang Q, Guo L, Li XH, Zhao XH, Song L B, Lin HX. Flotillin-2 is associated with breast cancer progression and poor survival outcomes. J Transl Med. 2013;11:190. doi: 10.1186/1479-5876-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Z, Wang J, Sun Z, Sun X, Wang Z, Xu H. Flotillin2 expression correlates with HER2 levels and poor prognosis in gastric cancer. PLoS One. 2013;8:e62365. doi: 10.1371/journal.pone.0062365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan S, Ramalingam SS, Kauh J, Xu Z, Khuri FR, Sun SY. Phosphorylated eukaryotic translation initiation factor 4 (eIF4E) is elevated in human cancer tissues. Cancer Biol Ther. 2009;8:1463–1469. doi: 10.4161/cbt.8.15.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 18.Petersen PE. Oral cancer prevention and control--the approach of the World Health Organization. Oral Oncol. 2009;45:454–460. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Kayamba V, Bateman AC, Asombang AW, Shibemba A, Zyambo K, Banda T, Soko R, Kelly P. HIV infection and domestic smoke exposure, but not human papillomavirus, are risk factors for esophageal squamous cell carcinoma in Zambia: a case-control study. Cancer Med. 2015;4:588–95. doi: 10.1002/cam4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 21.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–316. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 22.Xie S, Xu H, Shan X, Liu B, Wang K, Cai Z. Clinicopathological and prognostic significance of survivin expression in patients with oral squamous cell carcinoma: evidence from a meta-analysis. PLoS One. 2015;10:e0116517. doi: 10.1371/journal.pone.0116517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent-Chong VK, Salahshourifar I, Karen-Ng LP, Siow MY, Kallarakkal TG, Ramanathan A, Yang YH, Khor GH, Rahman ZA, Ismail SM, Prepageran N, Mustafa WM, Abraham MT, Tay KK, Cheong SC, Zain RB. Overexpression of MMP13 is associated with clinical outcomes and poor prognosis in oral squamous cell carcinoma. ScientificWorldJournal. 2014;2014:897523. doi: 10.1155/2014/897523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki Y, Oshima Y, Koyama R, Maruyama R, Akashi H, Mita H, Toyota M, Shinomura Y, Imai K, Tokino T. Identification of flotillin-2, a major protein on lipid rafts, as a novel target of p53 family members. Mol Cancer Res. 2008;6:395–406. doi: 10.1158/1541-7786.MCR-07-0108. [DOI] [PubMed] [Google Scholar]
- 25.Wang YL, Yao WJ, Guo L, Xi HF, Li SY, Wang ZM. Expression of flotillin-2 in human non-small cell lung cancer and its correlation with tumor progression and patient survival. Int J Clin Exp Pathol. 2015;8:601–607. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Lin L, Huang Z, Ji B, Mei S, Lin Y, Shen Z. High expression of flotillin-2 is associated with poor clinical survival in cervical carcinoma. Int J Clin Exp Pathol. 2015;8:622–628. [PMC free article] [PubMed] [Google Scholar]


