Abstract
Heat shock protein 90-beta (Hsp90-β) is associated with cell proliferation, differentiation and apoptosis and has been investigated as a prognostic factor in many cancers. However, Hsp90-β protein expression in lung adenocarcinoma (ADC) has not been thoroughly elucidated. The aim of this study was to determine the relationship between Hsp90-β expression, clinicopathological parameters and prognosis in lung adenocarcinomas. Seventy-five surgically resected lung adenocarcinomas and matched normal lung tissue samples were obtained to construct a tissue microarray (TMA), including 44 stage IA-IB cases. Then, Hsp90-β protein expression level in lung tissue was evaluated by immunohistochemistry. Kaplan-Meier survival analysis with a Log-rank significance test was used to estimate the survival differences among subgroups according to Hsp90-β expression in lung ADC tissues using SigmaPlot/SigmaStat v10 and 3.5, respectively. Hsp90-β protein expression was significantly upregulated in lung ADC tissues compared to that in the matched normal alveoli (P<0.001) and was associated with tumor differentiation (P<0.001). Furthermore, Hsp90-β over-expression was correlated with poor survival in stage I patients (P=0.026). Increased Hsp90-β expression was associated with reduced overall survival (HR, 2.440; 95% confidence interval, 1.076-5.530; P=0.033). To conclude, our data demonstrated that Hsp90-β protein was over-expressed in lung ADC tumor tissues and was associated with poor outcomes in early stage ADC patients and low pathological grade tumors. These data suggest that Hsp90-β could be a clinically useful biomarker for the prognosis of ADC and an effective anticancer target.
Keywords: Lung adenocarcinoma, Hsp90-beta, survival, early stage, anticancer target
Introduction
According to an annual report regarding the prevalence of cancer in China in 2010, lung cancer has become one of the most serious threats to human health in both urban and rural areas. Its incidence and mortality are listed first among a variety of tumors [1]. Approximately 50% of patients are diagnosed at late stages, leading to poor prognoses. The 5-year survival rates of patients with resected stage I non-small cell lung cancer (NSCLC) are as high as 50-70% [2]. Therefore, early detection and early cancer treatment are effective methods for reducing cancer mortality and morbidity. Histologically, adenocarcinoma (ADC) is the most prevalent type of lung cancer, accounting for almost half of all of the different types of lung cancers diagnosed [3]. More lung adenocarcinoma cases occur in non-smokers due to the decrease in smoking rates. According to a recent comprehensive molecular profiling study, high rates of somatic mutation were observed in lung ADC tumors, with human epidermal growth factor receptor (EGFR) mutations being more frequent in females and RBM10 mutations being more prevalent in male patients [4].
Heat shock proteins (Hsps) are evolutionarily conserved proteins. One member of the Hsp family is Hsp90, a highly abundant protein in eukaryotic cells that is involved in many important cellular processes. Hsp90-α and Hsp90-β are the two major isoforms of Hsp90 that are associated with cell proliferation, differentiation and apoptosis [5]. In normal conditions, Hsp90-β has a higher expression level than Hsp90-α in most cells. Furthermore, Hsp90-β plays an important role in early embryonic development and trophoblast differentiation; Hsp90-β-deficient mice failed to differentiate to form placental labyrinths [6,7].
It has been reported that Hsp90 over-expression in many types of tumors is significantly associated with decreased cancer survival in breast cancer, non-small cell lung cancer, gastric cancer, and conjunctival melanoma, suggesting that Hsp90 participates in tumorigenesis [8-11]. However, Hsp90-β protein expression in lung ADC has not been thoroughly elucidated. In this study, we identified the expression profile of the Hsp90-β protein in lung ADC tissue by TMA. We further investigated whether Hsp90-β upregulation is associated with lung cancer clinicopathological parameters and evaluated the potential use of Hsp90-β as a prognostic marker for lung ADC.
Methods
Clinical samples and histopathologic data
The 75 patients with primary lung adenocarcinoma included in this study underwent conventional radical tumor resections between July 2005 and December 2011 at the Liaoning Cancer Hospital & Institute, China. All of the patients provided informed consent. Lung cancer and matched normal tissue specimens were formalin-fixed and paraffin-embedded. There were 43 male and 32 female patients, who ranged in age from 32 to 80 years (median 59 years). Tumor stage was classified according to the 7th Union International Cancer Control (UICC) TNM staging system. There were 44 stage IA-IB patients, 28 stage IIA-IIB patients and 3 stage IIIA-IIIB patients. The ADCs were classified according to the International association for the study of cancer/American thoracic society/European respiratory society (IASLC/ATS/ERS) classification. The following major lung ADC histological types were represented: (1) 41.3% acinar, (2) 26.6% papillary, (3) 2.7% micropapillary, (4) 17.3% solid, (5) 1.3% lepidic, (6) 6.7% invasive mucinous, (7) 1.3% fetal and (8) 2.7% enteric adenocarcinomas (Table 1).
Table 1.
Clinicopathological features of lung adenocarcinomas (n=75)
| Groups | Characteristics | Number (%) |
|---|---|---|
| Gender | Male | 43 (57.3) |
| Female | 32 (42.7) | |
| Age | <60 | 39 (52.0) |
| ≥60 | 36 (48.0) | |
| Histology | Acinar predominant adenocarcinoma, APA | 31 (41.3) |
| Papillary predominant adenocarcinoma, PPA | 20 (26.6) | |
| Solid predominant adenocarcinoma, SPA | 13 (17.3) | |
| Micropapillary predominant adenocarcinoma, MPA | 2 (2.7) | |
| Lepidic predominant adenocarcinoma, LPA | 1 (1.3) | |
| Invasive mucinous adenocarcinoma | 5 (6.7) | |
| Fetal invasive adenocarcinoma | 1 (1.3) | |
| Enteric invasive adenocarcinoma | 2 (2.7) | |
| Pathologic grade | Well-differentiated | 19 (25.3) |
| Moderately differentiated | 27 (36.0) | |
| Poorly differentiated | 24 (32.0) | |
| Undifferentiated | 5 (6.7) | |
| TNM staging | IA-IB | 44 (58.7) |
| IIA-IIB | 28 (37.3) | |
| IIIA-IIIB | 3 (4.0) |
Follow-up
The patients had follow-up appointments every 3 months for the first year after surgery, every 6 months for the second year and then yearly thereafter. The follow-up deadline was June 2013. The median follow-up period was 24 months (ranging from 2-95 months), and 40 patients were alive at the time of the last follow-up.
Tissue microarray (TMA) construction and immunohistochemistry staining
The formalin-fixed and paraffin-embedded lung cancer and matched normal tissue samples were obtained from surgery. Before the construction of the tissue microarray (TMA), a pathologist reviewed the H&E stained slides and the localized tumor/normal areas. Briefly, a tissue core from the donor block was punched using a hollow needle with an inner diameter of 1.5 mm. Then, the samples from each tissue were precisely placed into a recipient block at specifically assigned locations. The array block was baked in an oven and tested with H&E staining and an immunohistochemistry (IHC) test.
IHC staining was performed using a standard streptavidin-biotin-peroxidase complex method as described previously [11]. TMA sections (5 m thick) were incubated at 4°C in a humid chamber overnight with a rabbit polyclonal antibody against human Hsp90-β (1:100, ABGENT, catalog #AP7867d, San Diego, CA, USA). PBS was used as a negative control. The nuclei were counterstained with hematoxylin. Two experienced pathologists independently scored the immunostaining results. The immunoreactivity scoring (IRS) criteria were graded according to the following scale [12]: (1) 0, no staining; (2) 1+, weak diffuse cytoplasmic staining (with stronger staining in <25% of the cells); (3) 2, moderate cytoplasmic staining in 25-50% of the cells; and (4) 3, intense staining in >50% of the cells. The samples ranked as IRS 0 and 1 were defined as low Hsp90-β expressers, IRS 2 as moderate Hsp90-β expressers, and IRS3 as high Hsp90-β expressers.
Western blot
Twenty four paired fresh cancer tissues and adjacent normal tissues were collected from May 2010 to December 2011, and the total protein was extracted. In all samples, 30 μg of total protein was loaded on a 10% SDS-PAGE gel and electrophoretically separated. The proteins were transferred to polyvinylidene difluoride membranes according to the routine protocol. The membranes were incubated with rabbit polyclonal antibody against human Hsp90-β (ABGENT, catalog #AP7867d, San Diego, CA, USA) at a dilution of 1:1000 overnight at 4°C. The primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies, the membrane were detected with the enhanced chemiluminescence method. And β-actin was used as a control for equivalent protein loading.
Statistical analysis
The associations between the expression status and clinicopathological parameters were analyzed using the chi-square test. Kaplan-Meier survival analysis with the Log-rank significance test was used to estimate the survival differences among various subgroups according to HSP90-β expression in lung ADC tissues using SigmaPlot/SigmaStat v10 and 3.5, respectively (Systat Software, San Jose, CA, USA). The significance level was defined as P<0.05.
Results
Expression of Hsp90-β in lung cancer and normal tissues
Seventy-five lung ADC tissue samples were examined by immunohistochemistry. Hsp90-β staining was predominantly located in the cytoplasm of the tumor cells. According to the IRS criteria, the expression intensities of Hsp90-β immunostaining were low (IRS0, 1), moderate (IRS2), and high (IRS3), as shown in Figure 1. Correlations between the clinicopathological features and Hsp90-β expression in lung adenocarcinoma are summarized in Table 2. The IHC results showed that moderate and high Hsp90-β protein expression was observed in 82.7% (62/75) of lung cancer samples, and almost no or low expression (75/75, 100.0%) was observed in the matched normal alveoli. In addition, Hsp90-β expression was markedly associated with tumor grade. The expression frequencies of Hsp90-β in the poorly-differentiated tumors were considerably higher than those detected in the well-differentiated tumors (45.8%, 34.6% and 0.0% expression for poorly, moderately and well-differentiated tumors, respectively, P<0.001). However, Hsp90-β expression did not correlate with other clinicopathologic factors, such as gender, age, histology, TNM stage, and lymph node metastasis. The Western blot results indicated high expression levels of Hsp90-β in the cancer tissues than the adjacent normal tissues (P<0.001, Figure 2).
Figure 1.
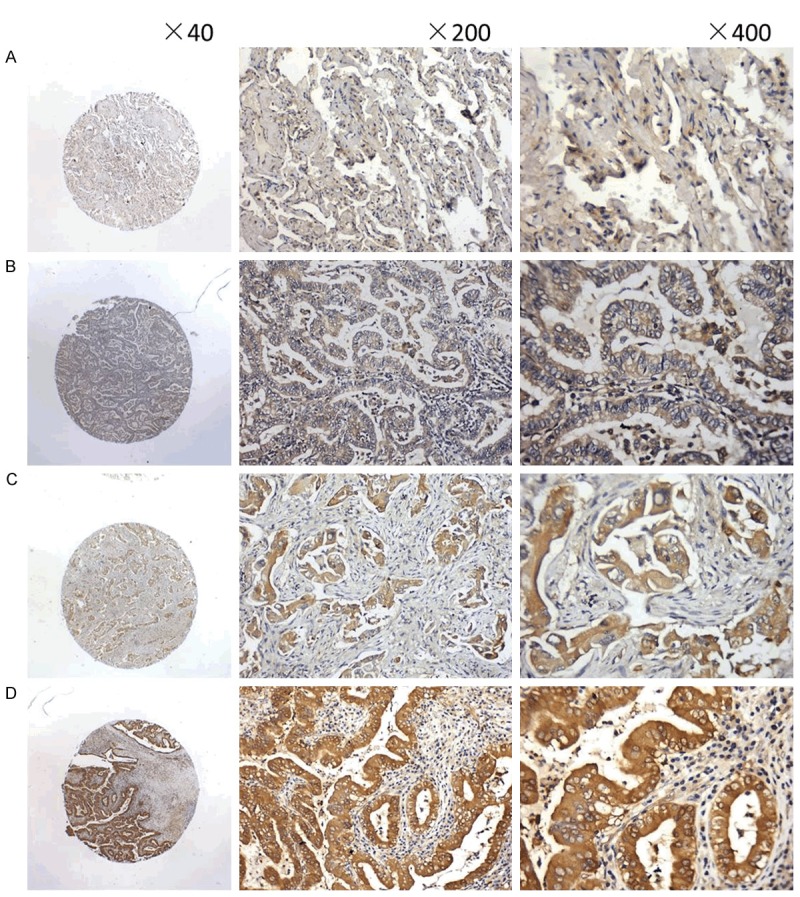
Hsp90-β protein expression in lung ADC tissues as observed by immunohistochemical staining with a rabbit polyclonal antibody against Hsp90-β applied to formalin-fixed and paraffin-embedded tissues in the manner of tissue microarrays. A. The expression intensity of Hsp90-β immunostaining in normal alveoli was IRS0; B-D. The expression intensities of Hsp90-β immunostaining in lung ADCs were IRS01, IRS2, and IRS3, respectively.
Table 2.
Correlation between clinicopathological features and Hsp90-β expression in lung adenocarcinomas
| Groups | Characteristics | N | Expression of Hsp90-beta | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Low (%) | Moderate (%) | High (%) | χ2 value | P value | |||
| Gender | Male | 43 | 7 (16.3) | 23 (53.5) | 13 (30.2) | 0.659 | 0.719 |
| Female | 32 | 6 (18.8) | 19 (59.4) | 7 (21.9) | |||
| Age | <60 | 39 | 8 (20.5) | 21 (53.8) | 10 (25.6) | 0.573 | 0.751 |
| ≥60 | 36 | 5 (13.9) | 21 (58.3) | 10 (27.8) | |||
| Histology | APA | 31 | 6 (19.4) | 17 (54.8) | 8 (25.8) | 6.758 | 0.344 |
| PPA | 20 | 4 (20.0) | 9 (45.0) | 7 (35.0) | |||
| SPA | 13 | 1 (7.7) | 7 (53.8) | 5 (38.5) | |||
| Others# | 11 | 2 (18.2) | 9 (81.8) | 0 (0.0) | |||
| Pathologic grade | Well | 23 | 11 (47.8) | 12 (52.2) | 0 (0.0) | 27.249 | <0.001 |
| Moderate | 26 | 2 (7.7) | 15 (57.7) | 9 (34.6) | |||
| Poor | 24 | 0 (12.5) | 13 (54.2) | 11 (33.3) | |||
| Undifferentiated | 2 | 0 (0.0) | 2 (100.0) | 0 (0.0) | |||
| TNM staging | I | 44 | 8 (18.2) | 20 (45.6) | 16 (36.4) | 5.912 | 0.052 |
| II+III | 31 | 5 (16.1) | 22 (71.0) | 4 (12.9) | |||
| Lymph node metastasis | N0 | 48 | 8 (16.7) | 25 (52.1) | 15 (31.3) | 1.450 | 0.484 |
| N1 | 27 | 5 (18.5) | 17 (63.0) | 5 (18.5) | |||
APA, Acinar predominant adenocarcinoma; PPA, Papillary predominant adenocarcinoma; SPA, Solid predominant adenocarcinoma.
others including Micropapillary predominant adenocarcinoma, Lepidic predominant adenocarcinoma, Invasive mucinous adenocarcinoma, Fetal invasive adenocarcinoma, and Enteric invasive adenocarcinoma.
Figure 2.
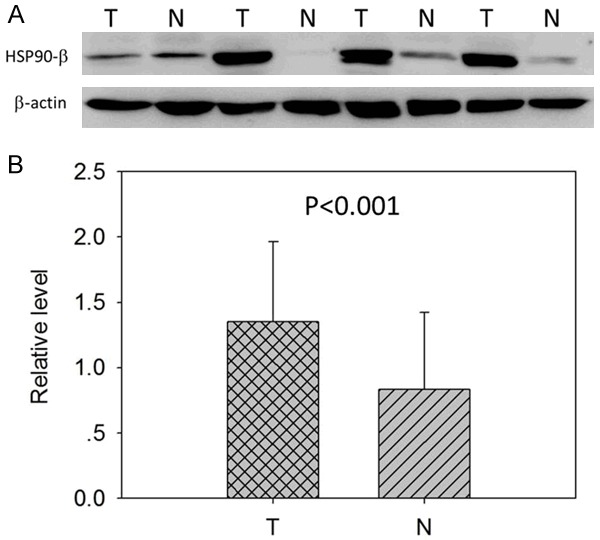
Protein expression of Hsp90-β in lung ADC tissues was detected by Western blotting. A. Hsp90-β expression in paired normal (N) and tumor (T) tissues of 24 ADC patients. B. The Western blot results indicated high expression levels of Hsp90-beta in the cancer tissues than the adjacent normal tissues (P<0.001).
Association of high levels of Hsp90-β protein in tumor tissue with poor prognosis in early stage patients
Overall survival (OS) was calculated from the date of surgery to the date of death or the last follow-up. Unexpectedly, there was no significant relationship between Hsp90-β expression levels and the prognosis of lung ADC patients. However, we further investigated the association between Hsp90-β expression levels and early stage lung ADC patient prognosis. There were 44 early stage (IA-IB) patients. The median follow-up period was 22 months (ranging from 2-95 months), and 20 patients were alive at the time of the last follow-up. The Kaplan-Meier survival analysis revealed that the patients with high (n=16) Hsp90-β expression had a significantly poorer outcome than those with moderate (n=20) or low (n=8) Hsp90-β expression (P=0.034) (Figure 3). The postoperative median OS of the patients with high, moderate and low staining were 22 months, 27 months, and 50 months, respectively.
Figure 3.
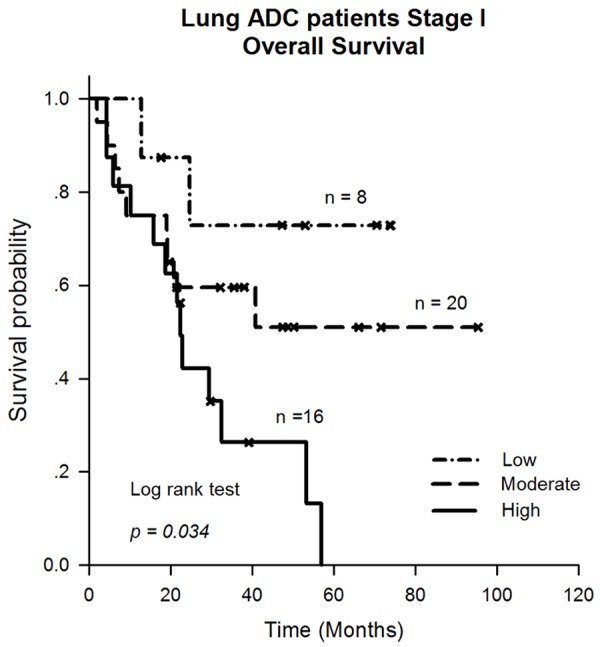
The Kaplan-Meier survival analysis revealed that the patients with high (n=16) Hsp90-β expression had a significantly poorer outcome than those with moderate (n=20) or low (n=8) Hsp90-β expression (P=0.034).
Furthermore, to evaluate the independent impact of Hsp90-β over-expression on OS, a multivariate Cox’s proportional hazards regression model adjusted for age, gender, histology, pathologic grade and Hsp90-β expression was performed. Our results showed that Hsp90-β expression was an independent prognostic factor for OS (Hazard ratios, 2.440; 95% confidence interval, 1.076-5.530; P=0.033) for early stage lung ADC cancer patients (Table 3).
Table 3.
Multivariate cox regression analysis for overall survival in early stage patients with lung ADC cancer
| Variable | Overall survival (n=44) | |
|---|---|---|
|
| ||
| Hazard ratios (95% CI) | P value | |
| Age | 0.991 (0.946-1.038) | 0.702 |
| Gender | 1.109 (0.435-2.828) | 0.829 |
| Histology | 0.991 (0.595-1.650) | 0.971 |
| Pathologic grade | 1.190 (0.529-2.676) | 0.674 |
| Hsp90-beta | 2.440 (1.076-5.530) | 0.033 |
CI, confidence interval.
Discussion
The incidence rates of lung adenocarcinoma are increasing rapidly in most countries among males and females and account for approximately 85% of non-small cell lung cancer (NSCLC) [13]. The highly conserved Hsp90, comprising 1-2% of cellular proteins, acts as an essential cytoplasmic chaperone complex and is involved in maintaining homeostasis by assisting protein folding [5]. In this study, the relationship between Hsp90-β expression and clinicopathological factors was evaluated. We observed that Hsp90-β was commonly over-expressed in lung ADC, even in very early stage (IA-IB) patients. By multivariate analysis, high levels of Hsp90-β expression remained an independent factor for overall survival in stage I ADC patients. More importantly, our data suggest that Hsp90-β over-expression was associated with high pathologic grade lung ADC.
Targeted therapy is currently available for lung ADC. The frequency of the EGFR (epidermal growth factor receptor) mutation is 15%-20% in white populations and over 50% in Asian patients with ADC [14]. Hsp90 binds some important transcription factor and signaling molecule gene promoters, such as c-myc, p53, scr, and rhomboid (rho) [15]. Furthermore, rho codes for a component the triggers the EGF pathway in Drosophila adult muscle precursors (AMPs), suggesting that Hsp90 is also implicated in the EGFR pathway [16]. The Hsp90 N-terminus contains an ATP-binding domain, which is important for its cellular functions [17]. Several Hsp90 inhibitors that are used to target cancers by inhibiting Hsp90’s ATPase activity have been successfully evaluated in clinical trials, for example, geldanamycin [18]. A recent study reported that combining a proteasome inhibitor with an Hsp90 antagonist might represent a novel strategy against NSCLC (non-small cell lung cancer) [19]. In our cohort study, Hsp90-β was significantly increased in 82.7% of lung ADC samples compared to that of normal alveoli and was associated with poor outcome in stage I patients after surgery. These clinical studies suggest that Hsp90 is an effective anticancer target [20].
In our study, significantly increased levels of Hsp90-β were detected in poorly differentiated lung ADC tissues, suggesting that Hsp70-β over-expression is responsible for the differentiation of lung ADC cells. It has been reported that Hsp90-β inhibits cell differentiation by preventing auto-ubiquitination and the degradation of its client protein, cellular inhibitor of apoptosis protein-1 (c-IAP1) [21]. The ubiquitin protein ligase activity of c-IAP1 regulates the tumor necrosis factor alpha (TNF-α) signaling pathway by expressing tumor necrosis factor receptor 2 (TNFR2) [22]. Hsp90 inhibitors have been shown to block cell differentiation [23]. Retaspimycin hydrochloride (IPI-504), one of the inhibitors, is in clinical trials for NSCLC patients (http://www.ClinicalTrial.gov). Furthermore, IPI-504 induced cell apoptosis and blocked the migration and invasion of high-grade gliomas (HGGs), indicating that IPI-450 could be a therapeutic strategy for gliomas [24].
In conclusion, our results show that Hsp90-β exhibited high expression levels in lung ADC tissues, particularly in poorly differentiated lung cancer. Additionally, increased Hsp90-β levels were an independent predictor of worse prognosis.
Acknowledgements
This work was supported by the Natural Science Foundation of Liaoning Province (20102120).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, He J. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hung JJ, Jeng WJ, Hsu WH, Chou TY, Huang BS, Wu YC. Predictors of death, local recurrence, and distant metastasis in completely resected pathological stage-I non-small-cell lung cancer. J Thorac Oncol. 2012;7:1115–23. doi: 10.1097/JTO.0b013e31824cbad8. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, Garg K, Austin JH, Asamura H, Rusch VW, Hirsch FR, Scagliotti G, Mitsudomi T, Huber RM, Ishikawa Y, Jett J, Sanchez-Cespedes M, Sculier JP, Takahashi T, Tsuboi M, Vansteenkiste J, Wistuba I, Yang PC, Aberle D, Brambilla C, Flieder D, Franklin W, Gazdar A, Gould M, Hasleton P, Henderson D, Johnson B, Johnson D, Kerr K, Kuriyama K, Lee JS, Miller VA, Petersen I, Roggli V, Rosell R, Saijo N, Thunnissen E, Tsao M, Yankelewitz D. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–50. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sreedhar AS, Kalmár E, Csermely P, Shen YF. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562:11–5. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- 6.Gruppi CM, Zakeri ZF, Wolgemuth DJ. Stage and lineage-regulated expression of two hsp90 transcripts during mouse germ cell differentiation and embryogenesis. Mol Reprod Dev. 1991;28:209–17. doi: 10.1002/mrd.1080280302. [DOI] [PubMed] [Google Scholar]
- 7.Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127:1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007;67:2932–37. doi: 10.1158/0008-5472.CAN-06-4511. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Wu Y, Wang L, Gao L, Wang Y, Liu X, Zhang K, Song J, Wang H, Bayer TA, Glaser L, Sun Y, Zhang W, Cutaia M, Zhang DY, Ye F. Protein signature for non-small cell lung cancer prognosis. Am J Cancer Res. 2014;4:256–69. [PMC free article] [PubMed] [Google Scholar]
- 10.Westekemper H, Karimi S, Süsskind D, Anastassiou G, Freistühler M, Steuhl KP, Bornfeld N, Schmid KW, Grabellus F. Expression of HSP 90, PTEN and Bcl-2 in conjunctival melanoma. Br J Ophthalmol. 2011;95:853–8. doi: 10.1136/bjo.2010.183939. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Cui S, Zhang X, Wu Y, Tang H. High expression of heat shock protein 90 is associated with tumor aggressiveness and poor prognosis in patients with advanced gastric cancer. PLoS One. 2013;8:e62876. doi: 10.1371/journal.pone.0062876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Xiao T, Zhang Y, Feng L, Lin D, Liu Y, Mao Y, Guo S, Han N, Di X, Zhang K, Cheng S, Gao Y. Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumour tissues of lung cancer patients. Lung Cancer. 2010;69:341–7. doi: 10.1016/j.lungcan.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–62. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawarkar R, Sievers C, Paro R. Hsp90 globally targets paused RNA polymerase to regulate gene expression in response to environmental stimuli. Cell. 2012;149:807–18. doi: 10.1016/j.cell.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 16.Figeac N, Jagla T, Aradhya R, Da Ponte JP, Jagla K. Drosophila adult muscle precursors form a network of interconnected cells and are specified by the rhomboid-triggered EGF pathway. Development. 2010;137:1965–73. doi: 10.1242/dev.049080. [DOI] [PubMed] [Google Scholar]
- 17.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–28. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 18.Barrott JJ, Haystead TA. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013;280:1381–1396. doi: 10.1111/febs.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zismanov V, Drucker L, Gottfried M. Combined inhibition of hsp90 and the proteasome affects NSCLC proteostasis and attenuates cell migration. Anticancer Drugs. 2014;25:998–1006. doi: 10.1097/CAD.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 20.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–16. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 21.Didelot C, Lanneau D, Brunet M, Bouchot A, Cartier J, Jacquel A, Ducoroy P, Cathelin S, Decologne N, Chiosis G, Dubrez-Daloz L, Solary E, Garrido C. Interaction of heat-shock protein 90 beta isoform (HSP90 beta) with cellular inhibitor of apoptosis 1 (c-IAP1) is required for cell differentiation. Cell Death Differ. 2008;15:859–66. doi: 10.1038/cdd.2008.5. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Conze DB, Hanover JA, Ashwell JD. Tumor necrosis factor receptor 2 signaling induces selective c-IAP1-dependent ASK1 ubiquitination and terminates mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:7777–82. doi: 10.1074/jbc.M609146200. [DOI] [PubMed] [Google Scholar]
- 23.Yun BG, Matts RL. Differential effects of Hsp90 inhibition on protein kinases regulating signal transduction pathways required for myoblast differentiation. Exp Cell Res. 2005;307:212–23. doi: 10.1016/j.yexcr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Di K, Keir ST, Alexandru-Abrams D, Gong X, Nguyen H, Friedman HS, Bota DA. Profiling Hsp90 differential expression and the molecular effects of the Hsp90 inhibitor IPI-504 in high-grade glioma models. J Neurooncol. 2014;120:473–81. doi: 10.1007/s11060-014-1579-y. [DOI] [PubMed] [Google Scholar]


