Abstract
Aim: To investigate the expression of silent information regulator 1 (SIRT1) in rats with polycystic ovary syndrome (PCOS) and its alteration after exenatide treatment. Methods: PCOS rat model was established by dehydroepiandrosterone induction. The animals were randomly divided into exenatide treatment group (EX group, n = 10), metformin treatment group (MF group, n = 10), PCOS group (PCOS group, n = 9) and normal control group (NC group, n = 10). Histological changes of the ovarian tissues were examined by HE staining. SIRT1 expression in the ovarian tissue was detected by RT-PCR and immunohistochemistry. Results: Rats in the PCOS group lost their estrous cycle. Histological observation of the ovary showed saccular dilatation of the follicle, decreased number of corpora lutea, fewer layers of granulosa cells aligned loosely, and thickened layer of theca cells. The changes in reproductive hormones and the development of insulin resistance suggested the successful establishment of the animal models. Immunohistochemistry and Q-PCR detected the mRNA and protein expressions of SIRT1 in the ovary tissues of rats in the normal control group. The SIRT1 expression was significantly lower in PCOS group than in control group (P < 0.05); after drug intervention, the SIRT1 expression significantly increased in EX and MF groups (compared with the PCOS group), whereas no significant difference was noted between the EX group and MF group. Conclusions: The SIRT1 expression in the ovary tissue decreases in PCOS rats (compare with the normal rats) but can be up-regulated after Ex or MF treatment. These drugs may affect the process and development of PCOS by regulating the SIRT1 expression. Exenatide may be therapeutic for PCOS by up-regulating the SITR1 expression.
Keywords: Polycystic ovary syndrome, silent information regulator 1, exenatide, dimethyl biguanide, ovary
Introduction
Polycystic ovary syndrome (PCOS) is a common reproductive endocrine disorder among adolescent and reproductive age women. The incidence of PCOS in women of reproductive age is up to 6-10%, with heterogeneous clinical manifestations. Insulin resistance (IR) and hyperinsulinemia form an important pathophysiological basis for PCOS [1].
Silent information regulator 1 (Sirtuin, SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase. In the process of glucose metabolism, SIRT1 can promote insulin secretion and increase insulin sensitivity. PCOS is a systemic endocrine disorder most characterized by IR, and it is associated with obesity as well as disorders of glucose and lipid metabolism. Drugs that can improve insulin sensitivity are gradually being used for the treatment of PCOS patients. Exenatide (EX) is the first drug with glucagon-like peptide-1 (GLP-1) activity available for clinical application and approved for market by the Food and Drug Administration of the United States. EX can stimulate the proliferation and differentiation of islet β cells and effectively improve their function. This drug plays an important role in delaying the development and progression of diabetes mellitus. Additionally, it indicates a new direction for study of the pathogenesis and treatment of PCOS [2]. To date, the therapeutic efficacy of EX in PCOS has rarely been reported. The present study evaluated the expression of SIRT1 in the ovarian tissue of PCOS rats using immunohistochemistry (IHC) and real time-polymerase chain reaction (RT-PCR). Additionally, PCOS rats were treated with EX or metformin (MF). SIRT1 expression and its alteration in ovarian tissue after drug intervention were examined. The discussion of the results explores the therapeutic effects of EX and MF in the development and outcome of PCOS to identify a new PCOS treatment at the molecular level.
Materials and methods
Experimental animals
Fifty female Sprague-Dawley (SD) rats were provided by the Experimental Animal Center at Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China (Certificate No. SCXK (Guangdong) 2009-0011). The animals were of specific-pathogen-free (SPF) grade and were 23 days old, with a mean body weight of 67.00 ± 15.96 g.
Reagents
Primary rabbit anti-mouse anti-SIRT1 polyclonal antibody was purchased from Santa Cruz (Cat#: sc-15404). The secondary antibody was from a Chem MateTM EnVision+/HRP kit produced by Guangzhou Jetway Biotech. Co., Ltd (Cat#: GK500705). For the RT-PCR assay, Trizol reagent was purchased from Invitrogen, a reverse transcription kit was purchased from TaKaRa (Cat#: DRR036), a RT-PCR kit was purchased from TaKaRa (Cat#: DRR420), and PCR primers were purchased from Invitrogen.
Replication of a PCOS animal model
Fifty 23-day-old female SD rats of SPF grade were randomly divided into two groups: the PCOS model group (n = 40), which was administered dehydroepiandrosterone (DHEA; Meck) at 6 mg/ (100 g.d) + 0.2 mL of injectable soybean oil via hypodermic injection for 20 consecutive days [3]; and the normal control (NC) group (n = 10), which was given 0.2 mL of injectable soybean oil via hypodermic injection over the same period. From day 21. The model was considered successfully established when rats of the PCOS model group lost their estrous cycle. Eleven PCOS rats were randomly selected for a vaginal smear, serum reproductive hormone test, and histological examination of ovarian tissue to further evaluate the efficiency of the model creation. The remaining rats of the PCOS model group were randomly divided into an EX group (n = 10), MF group (n = 10), and PCOS control group (n = 9) [4]. The MF group was given 300 mg·kg-1·d-1 of MF dissolved with sterile distilled water (dose calculated based on the kg body weight conversion factor for human to rat) via intragastric injection. The EX group was given 10 μg·kg-1·d-1 of EX via hypodermic injection [5]. The PCOS and NC groups were given a normal diet every day.
Specimen collection
After successful model establishment, the rats were grouped and treated with drug intervention for four weeks. Then, the animals were anesthetized with 10% chloral hydrate (3.5 μL/g) for laparotomy to collect ovarian tissue specimens. Then the ovary specimens were rapidly fixed in 10% neutral formaldehyde solution, embedded with paraffin, and cut into sections. Four weeks after modeling and drug intervention, the fasting insulin (FINS), OGTT, and HOMA-IR were measured to assess the IR degree. After drug intervention: T: significantly lower in MF, EX, and NC groups than in PCOS group (P < 0.05); LH: significantly lower in MF and EX groups than in PCOS group (P < 0.05); LH/FSH: significantly lower in EX group than in PCOS group (P < 0.05); serum FPG, FINS, and HOMA-IR: significantly lower in MF, EX, and NC groups than in PCOS group (P < 0.05); 30 min PG: significantly higher in MF group than in EX, PCOS, and NC groups (P < 0.05); 1 h PG: significantly higher in MF and PCOS groups than in EX and NC groups (P < 0.05); 2 h PG: significantly higher in PCOS group than in MF, EX, and NC groups (P < 0.05).
Histopathological examination
After 4 h of fixation in formalin, rat ovarian tissue specimens were subjected to conventional dehydration followed by paraffin embedding and sectioning (4 μm thick). Hematoxylin and eosin stain (HE) staining was performed to examine the pathological structures of the rat ovary.
Fluorescence quantitative RT-PCR
The four groups of ovarian tissue specimens were milled separately with a 5-mL tissue homogenizer. Total RNA was extracted from all specimens using Trizol reagent, and the RNA concentration was measured using a UV spectrophotometer. The extracted RNA (3 µL each for 10-µL reaction) was reverse-transcribed into cDNA, and 2.5 µL of the reverse transcription products was used as a template for PCR amplification. The primer sequences used for SIRT1 were as follows: upstream 5’-TCATTCCTGTGAAAGTGATGACGA-3’ and downstream 5’-CTGCCCTAGTGTCATATCATCCAA-3’. The primer sequences for the endogenous control GAPDH were upstream 5’-GGCACAGTCAAGGCTGAGAATG-3’ and downstream 5’-ATGGTGGTGAAGACGCCAGTA-3’. The amplification conditions were as follows: pre-denaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 3 s and annealing at 60°C for 30 s. Data were collected and used for relative quantitative analysis with the 2-ΔΔCT method. The relative mRNA expression level was obtained by comparing data of the experimental group with those of the control group. The experiment was repeated three times.
IHC staining
The EnVision method was used for IHC staining. Sections (3-4 mm thick) were cut following a conventional procedure. The section specimens were affixed to silicate glass and heated at 60°C for 1 h. After dewaxing in fresh xylene (5 min × 3 times), the specimens were treated with gradient ethanol dehydration (100%, 100%, 95%, and 80%, 5 min each) and rinsed with tap water, followed by two rinses with distilled water (1-2 min each). The rinsed specimens were placed in 3% hydrogen peroxide for microwave heating at low power for 3 min and then rinsed with tap water, followed by two rinses with distilled water (1-2 min each). The rinsed specimens were fixed with pH 8.0 ethylenediaminetetraacetic acid (EDTA) under high pressure and then cooled for 20 min. After rinsing with phosphate-buffered saline (PBS) three times (3 min each), 50 µL of primary antibody (SIRT1 dilution factor 1:100) was added to each specimen and incubated in a humidified chamber at 37°C for 1 h. The specimens were rinsed with PBS three times (5 min each), and each specimen was added with drops of secondary antibody and then incubated in a humidified chamber at 37°C for 30 min. After washing with PBS three times (5 min each), a 3,3’-diaminobenzidine (DAB) solution was added to the specimens for coloration, and the reaction was terminated with tap water. Hematoxylin was used for counterstaining, and tap water was used for bluing. The specimens were dried and mounted for microscopic examination and photographing. A negative control was prepared by substituting PBS for the primary antibody.
Statistical analysis
The statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The measurement data were expressed as the mean ± standard deviation (SD). A normality test and homogeneity of variance test were performed prior to comparison of the group means. An independent two-samples t test was used for homogeneity of variance, and the t’ test was used for heterogeneity of variance. Variables that did not meet normal distribution were analyzed using a non-parametric test. Comparisons of multiple samples were performed using one-way ANOVA at the 0.05 level.
Results
Results of estrous cycle monitoring
Twenty days after the DHEA treatment, rats in the modeling group lost their regular estrous cycles and all of them stayed in the anoestrus period. Microscopy of stained smears of vaginal secretion showed the presence of a large number of white blood cells, while few keratinized cells and non-keratinized cells and epithelial cells were visible, suggesting anovulation. In contrast, rats in the control group had a regular estrous cycle.
Light miscroscopy of the ovary
Morphological changes in the rat ovarian tissue specimens were examined by light microscopy. In the NC group, microscopic examination after HE staining revealed the presence of follicles of different developmental stages and a few corpora lutea; granulosa cells were orderly arranged with an intact form, mostly in 6-8 layers. Theca cells were arranged in a spindle pattern, and the theca and granulosa cells did not proliferate. In the PCOS group, oocyte or corona radiata disappeared within the follicle; follicles of different developmental stages and corpora lutea were rare in the cortex. The number of follicles with saccular dilatation significantly increased. Granulosa cells were arranged loosely in fewer (only 2-3) layers, and the theca and granulosa cells proliferated, with atresia of some follicles. In the MF and EX groups, the number of granulosa cell layers increased to approximately 5-6 and showed an intact form in an orderly arrangement. Theca cells were arranged in a spindle pattern, with follicular fluid and cumulus oophorus observed in dominant follicles. Additionally, theca cell layer became thinner while the number of corpora lutea increased and dilated follicles significantly decreased compared with those in the PCOS group (Figure 1).
Figure 1.
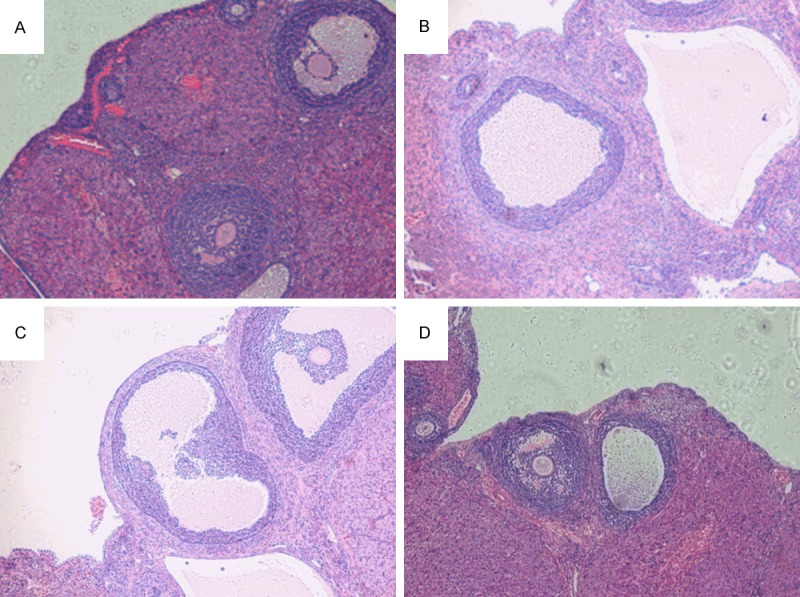
Comparison of rat ovarian follicle development before and after medication among the four groups (HE staining, magnification × 400) A. NC group; B. PCOS group; C. MF group; D. EX group.
IHC staining
SIRT1 positive staining in the rat ovary was mainly concentrated in the nucleus. The stain was light yellow to dark brown. The staining intensity was scored as follows [6]: 0, colorless; 1, light yellow; 2, brown yellow; and 3, brown (stain color compared with background color). Then, the percentage of positive cells was scored as follows: 0, negative; 1, positive cells ≤ 10%; 2, positive cells 11-50%; 3, positive cells 51-75%; and 4, positive cells > 75%. The product of the two scores was taken as the final score (Table 1; Figure 2).
Table 1.
Comparison of immunohistochemical scores for SIRT1 expression in rat ovarian tissue among the four groups (mean ± SD)
| Group | n | Score |
|---|---|---|
| MF group | 10 | 7.40 ± 1.26* |
| EX group | 10 | 8.00 ± 1.15*,# |
| PCOS group | 9 | 2.33 ± 0.71 |
| NC group | 10 | 9.10 ± 1.60* |
The means of both the MF and EX groups were compared withthat of the PCOS group, P < 0.05;
the mean of the EX groupwas compared with that of the MF group, P > 0.05 (P = 0.260).
Figure 2.
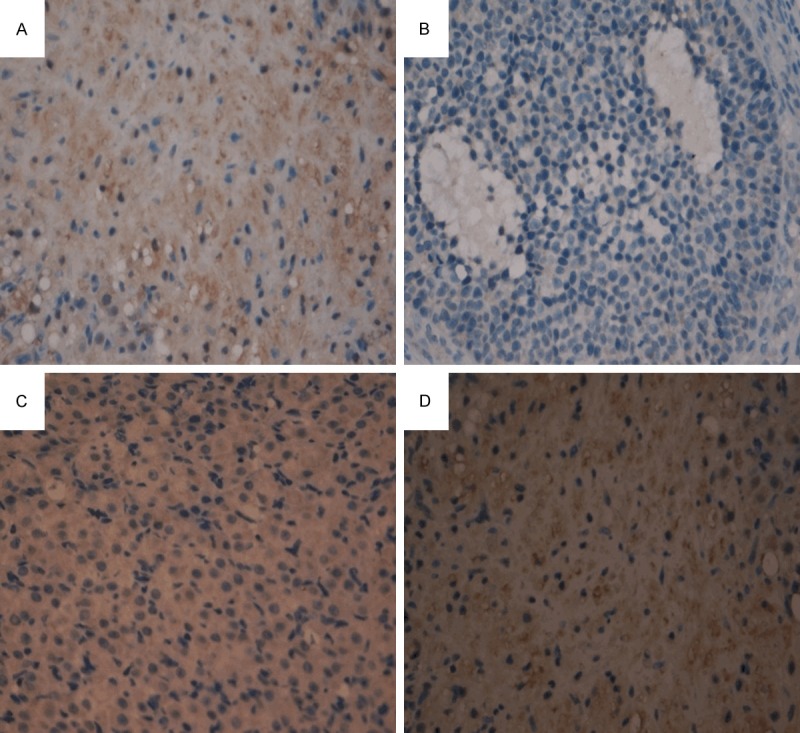
Expression of SIRT1 in rat ovarian tissue before and after medication among the four groups (IHC staining, magnification × 400) A. NC group; B. PCOS group; C. EX group; D. MF group.
Fluorescence quantitative RT-PCR
The expression level of SIRT1 mRNA in the rat ovarian tissue of the PCOS group was significantly lower than that in the NC group (P < 0.05). After drug intervention, SIRT1 mRNA expression was up-regulated to varying degrees in the EX and MF groups compared with that of the PCOS group (P < 0.05). There was no statistically significant difference between the EX and MF groups (P > 0.05) (Table 2; Figure 3).
Table 2.
Comparison of SIRT1 mRNA expression in rat ovariantissue among the four groups (mean ± SD)
The means of both the MF and EX groups were compared with that of the PCOS group, P < 0.05;
the mean of the EX group was compared with that of the MF group, P > 0.05 (P = 0.139).
Figure 3.
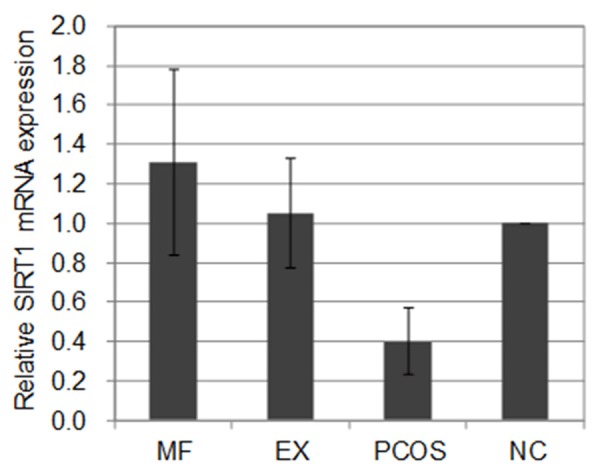
Comparison of SIRT1 mRNA expression in rat ovarian tissue among the four groups (mean ± SD).
Discussion
PCOS is an endocrine/metabolic disorder characterized by IR, which has multifactorial pathogenesis and heterogeneous clinical manifestations. Independent of whether the patients are obese, PCOS patients are associated with varying degrees of IR. Among the PCOS patients, the IR of classical (e.g., pancreas) and non-classical target tissues (e.g., ovary) can synergistically induce obesity, abnormal glucose metabolism, and changes in the reproductive function of the ovary.
SIRT1 and PCOS
SIRT1 is a NAD+-dependent histone deacetylase. In insulin-sensitive tissues and organs, SIRT1 can improve insulin sensitivity and IR as well as hyperinsulinemia by adjusting the interactions between insulin-related proteins and the insulin signal transduction pathway [7]. Decreased expression of SIRT1 or alteration of its activity may be related to the pathogenesis of IR-related diseases. Among PCOS patients, IR is not limited to pancreatic tissue but involves ovarian tissue itself and affects its excitometabolic signaling pathway. Sonntag et al. [8] suggest that insulin and insulin-like growth factors in ovarian tissue play important roles in steroid hormone synthesis and ovarian follicular development; thus, abnormal insulin signal transduction also exists in the local ovary. The results from the present study showed that rats in the PCOS model group had significant IR and hyperinsulinemia. It is currently believed that the decline in insulin sensitivity results in compensatory high insulin levels. This is an important cause of hyperandrogenism and ovarian function changes, as well as a pathological basis for metabolic abnormalities and reproductive dysfunction in PCOS. In the course of PCOS, including that characterized by IR, alteration of SIRT1 expression is still unclear. In this study, a dehydroepiandrosterone (DHEA)-induced PCOS model was constructed in female rats. It was found that SIRT1 expression in ovarian tissue was significantly lower in PCOS rats than in normal rats (P < 0.05). This finding suggests that the decline in SIRT1 expression may be related to local IR in the ovary.
Effect of MF on PCOS
MF is an insulin sensitizer and belongs to the biguanide class of oral hypoglycemic agents. Its anti-hyperglycemic effect enhances insulin sensitivity in the liver and peripheral tissues, reduces the generation of endogenous glycogen, and promotes glucose utilization of skeletal muscle and fat cells. Thus, MF can reduce fasting and postprandial blood glucose, alleviate the IR state, and improve hyperinsulinemia. MF is currently widely used to treat PCOS in obese patients [9]. The present study found that after MF intervention, PCOS rat ovarian tissue underwent a series of morphological changes with a thicker granule cell layer, thinner theca folliculi, increased corpora lutea, and recovery of ovulation.
MF improves the blood sugar level in diabetic patients by activating adenosine monophosphate-activated protein kinase (AMPK). AMPK is a receptor that senses the body’s energy state. It can sense changes in cellular adenosine triphosphate/adenosine monophosphate (ATP/AMP) levels and promote cells transformation from anabolism to catabolism to maintain the cellular energy balance. Research shows [10] that AMPK also simultaneously activates another energy sensor, SIRT1. The latter is capable of deacetylation of transcription factors such as Forkhead box protein O1 (FOXO1), FOXO3a, and peroxisome proliferator-activated receptor γ (PPAR-γ), further regulating energy metabolism in the body. Other studies [11,12] indicate that MF plays an independent role in addition to its insulin-sensitizing effects. However, it often causes serious gastrointestinal adverse reactions (e.g., nausea and vomiting). For patients with cardiac, hepatic, and renal insufficiency, MF treatment can increase the risk of lactic acidosis. It has been shown that long-term use of MF may lead to deficiency of vitamin B12. At present, commonly used insulin sensitizers (e.g., MF and thiazolidinediones) mainly target liver IR. Among PCOS patients, 31-35% has impaired glucose tolerance (IGT), and 7.5-10% present with diabetes [13], among whom the major type of IR is muscle IR.
The present study found that SIRT1 expression in the rat ovarian tissue of the MF group was significantly higher than that of the PCOS group (P < 0.05). To date, the mechanism through which MF improves SIRT1 expression in ovarian tissue is poorly understood. Whether it involves an indirect effect of reducing local ovarian IR or a direct effect of MF needs to be examined in a further study.
Effect of exenatide on PCOS
GLP-1 is an incretin, which can stimulate insulin biosynthesis and glucose-dependent insulin secretion. GLP-1 also increases the activity of islet β cells, promotes their proliferation and differentiation, and inhibits their apoptosis. Moreover, it reduces glucagon secretion and the rate of gastric emptying, promotes satiety, and inhibits food intake; thus, GLP-1 can effectively control body weight [14,15]. However, active GLP-1 has a very short half-life in vivo due to its degradation by specific enzymes and renal excretion, which severely limits the application of GLP-1. After structural modification of GLP-1, a novel medication with GLP-1 activity was obtained, i.e., EX. EX can better resist digestion by dipeptidyl-peptidase IV in vivo, significantly increasing its biological activity and action period in vivo. This drug has been used for clinical treatment of diabetes. The molecular mechanisms through which EX reduces IR in patients with obesity and type 2 diabetes are still unclear. As an important regulator of energy metabolism, SIRT1 may be involved in the regulation of the insulin signaling pathway. A study found [16] that SIRT1 expression in diabetic mice adipose tissue was significantly down-regulated at the mRNA and protein levels; after EX treatment, SIRT1 mRNA and protein expression in adipose tissue was up-regulated. This effect may be achieved through the improvement of p-JNK and p-CJUN protein expression in mouse adipose tissue and by up-regulating IRS1 and p-AKT protein levels in the insulin signaling pathway.
For PCOS similarly characterized by ovarian IR, Elkind-Hirsch et al. [2] directly used EX and MF for a randomized controlled treatment trial of PCOS patients. The results showed that EX relieved body weight, reduced abdominal circumference, and down-regulated androgen levels in vivo, thereby effectively improving hyperinsulinemia and increasing insulin sensitivity. The present study found that after EX intervention, PCOS rat ovarian tissue underwent histomorphological changes similar to those after MF intervention, including an increased number of granulosa cell layers, thinner theca cell layer, and more corpora lutea. Additionally, the expression of SIRT1 in ovarian tissue was significantly higher in the drug intervention group than in the PCOS group (P < 0.05). We inferred that EX may be involved in the regulation of the insulin signaling pathway by influencing SIRT1 expression.
In short, these results suggest that a decline in SIRT1 expression in the ovarian tissue of PCOS rats may be involved in the development and progression of PCOS. SIRT1 expression in ovarian tissue can be up-regulated through EX administration, which reverses the course of PCOS morphologically. Thus, SIRT1 can serve as a new molecular target, providing new options for PCOS treatment advances in the future.
Acknowledgements
This work was supported by Science and Technology Planning Project of Guangdong Province, China (2013B022000012).
Disclosure of conflict of interest
None.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ. The prevalence and features of polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of Single and Combined Treatment with Exenatide and Metformin on Menstrual Cyclicity in Overweight Women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2008;93:2670–2678. doi: 10.1210/jc.2008-0115. [DOI] [PubMed] [Google Scholar]
- 3.Anderson E, Lee GY, O’brein K. Polycystic ovarian condition in the dehydroepiandrosterone treated rat model: hyperandrogenism and the resumption of meiosis are major in initial events associated with cystogensis of antral follicle. Anat Rec. 1997;249:44–53. doi: 10.1002/(SICI)1097-0185(199709)249:1<44::AID-AR6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Tao X, Ge SJ, Chen J, et al. Effect of Exenatide on insulin resistance and reproductive hormone in polycystic ovary syndrome rats. Journal of Sun Yat-Sen University (Medical Sciences) 2014;35:6–10. [Google Scholar]
- 5.Wang XY, Zhang S, Li H. Study on the hypoglycemic effects of an exenatide analog in rats with type 2 diabetes mellitus. Chinese Journal of New Drugs. 2010;19:1347–1350. [Google Scholar]
- 6.Kohno M, Maruyama R, Kitagawa D, Sugimachi K, Kinjo M, Higashi H. Localized biphasic type malignant mesothelioma arising in the peritoneum: Report of a case. Thoracic Cancer. 2014;5:74–77. doi: 10.1111/1759-7714.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–19. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Sonntag B, Götte M, Wülfing P, Schüring AN, Kiesel L, Greb RR. Metformin alters insulin signaling and viability of human granulosa cells. Fertil Steril. 2005;84:1173–1179. doi: 10.1016/j.fertnstert.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 9.Leeman L, Acharya U. The use of metformin in the management of polycystic ovary syndrome and associated anovulatory infertility: the current evidence. J Obstet Gynaecol. 2009;29:467–472. doi: 10.1080/01443610902829414. [DOI] [PubMed] [Google Scholar]
- 10.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayrak A, Terbell H, Urwitz-Lane R, Mor E, Stanczyk FZ, Paulson RJ. Acute effects of metformin therapy include improvement of insulin resistance and ovarian morphology. Fertil Steril. 2007;87:870–875. doi: 10.1016/j.fertnstert.2006.08.096. [DOI] [PubMed] [Google Scholar]
- 12.Romualdi D, Giuliani M, Cristello F, Fulghesu AM, Selvaggi L, Lanzone A, Guido M. Metformin effects on ovarian ultrasound appearance and steroidogenic function in normal-weight normoinsulinemic women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Fertil Steril. 2010;93:2303–2310. doi: 10.1016/j.fertnstert.2009.01.114. [DOI] [PubMed] [Google Scholar]
- 13.Lewandowski KC, Szosland K, O’callaghan C, Tan BK, Randeva HS, Lewinski A. Adiponectin and resistin serum levels in women with polycystic ovary syndrome during oral glucose tolerance tests: a significant reciprocal correlation between adiponectin and resistin independent of insulin resistance indices. Mol Genet Metab. 2005;85:61–69. doi: 10.1016/j.ymgme.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Cornu M, Yang JY, Jaccard E, Poussin C, Widmann C, Thorens B. Glucagon-Like Peptide-1 Protects β-Cells Against Apoptosis by Increasing the Activity of an Igf-2/Igf-1 Receptor Autocrine Loop. Diabetes. 2009;58:1816–1825. doi: 10.2337/db09-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaime A, Davidson MD. Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors. Cleve Clin J Med. 2009;76:S28–S38. doi: 10.3949/ccjm.76.s5.05. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZL, Xu F, Gu HM, Yan JH, Liang H, Weng JP. Exenatide upregulates the expression of SIRT1 in adipose tissue and improves insulin resistance in db/db mice. Chinese Journal of Diabetes Mellitus. 2012;(Suppl 4) [Google Scholar]


