Abstract
Pancreatic ductal adenocarcinoma (PDA) is an aggressive malignant tumor with poor prognosis. Epidermal growth factor receptor (EGFR) is an important cell adhesion and signaling pathway mediator. The aim of this study was to evaluate the expression of EGFR in both pancreatic intraepithelial neoplasia (PanIN) and PDA and their relationship to clinicopathologic characteristics. Formalin-fixed, paraffin-embedded tissues including 81 cases with pancreatic ductal adenocarcinoma, 27 with normal pancreas, 16 with PanIN-1A, 18 with PanIN-1B, 11 with PanIN-2, and 24 with PanIN-3 were used for construction of tissue microarrays. Imunohistochemistry for EGFR was performed. Normal pancreatic ducts, PanIN-1A, and PanIN-1B did not show EGFR overexpression. EGFR overexpression was observed in 18.2% (2/9) of PanIN-2, 41.7% (10/14) of PanIN-3, and 64.2% (52/81) of PDA, respectively. Significantly higher EGFR overexpression was observed in PDAs than in PanIN lesions (P<0.05). No statistically significant correlation was observed between EGFR overexpression and patient age, sex, tumor location, size, histological grade, vascular invasion, lymph node metastasis and stage at presentation, respectively. In conclusion, EGFR expression increased from PanIN to PDA. EGFR may be involved in early stage in development of PDA.
Keywords: Epidermal growth factor receptor, pancreatic ductal adenocarcinoma, pancreatic intraepithelial neoplasia
Introduction
Pancreatic ductal adenocarcinoma (PDA) is the fourth leading cause of cancer deaths in the United States and has one of the highest mortality rates of any cancer [1]. Pancreatic intraepithelial neoplasia (PanIN) is a well-defined, noninvasive precursor lesion for PDA.
Epidermal growth factor receptor (EGFR) is a transmembrane growth factor receptor with tyrosine kinase activity. EGFR is frequently overexpressed in many tumors, including lung, breast, colorectal and vulvar cancer [2-4]. Its activation affects the signaling pathways affecting cellular growth, differentiation, and proliferation.
Although EGFR expression has been previously studied in PDAs, its expression has not been well characterized [5-10]. In PanIN lesions, knowledge regarding EGGFR expression is limited. The role of EGFR expression in carcinogenesis of PDA remains controversial.
The aim of this study was to examine the expression of EGFR in PanIN lesions and PDAs and their relationship to clinicopathologic features.
Materials and methods
Patients and specimen
Eighty-one patients with PDA were selected. All patients underwent surgical resection at Yeungnam University Hospital, South Korea, between 1986 and 2014. Twenty seven normal pancreas, 16 PanIN-1A, 18 PanIN-1B, 11 PanIN-2, and 24 PanIN-3 were collected, which were found incidentally in pancreas parenchyma adjacent to resected pancreatic specimens due to traumatic rupture, intraductal papillary mucinous neoplasm, and other tumors such as ampulla of Vater or common bile duct cancers. All tissues were fixed in 10% buffered formalin and embedded in paraffin. Representative blocks for each case were selected for construction of tissue microarrays. A pair of 2-mm-diameter tissue cores were retrieved and transferred to the recipient block. PanIN lesions and histological grade of PDA were classified according to the criteria described in the World Health Organization classification [11]. TNM stage was classified according to AJCC cancer staging [12]. Clinicopathologic parameters including patient age, gender, tumor size, histological grade, location, vascular invasion, perineural invasion, lymph node metastasis, and stage were evaluated by review of medical charts and pathologic records. This study was approved by the institutional review board of Yeungnam University Hospital (YUH-2015-05-023).
Immunohistochemistry of EGFR and assessment of immunoreactivity
Immunohistochemical staining was performed using the Ultra View Universal DAB detection kit on a BenchMark Series automatic stainer (Ventana Medical Systems, Tuscon, AZ, USA). The primary antibody was a mouse monoclonal CONFIRM anti-EGFR (3C6) (Ventana Medical Systems). Omission of primary antibody was used as a negative control of immunohistochemical reaction and perineural fibroblasts served as a positive internal control. Semiquantative assessment of EGFR immunostaining was performed, and a 4-point scale was used for scoring as follows; Score 0: no membrane staining or incomplete membrane staining in less than 10% of cells, Score 1+: weak incomplete membrane staining in more than >10% of cells, Score 2+: moderate and complete membrane staining in more than 10% of cells, Score 3+: strong and complete membrane staining in more than 10% of cells. Score 0 and 1+ were interpreted as negative. Score 2+ and 3+ were considered positive.
Statistical analysis
Statistical analysis was performed using SPSS for window version 18.0. The x2 test or Fisher exact test was used for determination of correlation between EGFR expression and clinicopathologic variables in PDA. Survival curves were calculated using the Kaplan-Meier method, and statistical significance between curves was tested using the Breslow test. Cox proportional hazard regression analyses were performed. A P value of less than 0.05 was considered statistically significant.
Results
Clinicopathologic characteristics and EGFR expression in PDAs
Clinicopathologic characteristics and EGFR expression in PDAs are shown in Table 1. The patient ranged in age from 32 to 81 years, with a median age of 60 years. The male-to-female ratio was 1.7:1. Forty-five cases (55.6%) of PDAs arose in the head of the pancreas, and the remainder in the body (28.4%) and tail (16.0%). Expression of EGFR was observed in 64.2% (52 of 81) of PDAs (Figure 1). EGFR expression was observed in 56.3% (9/16) of well differentiated PDAs, 61.2% (30/49) of moderately differentiated PDAs, and 81.3% (13/16) of poorly differentiated PDAs. No significant correlation was observed between EGFR expression and patient age, sex, tumor size, histological grade, location, vascular invasion, perineural invasion, lymph node metastasis, and stage.
Table 1.
Comparison of EGFR expression and clinicopathologic factors of pancreatic ductal adenocarcinoma
| No. Case (n=81) | EGFR expression | P | ||
|---|---|---|---|---|
|
| ||||
| Negative (n=29) | Positive (n=52) | |||
| Age (y) | ||||
| ≤60 | 39 | 11 | 28 | 0.169 |
| >60 | 42 | 18 | 24 | |
| Sex | ||||
| Male | 52 | 19 | 33 | 0.853 |
| Female | 29 | 10 | 19 | |
| Size (cm) | ||||
| ≤3 | 25 | 8 | 17 | 0.633 |
| >3 | 56 | 21 | 35 | |
| Grade | ||||
| Well | 16 | 7 | 9 | 0.265 |
| Moderately | 49 | 19 | 30 | |
| Poorly | 16 | 3 | 13 | |
| Location | ||||
| Head | 45 | 14 | 31 | 0.364 |
| Body | 23 | 11 | 12 | |
| Tail | 13 | 4 | 9 | |
| VS invasion | ||||
| Absent | 16 | 6 | 10 | 0.874 |
| Present | 65 | 23 | 42 | |
| PN invasion | ||||
| Absent | 27 | 6 | 21 | 0.104 |
| Present | 53 | 23 | 30 | |
| pT stage | 0.665 | |||
| pT1 | 0 | 0 | 0 | |
| pT2 | 2 | 1 | 1 | |
| pT3 | 75 | 26 | 49 | |
| pT4 | 4 | 2 | 2 | |
| LN metastasis | ||||
| Absent | 35 | 14 | 21 | 0.492 |
| Present | 46 | 15 | 31 | |
| DT metastasis | ||||
| Absent | 78 | 27 | 51 | 0.291 |
| Present | 3 | 2 | 1 | |
| Stage | ||||
| I | 2 | 1 | 1 | 0.547 |
| II | 74 | 26 | 48 | |
| III | 2 | 0 | 2 | |
| IV | 3 | 2 | 1 | |
VS, vascular; PN, perineural; LN, lymph node; DT, distant.
Figure 1.
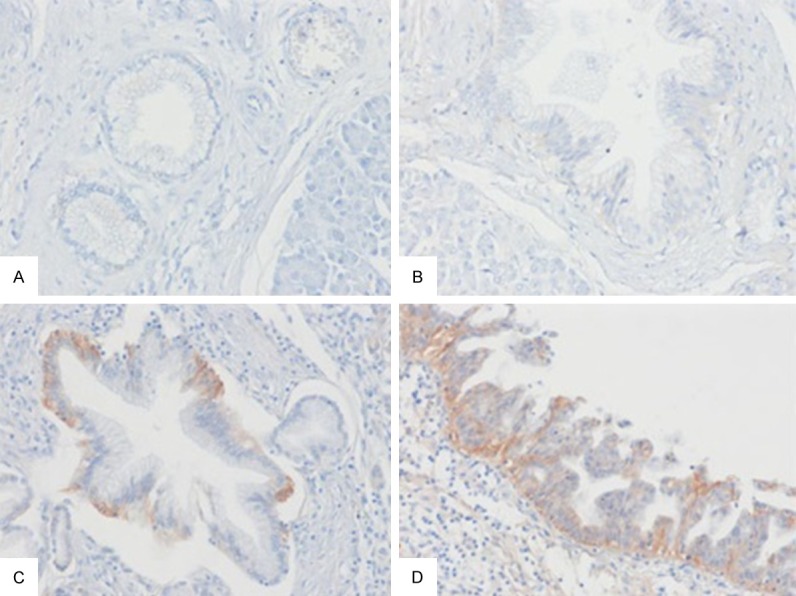
EGFR immunostaining in pancreatic intraepithelial neoplasia (PanIN). A. No EGFR expression in PanIN-1A. B. No EGFR expression in PanIN-1B. C. Score 2+ in PanIN-2. D. Score 2+ in PanIN-3.
EGFR expression in PanIN lesions
EGFR expression in PanIN lesions is summarized in Table 2. Normal pancreatic ducts, PanIN-1A, and PanIN-1B did not show EGFR expression. Expression of EGFR was detected in 18.2% (2/11) of PanIN-2 and in 41.7% (10/24) of PanIN-3 (Figure 2), respectively. Significantly higher EGFR expression was observed in PanIN-3 than in normal, PanIN-1A and PanIN-1B (P<0.01). Significantly higher frequency of EGFR expression was observed in PDA than in PanIN-1A, panIN-1B, and PanIN-2 (P<0.01). No significant difference in EGFR expression was observed between PanIN-3 and PDA.
Table 2.
EGFR expression in pancreatic intraepithelial neoplasia and ductal adenocarcinoma
| No. Case | EGFR expression | P | ||
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Normal | 27 | 27 (100) | 0 (0) | |
| PanIN-1A | 16 | 16 (100) | 0 (0) | |
| PanIN-1B | 18 | 18 (100) | 0 (0) | |
| PanIN-2 | 11 | 9 (81.8) | 2 (18.2) | |
| PanIN-3 | 24 | 14 (58.3) | 10 (41.7)* | |
| Adenocarcinoma | 81 | 29 (36.8) | 52 (64.2)** | |
P<0.01 versus normal, PanIN-1A, and PanIN-1B;
P<0.01 versus normal, PanIN-1A, PanIN-1B, PanIN-2, and P<0.05 versus PanIN-3.
Figure 2.
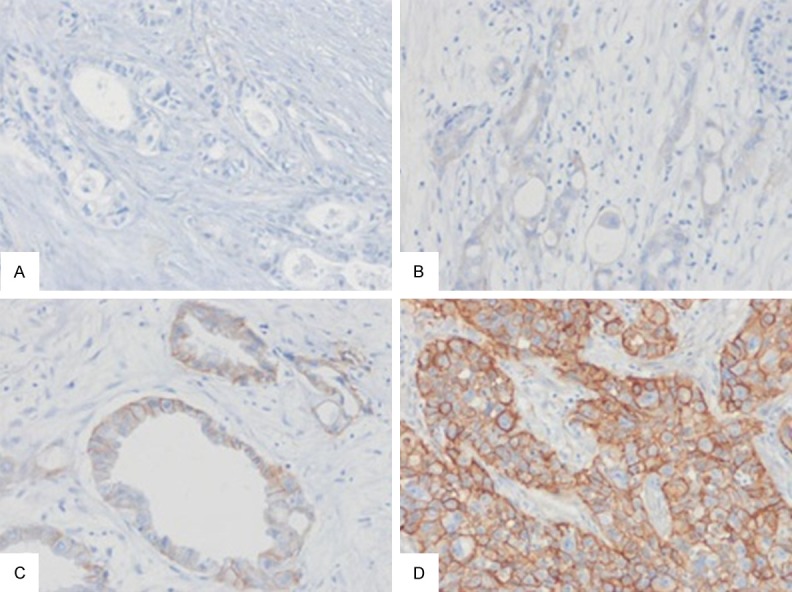
EGFR immunostaining in pancreatic ductal adenocarcinoma (PDA). A. Score 0 in moderately differentiated PDA. B. Score 1+ in poorly differentiated PDA. C. Score 2+ in well differentiated PAD. D. Score 3+ in poorly differentiated PDA.
Prognostic significance of EGFR expression
EGFR expression had no significant correlation with overall survival in patients with PDA (Figure 3). Results of univariate and multivariate analyses are shown in Table 3. Vascular invasion and lymph node metastasis were associated with unfavorable overall survival (P<0.05).
Figure 3.
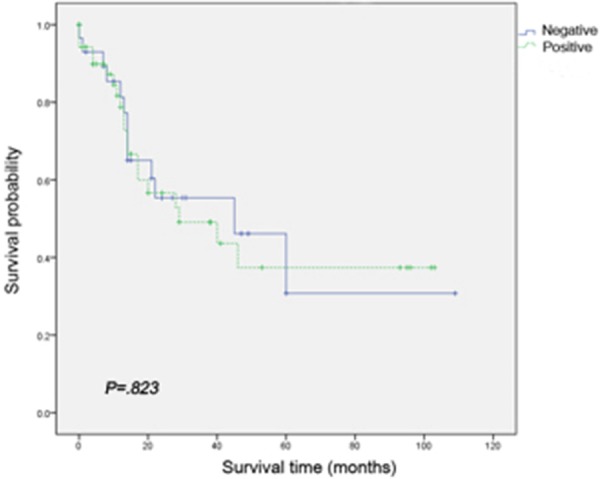
Kaplan-Meier curve demonstrates that 5-year survival is not significantly worse in cases with EGFR expression compared with EGFR-negative cases.
Table 3.
Univariate and multivariate analyses on the overall survival of pancreatic ductal adenocarcinoma
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Age >60 y | 0.712 | 0.354-1.434 | 0.342 | |||
| Tumor size >3 cm | 1.281 | 0.304-5.395 | 0.736 | |||
| Histologic grade | ||||||
| Well vs moderate | 2.300 | 0.874-6.050 | 0.091 | |||
| Well vs poor | 0.857 | 0.204-3.597 | 0.832 | |||
| Location | ||||||
| Head vs body | 1.081 | 0.508-2.300 | 0.840 | |||
| Head vs tail | 0.524 | 0.122-2.252 | 0.385 | |||
| Vascular invasion | 3.646 | 1.108-11.991 | 0.033 | 2.350 | 0.680-8.119 | 0.177 |
| PN invasion | 2.295 | 0.992-5.308 | 0.052 | |||
| LN metastasis | 2.164 | 1.043-4.489 | 0.038 | 2.094 | 0.959-4.573 | 0.064 |
| EGFR expression | 1.059 | 0.526-2.133 | 0.872 | |||
CI, confidence interval; PN, perineural; LN, lymph node.
Discussion
The aim of the current study was to examine the expression of EGFR in pancreatic intraepithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma (PDA) and their relationship to clinicopathologic features.
EGFR expression from 30.4% to 61.8% in PDAs has been reported [5-10]. In the current study, EGFR expression in PDAs was observed in 64.2% of PDAs when more than score 2(+) was considered positive. This difference may be due to the different types of antibody used, antigen retrieval methods, different criteria for assessing positivity, and heterogeneity of the samples (type of samples, fixation) [3]. The standardization of techniques to determine EGFR overexpression should be considered a priority [3].
In PDA, EGFR expression has been reported to show association with increased invasiveness and poor prognosis [6,9,10]. In contrast, meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancers showed no significant overall association between EGFR expression and survival [13]. Data regarding the prognostic role of EGFR expression is inconsistent. In our study, EGFR overexpression was not associated with a poorer prognosis. Univariate and multivariate analyses revealed that vascular invasion and lymph node metastasis were statistically significant factors for poorer prognosis.
EGFR expression has been reported to show association with tumor dedifferentiation, mitotic activity, and pleomorphism [10]. In our study, although no significant correlation was observed between EGFR expression and histological grade, EGFR expression tended to be higher in poorly differentiated PDA than in well differentiated and moderately differentiated PDA. No relationship was observed between EGFR expression and other clinicopathologic parameters. Accurate and conclusive evaluation of the clinical significance of EGFR expression is important in pancreatic cancer for selection of appropriate future molecular targets [8].
There is a progression in the pancreas from intraductal proliferation to invasive proliferation to invasive ductal carcinoma. This progression is associated with increasing degrees of cytological and architectural atypia, with accumulation of genetic alterations in cancer-associated genes [14]. To the best of our knowledge, no study on the interrelationship of EGFR expression in normal pancreas, PanIN, and PDA has been reported. In an attempt to examine interrelationship of EGFR expression by which pancreatic cancers progress, we compared normal pancreas, PanIN lesions and PDA with EGFR expression. In our study, neither normal pancreas, Pan-1A nor PanIN-1B showed EGFR expression. Significantly higher EGFR expression was observed in high-grade PanIN-3 rather than in low-grade PanIN-1A and PanIN-1B, and EGFR expression increased from PanIN to PDA. These results suggest that EGFR expression may be related to early event in carcinogenesis of PDA. Similar to PanIN lesions in pancreas, EGFR expression rate increased from normal epithelium to carcinoma in situ and microinvasive tumors in the lung [15]. EGFR overexpression is more common in PDAs, and therefore may prove to be a useful marker to aid in differentiating reactive atypia from a well differentiated adenocarcinoma. EGFR signaling inhibition may prevent development of PDA [16].
EGFR mutation rate of 1.5% to 3.6% in PDA has been reported [17-19]. The correlation between EGFR mutation and EGFR protein overexpression by immunohistochemical staining was not linear [3]. PDA cases with EGFR overexpression by immunohistochemistry failed in EGFR gene amplification by FISH [20]. Although EGFR alterations were not examined at the genetic level, further study is mandatory in order to understand their role in multistep carcinogenesis of PDA. Conduct of more studies on relationship between EGFR gene alterations and EGFR protein expression is needed.
In conclusion, EGFR expression increased from PanINs to PDAs. EGFR expression may be an early event in carcinogenesis of PDA and associated with tumor progression to invasive cancer. In the future, understanding the molecular control of progression of PanIN into PDA will be extremely critical for prevention and treatment of PDA.
Acknowledgements
This work was supported by the 2013 Yeungnam University Research Grant.
Disclosure of conflict of interest
None.
References
- 1.Hruban RH, Lacobuzio-Donahue CA. The Pancreas. In: Kumar V, Abbas AK, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. 9th edition. Philadelphia: Elsevier Saunders; 2015. pp. 890–895. [Google Scholar]
- 2.Yoo SB, Chung JH, Lee HJ, Lee CT, Jheon S, Sung SW. Epidermal growth factor receptor mutation and p53 overexpression during the multistage progression of small adenocarcinoma of the lung. J Thorac Oncol. 2010;5:964–969. doi: 10.1097/JTO.0b013e3181dd15c0. [DOI] [PubMed] [Google Scholar]
- 3.Penault-Llorca F, Cayre A, Arnould L, Bibeau F, Bralet MP, Rochaix P, Savary J, Sabourin JC. Is there an immunohistochemical technique definitively valid in epidermal growth factor receptor assessment? Oncol Rep. 2006;16:1173–1179. [PubMed] [Google Scholar]
- 4.de Melo Maia B, Fontes AM, Lavorato-Rocha AM, Rodrigues IS, de Brot L, Baiocchi G, Stiepcich MM, Soares FA, Rocha RM. EGFR expression in vulvar cancer: clinical implications and tumor heterogeneity. Hum Pathol. 2014;45:917–925. doi: 10.1016/j.humpath.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Dong M, Nio Y, Guo KJ, Tamura K, Tian YL, Dong YT. Epidermal growth factor and its receptor as prognostic indicators in Chinese patients with pancreatic cancer. Anticancer Res. 1998;18:4613–4619. [PubMed] [Google Scholar]
- 6.Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, Tamai S, Matsubara O, Hatsuse K, Mochizuki H. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 7.Tsiambas E, Karameris A, Lazaris AC, Talieri M, Triantafillidis JK, Cheracakis P, Manaios L, Gerontopoulos K, Patsouris E, Lygidakis NJ. EGFR alterations in pancreatic ductal adenocarcinoma: a chromogenic in situ hybridization analysis based on tissue microarrays. Hepatogastroenterology. 2006;53:452–457. [PubMed] [Google Scholar]
- 8.Luo G, Long J, Qiu L, Liu C, Xu J, Yu X. Role of epidermal growth factor receptor expression on patient survival in pancreatic cancer: a meta-analysis. Pancreatology. 2011;11:595–600. doi: 10.1159/000334465. [DOI] [PubMed] [Google Scholar]
- 9.Valsecchi ME, McDonald M, Brody JR, Hyslop T, Freydin B, Yeo CJ, Solomides C, Peiper SC, Witkiewicz AK. Epidermal growth factor receptor and insulin-like growth factor 1 receptor expression predicts poor survival in pancreatic ductal adenocarcinoma. Cancer. 2012;118:3484–3493. doi: 10.1002/cncr.26661. [DOI] [PubMed] [Google Scholar]
- 10.Handra-Luca A, Hammel P, Sauvanet A, Lesty C, Ruszniewski P, Couvelard A. EGFR expression in pancreatic adenocarcinoma. Relationship to tumour morphology and cell adhesion proteins. J Clin Pathol. 2014;67:295–300. doi: 10.1136/jclinpath-2013-201662. [DOI] [PubMed] [Google Scholar]
- 11.Hruban RH, Boffetta R, Hiraoka N, Iacobuzio-Donahue C, Kato Y, Kern SE, Klimstra DS, Klöppel G, Maitra A, Offerhaus GJA, Pitman MB. Ductal adenocaricnoma of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4 th edition. Lyon: International Agency for Research on Cancer (IARC); 2010. pp. 281–291. [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer. 7 th edition. New York: Springer-Verlag; 2010. AJCC Cancer staging manual; pp. 241–249. [Google Scholar]
- 13.Smith RA, Tang J, Tudur-Smith C, Neoptolemos JP, Ghaneh P. Meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br J Cancer. 2011;104:1440–1451. doi: 10.1038/bjc.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira-Cunha M, Siriwardena AK, Byers R. Molecular diagnosis in pancreatic cancer. Diagn Histopathol. 2008;14:214–222. [Google Scholar]
- 15.Meert AP, Verdebout JM, Martin B, Ninane V, Feoli F, Sculier JP. Epidermal growth factor receptor expression in pre-invasive and early invasive bronchial lesions. Eur Respir J. 2003;21:611–615. doi: 10.1183/09031936.03.00064902. [DOI] [PubMed] [Google Scholar]
- 16.He X, Zhang H, Xiao M, Kong Y, Li W, Zhang H. Inhibition of progression of PanIN through antagonizing EGFR. Tumour Biol. 2015;36:3245–9. doi: 10.1007/s13277-014-2953-2. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Jang KT, Ki CS, Lim T, Park YS, Lim HY, Choi DW, Kang WK, Park K, Park JO. Impact of epidermal growth factor receptor (EGFR) kinase mutations, EGFR gene amplifications, and KRAS mutations on survival of pancreatic adenocarcinoma. Cancer. 2007;109:1561–1569. doi: 10.1002/cncr.22559. [DOI] [PubMed] [Google Scholar]
- 18.Weiss GA, Rossi MR, Khushalani NI, Lo K, Gibbs JF, Bharthuar A, Cowell JK, Iyer R. Evaluation of phosphatidylinositol-3-kinase catalytic subunit (PIK3CA) and epidermal growth factor receptor (EGFR) gene mutations in pancreaticobiliary adenocarcinoma. J Gastrointest Oncol. 2013;4:20–29. doi: 10.3978/j.issn.2078-6891.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak EL, Jankowski J, Thayer SP, Lauwers GY, Brannigan BW, Harris PL, Okimoto RA, Haserlat SM, Driscoll DR, Ferry D, Muir B, Settleman J, Fuchs CS, Kulke MH, Ryan DP, Clark JW, Sgroi DC, Haber DA, Bell DW. Epidermal growth factor receptor kinase domain mutations in esophageal and pancreatic adenocarcinomas. Clin Cancer Res. 2006;12:4283–4287. doi: 10.1158/1078-0432.CCR-06-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dancer J, Takei H, Ro JY, Lowery-Nordberg M. Coexpression of EGFR and HER-2 in pancreatic ductal adenocarcinoma: a comparative study using immunohistochemistry correlated with gene amplification by fluorescent in situ hybridization. Oncol Rep. 2007;18:151–155. [PubMed] [Google Scholar]


