Abstract
We evaluated the diagnostic value of hepatocyte nuclear factor 1 beta (HNF-1β) and napsin A for diagnosing ovarian clear cell carcinoma. Immunohistochemical EnVision was used to measure HNF-1β and napsin A expression in 38 cases of ovarian clear cell carcinoma, 30 cases of high-grade serous carcinoma, 22 cases of endometrioid adenocarcinoma, and 16 metastatic Krukenberg tumor cases. Then we found that HNF-1β appeared in all ovarian clear cell carcinoma and was less common in high-grade serous and endometrioid adenocarcinoma (P < 0.05). However, no significant difference in HNF-1β between clear cell carcinoma and metastatic Krukenberg tumor was found (P > 0.05). Napsin A was expressed in 97.4% of ovarian clear cell carcinoma, 6.7% high-grade serous carcinoma, 22.7% endometrioid adenocarcinoma, and 0% metastatic Krukenberg tumors. Napsin A in clear cell carcinoma was greater than that found in high-grade serous carcinoma, endometrioid adenocarcinoma, and metastatic Krukenberg tumor (P < 0.05). Sensitivity and specificity of HNF-1β and napsin A for diagnosing ovarian clear cell carcinoma was 100% and 54.4%, and 97.4% and 89.7%, respectively. Sensitivity and specificity of HNF-1β and napsin A for diagnosing ovarian clear cell carcinoma was 97.4% and 91.2%, respectively. So it is concluded that HNF-1β and napsin A are more sensitive than currently used markers for diagnosing ovarian clear cell carcinoma. Moreover, napsin A is more specific than HNF-1β. Combining HNF-1β and napsin A may distinguish clear cell carcinoma from high-grade serous carcinoma, endometrioid adenocarcinoma and metastatic Krukenberg tumors.
Keywords: Ovarian neoplasm, clear cell carcinoma, hepatocyte nuclear factor 1 beta (HNF-1β), napsin A, serous carcinoma, endometrioid adenocarcinoma
Introduction
Ovarian cancer is one of the most common malignant tumors of the female reproductive system. Using molecular pathogenic data, ovarian cancer can be classified into types I and II. Type II ovarian cancer includes ovarian clear cell carcinoma and high-grade ovarian serous carcinoma and both have poor prognoses. Moreover, ovarian clear cell carcinoma is insensitive to cisplatin-based chemotherapy. The typical histopathological features of ovarian clear cell carcinoma are transparent and hobnail-like cells arranged in tubulocystic, papillary, or solid structures. Nevertheless, ovarian serous and endometrioid-like cancers may also have clear cell changes. In addition, high-grade ovarian serous carcinoma has papillary or hobnail-like structures. Thus, these cases are difficult to differentiate. Our previous work confirmed that HNF-1β had high diagnostic sensitivity for the ovarian clear cell carcinoma but low specificity [1]. Napsin A is an aspartic protease highly expressed in normal lung and kidney tissues. It is mainly used for diagnosis and differential diagnosis of lung adenocarcinoma. Recent studies indicate that napsin A is expressed in ovarian and endometrioid clear cell carcinoma [2,3]. However, a comparative study of HNF-1β and napsin A expression in ovarian clear cell carcinoma has not been reported. Thus, we used immunohistochemical staining to measure expression of HNF-1β and napsin A in ovarian cancer samples and evaluated the diagnostic value of dual detection of HNF-1β and napsin A in ovarian clear cell carcinoma.
Materials and methods
Clinical data
A total of 106 archived cases of epithelial ovarian cancers were collected. All these patients had been treated with gynecologic surgery and were diagnosed in the department of pathology, at the First Hospital of Nanjing and Nanjing Maternity and Child Health Care Hospital, Nanjing, China, from June 2006 to September 2014. Collected cases included 38 cases of ovarian clear cell carcinoma, 30 cases of high-grade serous carcinoma, 22 cases of endometrioid adenocarcinoma, and 16 cases of metastatic Krukenberg tumor. Pathological diagnosis of all cases was confirmed by two senior pathologists after reviewing tissue sections under light microscopy. No case had a history of preoperative chemotherapy or radiotherapy.
Methods
All histopathological specimens were fixed in 10% neutral buffered formalin, followed by paraffin embedding and tissue sectioning (5-µm thickness). Immunohistochemistry using the EnVision method was performed following antigen retrieval. All tissue sections were submerged in a citrate buffer (pH 6.0) and heated in a pressure cooker during antigen retrieval. Immunohistochemical staining was carried out in a Benchmarker instrument according to the operating instructions provided by the manufacturer. Among the total mouse anti-human HNF-1β monoclonal antibody purchased from Santa Cruz Biotechnology, Inc (Dallas, TX). Antibody was diluted to 1:200. And mouse anti-human napsin A monoclonal antibody purchased from Dako (Agilent Technologies, Santa Clara, CA) was ready to use. Tissue sections with known HNF-1β positive staining was used as a positive control for anti-HNF-1β, and tissue sections of lung adenocarcinoma was used as a positive control for anti-napsin A; PBS replaced primary antibodies on corresponding tissue sections as negative controls for anti-HNF-1β and anti-napsin A. Diaminobenzidine (DAB) coloring and hematoxylin counterstaining were performed prior to microscopic analysis.
Result analysis
Positive staining of nuclear HNF-1β was based on staining intensity and percent positive cells. Staining intensity of HNF-1β was scored as follows: no staining (Score 0), light yellow (Score 1), yellow (Score 2), brownish yellow (Score 3), and brown (Score 4). Next, when the percent of HNF-1β positive cells < 5%, the tumor was scored as 0, 5-25% positivity was scored as 1, 26-50% positivity was scored as 2, and > 50% positivity was scored as 3. Staining scores and percent positive cells were summed to obtain a total immunostaining score. When HNF-1β immunostaining scores ≥ 2, this was defined as positive staining. Positive staining of napsin A was granular and localized to the cytoplasm. Staining intensity of napsin A was ranked as follows: no/negative staining (0), light yellow (1+), and brownish yellow (2+). To facilitate analysis of napsin A, we only considered the staining intensity but not the percent positive cells (i.e., any positive staining (> 0) was considered to be napsin A positive) in tissue sections.
Statistical analysis
All data were statistically analyzed using the SPSS 20.0 software (SPSS, Chicago, IL). HNF-1β and napsin A positive rates in different tumor types were calculated using χ2-test or Fisher’s exact method. A p-value of less than 0.05 was considered statistically significant.
Results
HNF-1β expression in ovarian cancer
All 38 cases of ovarian clear cell carcinoma had different amounts of HNF-1β nuclear staining and this was 100% positive. Of the 38 cases, 24 cases scored 7 for immunostaining; 10 cases scored 6; 2 cases scored 4, and 2 cases scored 3. Of the 30 cases of high-grade ovarian serous carcinoma, 4 cases expressed HNF-1β (13.3% positive). Of the 22 cases of endometrioid adenocarcinoma, 11 cases expressed HNF-1β (50% positive). All 16 cases of Krukenberg tumor expressed HNF-1β (100% positive). HNF-1β positivity in ovarian clear cell carcinoma was significantly higher than ovarian serous carcinoma and endometrioid adenocarcinoma (P < 0.05). However, no significant difference in HNF-1β positivity was found between ovarian clear cell carcinoma and Krukenberg tumor samples (P > 0.05). HNF-1β expression in different types of ovarian cancer is shown in Figures 1, 2 and Table 1.
Figure 1.
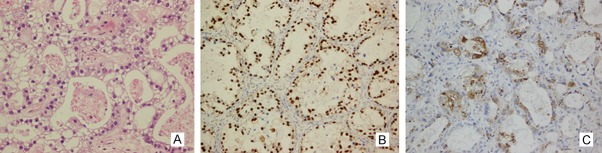
HNF-1β and napsin A protein expression was observed in the nuclear and cytoplasm of ovarian clear cell carcinoma by EnVision immunohistochemistry. In (A) ovarian clear cell carcinoma, (B) HNF-1β expression was observed in the nuclear, (C) napsin A expression was observed in the cytoplasm (Magnification, × 200).
Figure 2.
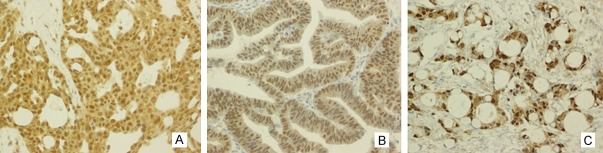
HNF-1β protein expression was observed in the nuclear of others ovarian neoplasm. In (A) high-grade ovarian serous carcinoma, (B) endometrioid adenocarcinoma, (C) krukenberg tumor (Magnification, × 200).
Table 1.
HNF-1β and napsin A expression in ovarian cancer
| Histological type | Sample number | HNF-1β | napsin A | ||
|---|---|---|---|---|---|
|
| |||||
| + (%) | - | + (%) | - | ||
| Ovarian clear cell carcinoma | 38 | 38 (100) | 0 | 37 (97.4) | 1 |
| Ovarian serous carcinoma | 30 | 4 (13.3) | 26 | 2 (6.7) | 28 |
| Endometrioid adenocarcinoma | 22 | 11 (50.0) | 11 | 5 (22.7) | 17 |
| Krukenberg tumor | 16 | 16 (100) | 0 | 0 (0) | 16 |
Napsin A expression in ovarian cancer
Figures 1 and 3 shows cytoplasmic expression of napsin A in tumor cells. Of the 38 cases of ovarian clear cell carcinoma, 37 cases were positive for napsin A (97.4% positive). Of the 30 cases of high-grade ovarian serous carcinoma, only 2 cases were positive for napsin A (6.7% positive). Of the 22 cases of endometrioid adenocarcinoma, 5 cases expressed napsin A (22.7% positive). Of the 16 cases of Krukenberg tumor, all were negative for napsin A. Napsin A positivity in ovarian clear cell carcinoma was significantly higher than in ovarian serous carcinoma, endometrioid adenocarcinoma and Krukenberg tumor (P < 0.05). Table 1 shows napsin A expression in different types of ovarian cancer.
Figure 3.
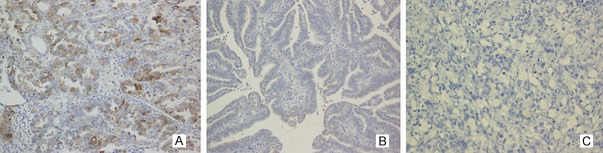
Napsin A protein expression was observed in the cytoplasm of others ovarian neoplasm. In (A) high-grade ovarian serous carcinoma, (B) endometrioid adenocarcinoma, (C) krukenberg tumor (Magnification, × 200).
Diagnostic sensitivity and specificity of HNF-1β and napsin A in ovarian clear cell carcinoma
Diagnostic sensitivity and specificity of HNF-1β in ovarian clear cell carcinoma were 100% and 54.4%, respectively. Diagnostic sensitivity and specificity of napsin A in ovarian clear cell carcinoma were 97.4% and 89.7%, respectively. It was observed that 37 cases of ovarian clear cell carcinoma, 1 cases of high-grade serous carcinoma and 5 cases of endometrioid adenocarcinoma expressed HNF-1β and napsin A. Diagnostic sensitivity and specificity of HNF-1β and napsin A double positivity in ovarian clear cell carcinoma were 97.4% and 91.2%, respectively.
Discussion
Ovarian clear cell carcinoma accounted for 5-6% of the ovarian malignant tumors [4]. The typical features of ovarian clear cell carcinoma are tubular, cystic, papillary, and solid structures. These tumor cells have hobnail features, and the cytoplasm is transparent and bright. Many studies indicate that ovarian clear cell carcinoma has a unique, aggressive biological behavior. Compared with other ovarian cancers, ovarian clear cell carcinoma responded poorly to cisplatin [5]. Papillary structures and clear cell features of ovarian clear cell carcinoma were also present in ovarian serous carcinoma and endometrioid adenocarcinoma. Hence, a correct diagnosis of ovarian clear cell carcinoma is essential for clinical treatment plans.
HNF-1β is a transcription factor involved in embryonic organ development (liver, kidneys, pancreas, and Müllerian/Woolfian ducts) [6-8]. It is also a regulatory factor for glucose homeostasis [9]. Tsuchyia’s group applied real-time quantitative PCR, Western blot, and immunohistochemistry to confirm that HNF-1β is a specific marker for ovarian clear cell carcinoma [10]. Subsequently, Kato and colleagues confirmed that HNF-1β was a suitable marker for identification of benign, borderline, and malignant ovarian clear cell neoplasms [11]. Given the clear cell changes in ovarian serous carcinoma and endometrioid carcinoma, Kao [12] and DeLair [13] performed HNF-1β immunohistochemical staining in ovarian clear cell carcinoma and ovarian serous cancer and endometrioid carcinoma with clear cell changes (also called mixed serous and clear cell carcinoma) and confirmed that HNF-1β was not only a sensitive and specific marker for ovarian clear cell carcinoma, but could also verify that mixed serous and clear cell carcinoma accompanied by the changes of clear cells in ovarian serous carcinoma. Using cytological specimens, Higashiguchi and colleagues reported that HNF-1β was specifically expressed in clear cell carcinoma and may be a differential diagnostic marker for identifying clear cell carcinoma from mesothelial cells [14]. However, Higashiguchi’s group found four cases of endometrioid-like cancers also expressing HNF-1β. Here, we report that HNF-1β was expressed in all ovarian clear cell carcinoma. However, 22.7% of endometrioid adenocarcinoma samples also expressed HNF-1β, data that were consistent with results from Higashiguchi’s work. HNF-1β positivity in endometrioid adenocarcinoma is likely due to sharing common pathogenic process with clear cell carcinoma; they are both related to endometriosis. Metastatic ovarian Krukenberg tumors can be misdiagnosed as ovarian clear cell carcinomas, when there is a lack of clinical history, tumor cells from glandular ducts or diffused solid lesions, and the cytoplasm of tumor cells become transparent. Therefore, we also included cases of metastatic ovarian Krukenberg tumor and report that 100% of the cases expressed HNF-1β. Although we confirmed that HNF-1β was a highly sensitive marker for ovarian clear cell carcinoma, because of the high expression of HNF-1β in metastatic ovarian Krukenberg tumors, data interpretation should always rely on a combination of staining results and clinical observations.
Napsin A is a member of the aspartic protease family and plays an important role in maturation of surfactant protein B. Under normal conditions, napsin A is chiefly expressed in the cytoplasm of type II alveolar cells and macrophages in the lung. In pathological conditions, napsin A is a sensitive marker for lung adenocarcinoma that has been used with thyroid transcription factor 1 (TTF1) for the differential diagnosis of lung adenocarcinoma and squamous cell carcinoma [15]. Recent studies indicate showed that napsin A was highly positively expressed in ovarian clear cell carcinoma. Skirnisdottir’s group studied napsin A expression in 15 cases of ovarian clear cell carcinoma (type I = 40 cases; type II = 75 cases) and reported that 80% of the ovarian clear cell carcinomas had positive expression of napsin A; while only 4% of type I and type II ovarian cancers were positive for napsin A expression [3]. Yamashita and colleagues reported that 83% of ovarian clear cell carcinoma and 100% of clear cell adenofibroma were positive expression for napsin A [16]. None of the non-clear cell carcinomas (i.e., 30 cases of serous adenocarcinoma, 11 cases of serous adenoma and borderline tumor, 19 cases of endometrioid adenocarcinoma, 22 cases of mucinous adenoma and borderline tumor; 10 cases of mucinous adenocarcinoma, and 3 cases of yolk sac tumor) expressed napsin A. Similarly, Kandalaft and co-workers reported that napsin A expression occurred in 100% of ovarian clear cell carcinoma sample but not in serous borderline tumor and high-grade serous carcinomas [17]. In addition, napsin A was expressed focally in 10% of endometrioid adenocarcinomas. In our work, 97.4% of ovarian clear cell carcinoma samples expressed napsin A, which was greater than expression in serous carcinoma (6.7%) and endometrioid adenocarcinoma (22.7%). And the same time, there was no metastatic Krukenberg tumors expressed napsin A. Thus, napsin A appears to be a sensitive marker for diagnosing ovarian clear cell carcinoma. It can be used to distinguish ovarian clear cell carcinoma from serous carcinoma, endometrioid-like cancers and metastatic Krukenberg tumors as well.
Here we compared the diagnostic sensitivity and specificity of HNF-1β and napsin A in ovarian clear cell carcinoma and confirmed that the diagnostic sensitivity of HNF-1β in ovarian clear cell carcinoma was slightly higher than napsin A. However, the diagnostic specificity of HNF-1β in ovarian clear cell carcinoma was lower than napsin A. Positive expression of both HNF-1β and napsin A indicated had diagnostic sensitivity and specificity in ovarian clear cell carcinoma, suggesting that dual detection may significantly improve accuracy of diagnosis for ovarian clear cell carcinoma.
To conclude, this study indicated that HNF-1β and napsin A were sensitive markers for diagnosing ovarian clear cell carcinoma. Combined marker detection may be useful for distinguishing between ovarian clear cell carcinoma and high-grade ovarian serous carcinoma/endometrioid adenocarcinoma and metastatic Krukenberg tumor.
Acknowledgements
The authors would like to thank Mr. Jingsong Wang of the Department of Pathology, Nanjing First Hospital, for his excellent technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Huang WB, Wang JS, Cheng X, JI J, Zhang JM, Li Q. The application value of HNF-1β transcription factor in the diagnosis of ovarian clear cell carcinoma. Int J Gynecol Pathol. 2015 doi: 10.1097/PGP.0000000000000213. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Fadare O, Desouki MM, Gwin K, Hanley KZ, Jarboe EA, Liang SX, Quick CM, Zheng W, Parkash V, Hecht JL. Frequent expression of napsin A in clear cell carcinoma of the endometrium: potential diagnostic utility. Am J Surg Pathol. 2014;38:189–196. doi: 10.1097/PAS.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 3.Skirnisdottir I, Bjersand K, Akerud H, Seidal T. Napsin A as a marker of clear cell ovarian carcinoma. BMC Cancer. 2013;13:524. doi: 10.1186/1471-2407-13-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zannoni GF, Morassi F, Prisco MG, De Stefano I, Vellone VG, Arena V, Scambia G, Gallo D. Clinicopathologic and immunohistochemical features of ovarian clear cell carcinomas in comparison with type I and type II tumors. Int J Gynecol Pathol. 2012;31:507–516. doi: 10.1097/PGP.0b013e3182518557. [DOI] [PubMed] [Google Scholar]
- 5.Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–376. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Igarashi P, Shao X, McNally BT, Hiesberger T. Roles of HNF-1β in kidney development and congenital cystic diseases. Kidney Int. 2005;68:1944–1947. doi: 10.1111/j.1523-1755.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 7.Bohn S, Thomas H, Turan G, Ellard S, Bingham C, Hattersley AT, Ryffel GU. Distinct molecular and morphogenetic properties of mutations in the human HNF1beta gene that lead to defective kidney development. J Am Soc Nephrol. 2003;14:2033–2041. doi: 10.1097/01.asn.0000078808.70309.c4. [DOI] [PubMed] [Google Scholar]
- 8.Coffinier C, Gresh L, Fiette L, Tronche F, Schütz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1β. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- 9.Pontoglio M. Hepatocyte nuclear factor 1, a transcription factor at the crossroads of glucose homeostasis. J Am Soc Nephrol. 2000;11(Suppl 16):S140–143. [PubMed] [Google Scholar]
- 10.Tsuchiya A, Sakamoto M, Yasuda J, Chuma M, Ohta T, Ohki M, Yasugi T, Taketani Y, Hirohashi S. Expression profiling in ovarian clear cell carcinoma: identification of hepatocyte nuclear factor-1ß as a molecular marker and a possible molecular target for therapy of ovarian clear cell carcinoma. Am J Pathol. 2003;163:2503–2512. doi: 10.1016/s0002-9440(10)63605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato N, Sasou S, Motoyama T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol. 2006;19:83–89. doi: 10.1038/modpathol.3800492. [DOI] [PubMed] [Google Scholar]
- 12.Kao YC, Lin MC, Lin WC, Jeng YM, Mao TL. Utility of hepatocyte nuclear factor-1β as a diagnostic marker in ovarian carcinomas with clear cells. Histopathology. 2012;61:760–768. doi: 10.1111/j.1365-2559.2012.04267.x. [DOI] [PubMed] [Google Scholar]
- 13.DeLair D, Han G, Irving JA, Leung S, Ewanowich CA, Longacre TA, Gilks CB, Soslow RA. HNF-1β in ovarian carcinomas with serous and clear cell change. Int J Gynecol Pathol. 2013;32:541–546. doi: 10.1097/PGP.0b013e318273fd07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higashiguchi A, Yamada T, Susumu N, Mori T, Suzuki A, Aoki D, Sakamoto M. Specific expression of hepatocyte nuclear factor-1β in the ovarian clear cell adenocarcinoma and its application to cytological diagnosis. Cancer Sci. 2007;98:387–391. doi: 10.1111/j.1349-7006.2007.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao Q, Gu XW, Tian XC, et al. p40, TTF1 and napasin A expression in non-small cell lung cancer. Chinese Journal of Diagnostic Pathology. 2014;21:182–183. [Google Scholar]
- 16.Yamashita Y, Nagasaka T, Naiki-Ito A, Sato S, Suzuki S, Toyokuni S, Ito M, Takahashi S. Napsin A is a specific marker for ovarian clear cell adenocarcinoma. Mod Pathol. 2015;28:111–117. doi: 10.1038/modpathol.2014.61. [DOI] [PubMed] [Google Scholar]
- 17.Kandalaft PL, Gown AM, Isacson C. The lung-restricted marker napsin A is highly expressed in clear cell carcinomas of the ovary. Am J Clin Pathol. 2014;142:830–836. doi: 10.1309/AJCP8WO2EOIAHSOF. [DOI] [PubMed] [Google Scholar]


