Abstract
The prevalence of type 1 cardiorenal syndrome (CRS) is increasing and strongly associated with long-term mortality. However, lack of reliable animal models and well-defined measures of renoprotection, made early diagnosis and therapy difficult. We previously successfully established the swine acute myocardial infarction (AMI) model of ischemia-reperfusion by blocking left anterior descending branch (LAD). Reperfusion was performed after 90-minute occlusion of the LAD. AMI was confirmed by ECG and left ventricular angiography (LVG). Then those 52 survived AMI reperfusion swine, including ventricular fibrillation-cardiac arrest after restoration of blood flow, were randomly divided into four groups (four/group) according to different interventions: resuscitation in room temperature, resuscitation with 500 ml saline in room temperature, resuscitation with 4°C 500 ml saline and normal control (with no intervention of resuscitation). Each group was further observed in four groups according to different time of resuscitation after ventricular arrhythmias: 1, 3, 5, 10-minute reperfusion after ventricular arrhythmias. Plasma and random urine were collected to evaluate renal function and test renal biomarkers of acute kidney injury (AKI). Our swine AMI model of ischemia-reperfusion provoked subclinical AKI with the elevation of the tubular damage biomarker, NGAL, IL-18 and L-FABP. Renal damage rapidly observed after hemodynamic instability, rather than observation after several hours as previously reported. The increasing rate of biological markers declined after interventions, however, its impact on the long-term prognosis remains to be further studied. These data show that elevation of tubular damage biomarkers without glomerular function loss may indicate appropriate timing for effective renoprotections like hypothermia resuscitation in type 1 CRS.
Keywords: Cardiorenal syndrome, biomarkers, ischemia-reperfusion, acute kidney injury, NGAL, IL-18, L-FABP
Introduction
The complex interdependent relationship between the heart and kidney is often evident when either organ becomes severely injured. Previous studies have revealed that acute myocardial infarction (AMI) with acute kidney injury (AKI), about 17%, is strongly related to long-term mortality and heart failure [1-4]. An acute worsening in renal function is prognostically important in patients with decompensated heart failure; and after surgery, where even a small change in renal function is associated with increased mortality [5-8]. This clinical entity with regard to heart-kidney crosstalk was recently redefined as cardiorenal syndrome (CRS) according to the time frame and order of occurrence [9]. When cardiac dysfunction leads to renal injury, it is classified as either type 1 CRS if acute or type 2 CRS if chronic. In a retrospective study of 438 patients diagnosed with CRS, type 1 CRS was most frequent (48.2%) followed by Type 2 CRS (21.9%) [10].
Despite decades of pioneering basic research and important technical advances in our understanding of the dynamic changes in renal function during or after cardiac dysfunction, the prognosis for patients with AKI remains poor. Several major problems have plagued the field and hindered progress. Scant information is available for studying CRS with animal models. Renal ischemia and reperfusion models were often established by clamping both renal arteries, which could not well elucidate the pathophysiologic characteristics of AKI with cardiovascular disease. Our current knowledge regarding CRS is mainly based on single-or multiple-center clinical studies. However, those “hot-spot” researches still cannot make us reach an agreement of optimal treatment strategy of CRS. Firstly, clinical evaluation of acute loss of renal function is not early, accurate, and reliable enough for predicting the occurrence of AKI. Serum creatinine level and urine output do not change until approximately half the kidney function has been lost and they may be affected by sex, muscle mass, and the hydration status of the patient [11]. Thus, an early diagnosis of AKI by using tubular damage biomarkers preceding filtration function loss has aroused great interests in recent years. Secondly, the pathophysiologic mechanisms involved in CRS are complex and incompletely understood, and thus effective management of these syndromes remains a challenge [12]. Besides, several interventions for AKI which have been proved ineffective [13,14], hinders the progress of reaching a consensus about timely renal salvage.
Great progress has been made in recent years that molecules such as NGAL (neutrophil gelatinase-associated lipocalin), L-FABP (L-type fatty acid-binding protein) and IL-18 (Interleukin-18) have demonstrated to allow a kidney injury to be diagnosed even in the absence of a subsequent manifest dysfunction [15]. Those biomarkers are specific for kidney injury, sensitive enough to detect even less severe insults, easy and rapid to measure, and inexpensive enough to make their use sustainable, which complement clinical assessments and enable improved therapeutic decision-making. Furthermore, a body of evidence from experimental and clinical studies has now established a plausible biological role for biomarkers of tubular damage, and presented strong proof of the concept that subclinical AKI should be added in the spectrum of AKI diagnosis [16,17]. The term ‘subclinical’ AKI referring to patients who are biomarker-positive and creatinine-negative challenges the traditional view that a kidney problem is clinically relevant, only when a loss of filtration function becomes apparent and distinguishes renal function loss from AKI with tubular damage, which may have a profound impact on the epidemiology, prevention, and management of AKI [18].
Materials and methods
Renal I/R model
The swine AMI model of ischemia-reperfusion was establish as described previously [19]. Blood restoration was performed after 90-minute occlusion of the LAD (left anterior descending branch). AMI was confirmed by ECG and left ventricular angiography (LVG). Then those 52 survived AMI reperfusion swine, including ventricular fibrillation-cardiac arrest after restoration of blood flow, were randomly divided into four groups (four/group) according to different interventions: resuscitation in room temperature, resuscitation with 500 ml saline in room temperature, resuscitation with 4°C 500 ml saline and normal control (with no intervention of resuscitation). Each group was further observed in four groups according to different time of resuscitation after ventricular arrhythmias: 1, 3, 5, 10-minute reperfusion after ventricular arrhythmias. Plasma and random urine were collected to evaluate renal function and test renal biomarkers of AKI. Subclinical AKI is confirmed by biomarker positivity and RIFLE (RIFLE, risk, injury, failure, loss of function and end-stage renal disease) negative. RIFLE referrers as diagnostic criteria for AKI based on loss of renal excretory function. All experiments were performed under the Guidelines for Animal Experimentation (Jiangsu, China).
Measurement of renal function
The blood samples and random urine were collected in lithium-heparinized tubes (4 ml, Venosafe, Terumo Europe N.V., 3001 Leuven, Belgium) and plastic tubes (10 ml, cent tube nat screw, Sarstedt Australia Pty Ltd Technology Park, SA 5095 Australia) respectively. After centrifuging at 3500 rpm at 4°C for 10 min, the plasma and the supernatant of urine were and analyzed with automatic biochemical analyzer (OLYMPUS AU5400).
Measurement of plasma and urinary neutrophil gelatinase-associated lipocalin (NGAL) by ELISA
The NGAL in plasma and urine was measured using a commercially available pig ELISA kit (KIT 044, Bio-Porto Diagnostics, Gentofte, DK). The analysis was carried out in accordance with the instructions given by the manufacturer.
When intraassay reproducibility was determined by the same sample eight times, the coefficient of variation for the obtained value was within 10%. The measurable range of this kit was between 10 and 400 ng/ml. Four different dilutions of urine (U1-4) and EDTA plasma (P1-4) were spiked with calibrator material and analyzed in the assay after appropriate further dilution, the mean recovery (P) of which was 98%. This assay system detects the pig NGAL in urine, plasma, serum, tissue extracts or culture media.
Measurement of plasma and urinary L-type fatty acid-binding protein (L-FABP) by ELISA
The L-FABP in plasma and urine was measured using a commercially available pig ELISA kit (KIT ab156517, Abcam, UK). The analysis was carried out in accordance with the instructions given by the manufacturer.
The sensitivity in this assay is 0.1 ng/mL, and the range of spike recovery is from 94% to 103%. The measurable range of this kit is between 0 and 10 ng/ml. This assay system detects the pig L-FABP in serum, plasmas, tissue homogenates, body fluids and cell culture supernatants.
Measurement of plasma and urinary Interleukin -18 (IL-18) by ELISA
The IL-18 in plasma and urine was measured using a commercially available pig ELISA kit (KIT BMS672/BMS672TEN, eBioscience, US). The analysis was carried out in accordance with the instructions given by the manufacturer.
Reproducibility within the assay was evaluated in 3 independent experiments, each assay of which was carried out with 6 replicates of 4 serum samples. The calculated overall intra-assay coefficient of variation value was 6.8%. The measurable range of this kit was between 39.1 and 2500 pg/ml. Recoveries were determined in 3 independent experiments with 6 replicates each, and the overall mean recovery was 110%.
Statistical analysis
Statistical analysis was performed using SAS 6.12 software (SAS Institute Inc., USA). Data are given as the mean ± SD analysed using one-way ANOVA with Dunnett’s significance correction test. Differences were considered significant at P < 0.05.
Statement of ethical approval
All animal work was conducted in accordance with the Chinese Animal Protection Law, compliance with the Guide for the Care and Use of Laboratory Animals for non-European authors and was approved by the Ethics Review Committee for laboratory animals of the district government of Jiangsu, China.
Results
Establishment of the swine AMI model of ischemia-reperfusion-interventions
There were 62 swine in total enrolled in the AMI model of ischemia-reperfusion-interventions. Firstly, we established the swine model of AMI-reperfusion as the method mentioned above. All the animals developed ventricular arrhythmias in 30 minutes after reperfusion. 10 swine died after reperfusion because of recurrent ventricular fibrillation despite the defibrillation (360 J) and chest compressions. Those survived swine including ones with ventricular fibrillation-cardiac arrest intervened by 4 different resuscitation methods and then sacrificed on Day 1 post-surgery.
AMI and reperfusion-induced subclinical acute kidney injury
Plasma parameters reflecting renal function in experiment groups were changed unparalleled to clinical AKI. Creatinine (Scr) and blood urea nitrogen (BUN) level in all resuscitation groups were lower than normal control group. Albumin level were significantly higher in 1, 5, 10-min resuscitation of group 1 especially 10-min resuscitation of group 2, while significantly lower in 10-min resuscitation of group 3. Those renal biomarkers including plasma and urine NGAL, IL-18, L-FABP were almost all significantly higher in experimental groups than control normal, suggesting that AMI-reperfusion swine provoked subclinical AKI (Table 1). Plus, urine albumin creatinine ratio and Cys C level reflecting early renal damage in resuscitation groups were mostly statistically higher than normal control.
Table 1.
Parameters of renal function and biomarkers in different groups
| Groups | Group 1 (resuscitation in room temperature) | Group 2 (resuscitation with 500 ml saline in room temperature) | Group 3 (resuscitation with 4°C 500 ml saline) | Nor-mal control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroups# | 1 min | 3 min | 5 min | 10 min | 1 min | 3 min | 5 min | 10 min | 1 min | 3 min | 5 min | 10 min | |
| ALB (g/l) | 16.22±1.67Δ | 12.07±0.64 | 17.38±3.18Δ | 19.15±1.84Δ | 12.22±1.42 | 11.93±0.24 | 13.48±1.27 | 21.15±2.93Δ | 12.85±1.49 | 11.55±1.78 | 11.60±1.29 | 8.67±0.79Δ | 14.20±2.39 |
| SCR(μmol/l) | 59.80±17.17 | 64.2±8.79Δ | 65.9±9.21Δ | 53.2±3.03Δ | 61.28±15.09 | 68.87±10.85Δ | 39.07±3.01Δ | 51.87±6.17Δ | 61.28±23 | 35.38±3.96Δ | 40.05±10.14Δ | 28.88±2.26Δ | 76.78±8.20 |
| BUN (mmol/l) | 2.98±0.73 | 3.02±0.40Δ | 3.29±0.62 | 3.25±0.27 | 3.03±0.66 | 3.28±0.51 | 2.44±0.21Δ | 3.57±0.44Δ | 2.85±0.73 | 1.96±0.33Δ | 2.03±0.47Δ | 1.44±0.14Δ | 3.23±0.29 |
| Cys C (mg/l) | 0.04±0.038 | 0.00±0.01 | 0.04±0.37 | 0.05±0.03Δ | 0.06±0.05Δ | 0.03±0.028Δ | 0.096±0.04Δ | 0.12±0.09 | 0.035±0.028Δ | 0.06±0.018Δ | 0.00±0.18 | 0.017±0.04 | 0.01±0.16 |
| Urine A/C (μg/μmol) | 830.67±219.54Δ | 802.61±345.47Δ | 1247.69±247.60Δ | 1332.82±179.65Δ | 249.58±85.28Δ | 428.67±55.76Δ | 854.69±346.28Δ | 1286.52±109.59Δ | 348.78±212.27Δ | 367.06±115.50Δ | 391.19±51.53Δ | 1324.56±420.87Δ | 46.34±2.25 |
| Plasma NGAL (ng/ml) | 155.43±4.14Δ | 159.92±1.33Δ | 154.61±9.05Δ | 168.90±15.35Δ | 152.93±4.32Δ | 158.88±2.83Δ | 147.85±0.36Δ | 163.17±9.15Δ | 161.19±15.23Δ | 171.88±15.33Δ | 147.63±8.52Δ | 147.03±1.50Δ | 43.43±3.69 |
| Urine NGAL (ng/mg Cr) | 687.13±58.18Δ | 738.71±115.48Δ | 900.99±44.27Δ | 866.27±49.09Δ | 911.99±237.55Δ | 882.59±123.94Δ | 753.52±54.13Δ | 813.94±75.19Δ | 1129.97±329.69Δ | 1025.52±111.36Δ | 767.51±69.94Δ | 1192.63±148.32Δ | 149.88±20.23 |
| Plasma IL-18 (pg/ml) | 76.54±7.13Δ | 81.61±8.1Δ | 64.84±17.88Δ | 92.41±13.98Δ | 72.17±10.13Δ | 85.55±7.62Δ | 65.76±15.62Δ | 82.29±1.37Δ | 76.87±4.5Δ | 94.69±8.56Δ | 84.35±7.23Δ | 80.14±3.22Δ | 35.47±5.93 |
| Urine IL-18 (pg/mg Cr) | 132.68±14.53Δ | 108.42±19.01Δ | 58.39±13.53Δ | 54.77±12.27Δ | 22.50±9.79Δ | 130.95±17.78Δ | 80.70±18.00Δ | 93.92±8.13Δ | 61.16±25.49 | 128.34±17.51Δ | 120.18±3.26Δ | 171.19±8.07Δ | 36.09±5.06 |
| Plasma L-FABP (ng/ml) | 31.52±4.47Δ | 28.12±1.92Δ | 23.97±3.94Δ | 39.85±4.29Δ | 15.38±1.9Δ | 24.61±2.41Δ | 17.43±4.23Δ | 38.45±4.93Δ | 1.46±5.8 | 38.08±6.9Δ | 16.75±4.58Δ | 16.78±3.32Δ | 9.39±1.09 |
| Urine L-FABP (ng/mg Cr) | 305.47±86.69Δ | 484.19±153.14Δ | 378.49±83.59Δ | 533.93±102.88Δ | 1388.96±718.99Δ | 1025.13±269.15Δ | 473.56±136.79Δ | 443.40±326.55Δ | 456.62±102.76Δ | 909.03±183.12Δ | 817.48±136.63Δ | 1085.89±97.2Δ | 92.93±8.38 |
Values are means ± s.d.
Compared with normal control, P < 0.05;
Each group was further observed in four groups according to different time of resuscitation after cardiac arrest: 1, 3, 5, 10-minute reperfusion after cardiac arrest. (min: minute).
ALB: albumin; SCR: serum creatinine; BUN: blood urea nitrogen; Cys C: cystatin C; A/C: albumin creatinine ratio; NGAL: Neutrophil Gelatinase-Associated Lipocalin; IL-18:Interleukin -18; L-FABP: L-Type Fatty Acid-Binding Protein.
Effective interventions for the subclinical acute kidney injury based on the changes of clinical renal parameters
To compare optimal time and appropriate fluid supply for the salvage of subclinical acute kidney injury, we used 4 different interventions in 4 different-time resuscitation. Firstly, we observed the clinical parameters of renal function (Figure 1). Along with the time axis, albumin creatinine ratio (AC) level increased significantly in 5, 10-min resuscitation compared with 1, 3 min in each intervention. When we supplied them 500 ml saline to resuscitate, AC in 10-min resuscitation were notablely higher than in other 3 time-point resuscitation. Moreover, in each time-point resuscitation except for the 10-min resuscitation, AC decreased overwhelmingly in group 2, 3 compared with group 1 (Figure 1A). For the parameter of Cys C (Figure 1B), value of 3-min resuscitation of group 1 was significantly lower than other 3 time point. Conversely, it of group 3 significantly transcended other 3 time point. The trend of Scr and BUN levels were unexpectedly declined over time in groups of resuscitating with 500 ml saline, and in 5-min resuscitation of group 2, both values were significantly lower than other time point (Figure 1C and 1D). Alb levels of 10-min resuscitation exceed other 3 time point in group 1, 2, whereas dropped the lowest in group 3. Besides, the values of 1, 3-min resuscitation in group 2, 3 were significantly lower than in group 1 (Figure 1E).
Figure 1.
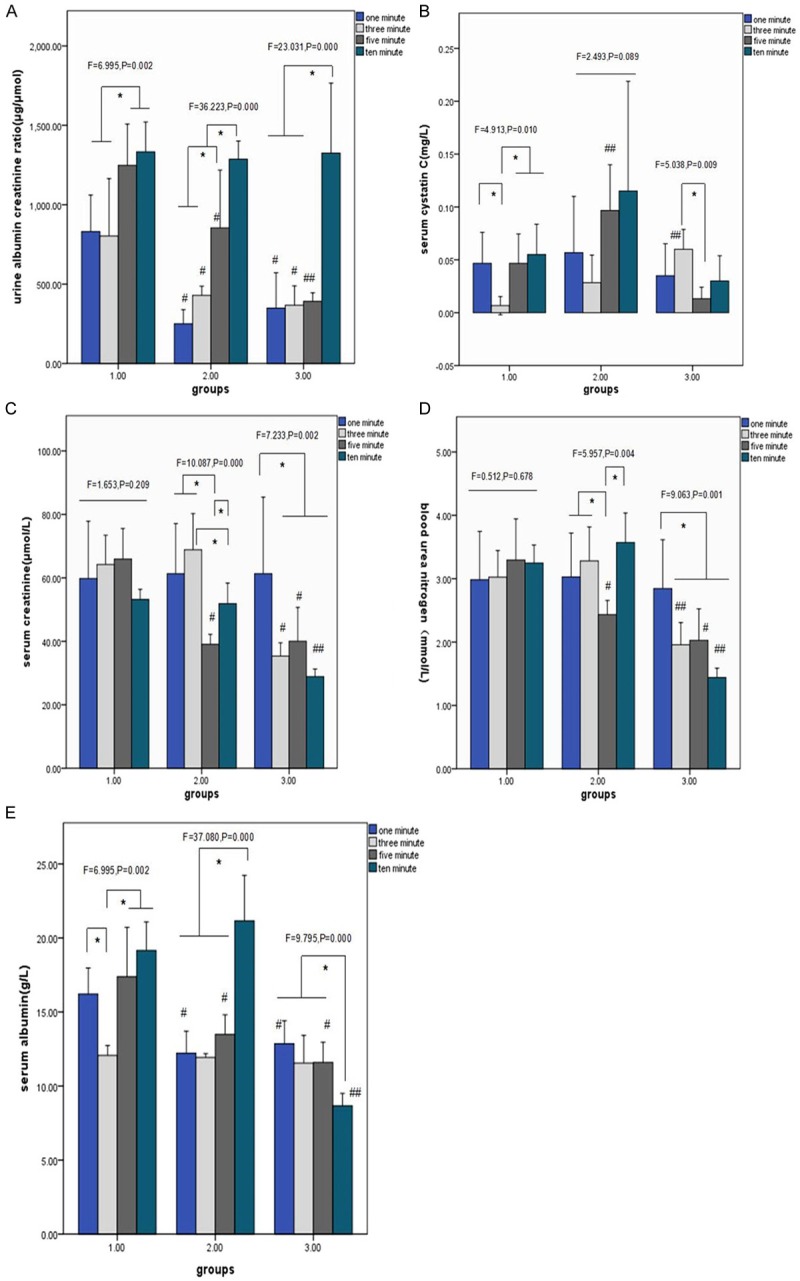
Changes of clinical renal parameters fewer than 3 different interventions in 4 different-time resuscitation. *P < 0.05 compared 4 different-time resuscitation within each group, # < 0.05 compared with group 1 in each-time resuscitation, ## < 0.05 versus other 2 groups in each-time resuscitation. A-E shows the comparison of mean values of clinical renal parameters (AC, Cys C, Scr, BUN, Alb) under different interventions. A: Versus 3 groups, in 1-min resuscitation: F=17.299, P < 0.001; in 3-min resuscitation: F=7.364, P=0.006; in 5-min resuscitation: F=17.994, P < 0.001; in 10-min resuscitation: F=0.05, P=0.092; B: Versus 3 groups, in 1-min resuscitation: F=0.504, P=0.614; in 3-min resuscitation: F=12.907, P=0.001; in 5-min resuscitation: F=12.566, P=0.001; in 10-min resuscitation: F=3.106, P=0.074; C: Versus 3 groups, in 1-min resuscitation: F=0.013, P=0.988; in 3-min resuscitation: F=28.094, P<0.001; in 5-min resuscitation: F=21.180, P < 0.001; in 10-min resuscitation: F=64.251, P < 0.001; D: Versus 3 groups, in 1-min resuscitation: F=0.013, P=0.988; in 3-min resuscitation: F=16.573, P < 0.001; in 5-min resuscitation: F=11.498, P=0.001; in 10-min resuscitation: F=82.050, P < 0.001; E: Versus 3 groups, in 1-min resuscitation: F=11.849, P=0.001; in 3-min resuscitation F=0.355, P=0.707; in 5-min resuscitation: F=11.695, P=0.001; in 10-min resuscitation: F=64.097, P < 0.001.
Role of renal biomarkers in comparing optimal interventions for the subclinical acute kidney injury
Then we compared the changes of plasma and urine biomarkers including NGAL, IL-18 and L-FABP under different interventions (Figure 2). In the condition of resuscitation in room temperature, plasma NGAL levels (Figure 2A) had no fluctuation along with the time axis, while the level of plasma L-FABP (Figure 2E) in 10-min resuscitation significantly exceed other 3 time point, with the plasma IL-18 level (Figure 2C) of 5-min resuscitation significantly lower than 10-min resuscitation. The values of urine NGAL (Figure 2B) in 5, 10-min resuscitation of group 1 surpassed 1,3-min resuscitation, with urine L-FABP levels (Figure 2F) in 3, 10-min resuscitation higher than 1-min resuscitation. Conversely, the urine IL-18 (Figure 2D) of group 1 showed the opposite trend that values in 5, 10-min resuscitation were significantly lower than other time point. The 3 plasma biomarkers in group 2 and 3 displayed similar fluctuation in the wake of time that values of group 2 dropped at 5-min resuscitation and raised at 10-min resuscitation, while 3-min resuscitation of group 3 significantly transcended others. Urine IL-18 level of group 2 at 3-min resuscitation exceeded other 3 time point, while L-FABP levels peaked at 1-min resuscitation. There was the same trend of urine IL-18 and L-FABP levels in group 3 that values hit the lowest at 1-min resuscitation and reached the highest at 10-min resuscitation, while values at 5-min resuscitation of urine NGAL were significantly below others. Comparing 3 different interventions at the same time point, plasma NGAL and IL-18 seemed no significant changes, while plasma L-FABP indicated significant fluctuation in resuscitation with 500 ml saline especially under 4°C. At 1-min resuscitation, the 3 urine biomarkers showed different trends in different groups that NGAL levels in group 3 were significantly higher than group 1, while group 2 of L-FABP levels were the highest than other 2 groups which of IL-18 were the lowest. Besides, urine NGAL levels at 5-min resuscitation in group 2 and 3 were significantly lower than group 1, on the contrary, urine IL-18 and L-FABP levels had an upward trend from group 1 to 3 that values in group 3 were significantly higher than that of 2 groups. There was a consistently upward trend of the 3 urine biomarkers at 10-min resuscitation from group 1 to 3 that they all culminated in group 3.
Figure 2.
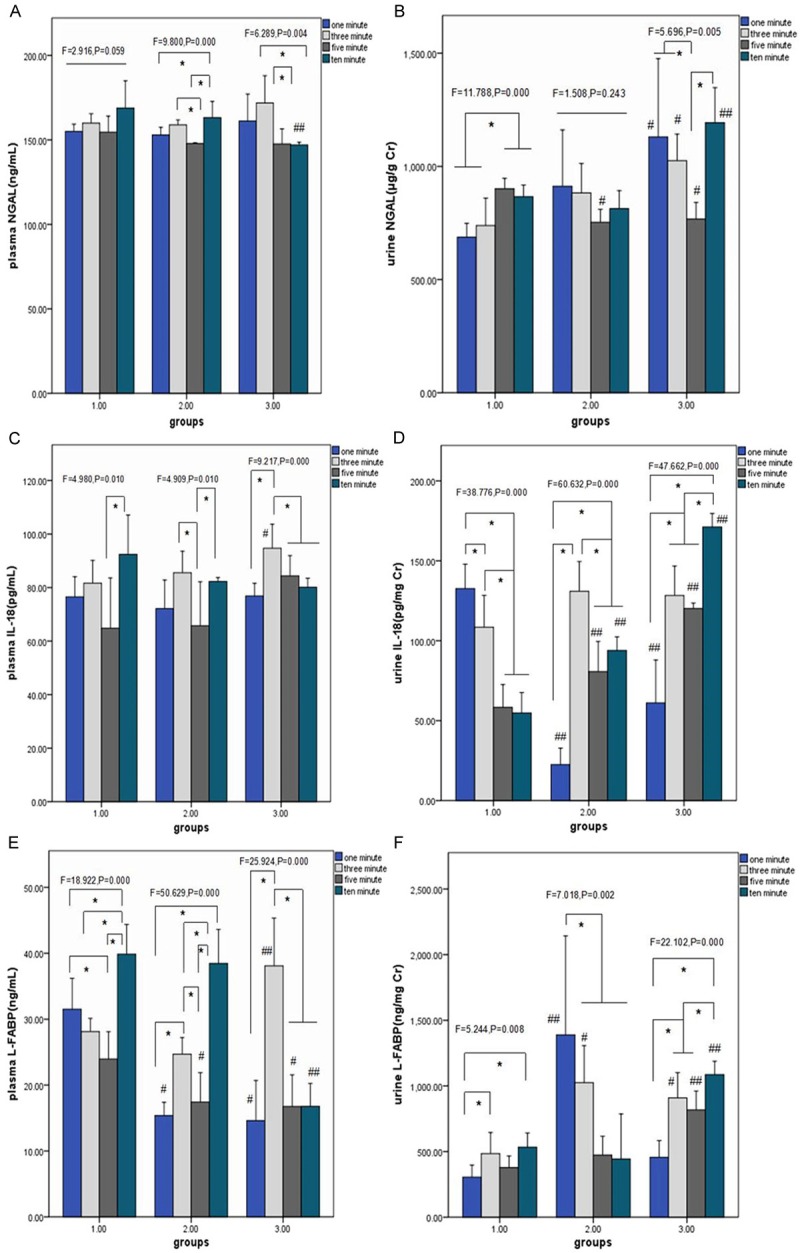
Comparison of renal biomarkers under 3 different interventions in 4 different-time resuscitation. *P < 0.05 compared 4 different-time resuscitation within each group, # < 0.05 compared with group 1 in each-time resuscitation, ## < 0.05 versus other 2 groups in each-time resuscitation. (A, C and E) shows the comparison of mean values of plasma renal biomarkers (NGAL, IL-18, L-FABP) under different interventions, with (B, D and F) displaying them of urine samples. (A) Versus 3 groups, in 1-min resuscitation: F=1.237, P=0.318; in 3-min resuscitation: F=3.459, P=0.058; in 5-min resuscitation: F=1.813, P=0.194; in 10-min resuscitation: F=7.204, P=0.006; (B) Versus 3 groups, in 1-min resuscitation: F=5.237, P=0.019; in 3-min resuscitation: F=9.007, P=0.003; in 5-min resuscitation: F=12.196, P=0.001; in 10-min resuscitation: F=25.210, P < 0.001; (C) Versus 3 groups, in 1-min resuscitation: F=0.712, P=0.507; in 3-min resuscitation: F=4.105, P=0.038; in 5-min resuscitation: F=3.541, P=0.055; in 10-min resuscitation: F=3.722, P=0.049; (D) Versus 3 groups, in 1-min resuscitation: F=58.748, P < 0.001; in 3-min resuscitation: F=2.778, P=0.094; in 5-min resuscitation: F=34.030, P < 0.001; in 10-min resuscitation: F=224.256, P < 0.001; (E) Versus 3 groups, in 1-min resuscitation: F=28.627, P < 0.001; in 3-min resuscitation: F=15.255, P=0.000; in 5-min resuscitation: F=5.254, P=0.019; in 10-min resuscitation: F=56.010, P < 0.001; (F) Versus 3 groups, in 1-min resuscitation: F=11.498, P=0.001; in 3-min resuscitation: F=11.279, P=0.001; in 5-min resuscitation: F=21.640, P=0.019; in 10-min resuscitation: F=17.184, P < 0.001.
Discussion
In this study using the swine AMI-reperfusion-resuscitation model, we demonstrated the three novel findings. First, AMI and reperfusion induced subclinical AKI, which was detected by increases of urine albumin creatinine ratio, plasma Cys C level, and novel renal biomarkers, including plasma and urine NGAL, IL-18, L-FABP, consistent with the establishment of type 1 CRS. Secondly, we confirmed the critical role of renal biomarkers in the timely observation and comparison of effective interventions for renal salvage in type 1 CRS. Thirdly, in the very early stage of type 1 CRS, plasma parameters of renal function monitored in clinic including Scr, BUN, Alb levels were not sensitive enough to reflect the development and progression of renal damage. Furthermore, this swine AMI-reperfusion-resuscitation induced subclinical AKI being closer to the human physiological status, less trauma, being able for repeated coronary angiography and electrical physiological examination, etc, which provides a good base of animal model for the further research on heart-kidney crosstalk.
CRS is defined as a complex pathophysiological disorder of the heart and the kidneys in which acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ. This has been recently classified into five subtypes on the basis of the primary organ dysfunction (heart or kidney) and on whether the organ dysfunction is acute or chronic [20]. Of particular interest to the critical care specialist are CRS type 1 (acute cardiorenal syndrome), which has been described in 27-45% of hospitalized AHF (acute decompensated heart failure) patients [21,22] and in 9-54% of ACS patients [23-26]. CRS type 1 is characterized by an acute deterioration in cardiac function that leads to AKI; the spectrum of acute cardiac dysfunction that could result in AKI includes AHF, acute coronary syndrome (ACS), and postcardiotomy low cardiac output syndrome, among others [27].
In our swine model, AMI and reperfusion provoked only subclinical AKI, which is mainly diagnosed by the elevation of the tubular damage biomarker, NGAL, IL-18 and L-FABP. The concept of subclinical AKI has recently emerged as a novel entity as the role of biomarkers has become better established [28]. Emerging evidence suggests that 15-20% of patients who do not fulfill current serum-creatinine-based consensus criteria for AKI are nevertheless likely to have acute tubular damage, which is associated with adverse outcomes [16]. So, new diagnostic criteria of AKI that utilizes not only glomerular filtration rate/urine output, but also tubular damage markers, have been proposed recently [16]. Patients with subclinical AKI (tubular damage biomarker positivity without dysfunction), who might not have been recognized before the biomarker era, are now known to be at an increased risk of progression to clinical AKI and, more importantly, are at an increased risk of adverse outcomes [29]. Therefore, making an early diagnosis, by which we can identify patients who are likely to benefit from intensive monitoring and precaution for further damage is vital in clinic.
Genomic, transcriptomic, and proteomic techniques have identified NGAL as an early marker of AKI [30,31]. It has been investigated across a range of different clinical settings of AKI, such as after cardiac surgery [32,33], in critically ill patients, in patients receiving intravenous contrast media infusion for coronary angiography, and in patients admitted to the emergency department [34,35]. Our model showed significantly elevated levels of plasma and urinary NGAL compared with normal control swine. This is comparable with a recent report that showed the MI rats had higher levels of plasma and urinary NGAL compared with rats in the sham or volume depletion groups and supports the key role of measuring the plasma or urine NGAL level in MI patients with subclinical AKI [28].
IL-18 is a mediator of inflammation and ischemic tissue injury in many organs [36]. Using an ischemia-reperfusion model of human atrial myocardium, inhibition of IL-18 with IL-18-binding protein was associated with improved cardiac contractile force [37]. In patients with myocardial ischemia, IL-18 levels were increased in serum compared with healthy controls [38]. In addition, IL-18 was a mediator of ischemic ARF in mice, and production of IL-18 was independent of inflammation caused by neutrophils [39,40]. In the latter study, IL-18 was detected in isolated mouse proximal tubules, and the addition of IL-18 to the tubules exacerbated tubular necrosis. Consistently, we found that plasma and urine IL-18 were sensitive in AMI-reperfusion swine that it increased by several-fold after ischemia-reperfusion injury. It is produced by proximal tubules, activated by caspase 1, and then excreted into urine after ischemic injury, thus providing a rationale for its application as a subclinical AKI biomarker [41].
In human kidney, L-FABP is expressed predominantly in proximal epithelial tubules, where FABP serves as a target of the highly cytotoxic aldehydes that are inevitably generated from lipid peroxidation reaction during reperfusion [42]. To evaluate the potential role of L-type FABP (L-FABP) as a biomarker of renal ischemia in both human kidney transplant patients and animal models, Tokunori Yamamoto confirmed that increased urinary L-FABP after ischemic-reperfusion injury may find future use as a biomarker of acute ischemic injury [42]. Our observation that plasma and urinary L-FABP level increased significantly in AMI-reperfusion-resuscitation swine confirmed its function in early diagnosis of AKI.
To compare the role of renal biomarkers in the subclinical AKI model, we observed that changes of urine biomarkers were not unanimous with plasma ones except for group 3 in which we resuscitated AMI-reperfusion swine with 4°C 500 ml saline . We consider that the post-ischemic kidney displays peculiar regional alterations in blood flow patterns that there is marked congestion and hypoperfusion of the outer medulla that persist even though cortical blood flow improves during reperfusion after an ischemic insult [43-45]. If we resuscitate with less effective interventions such as group 1 and group 2, the characteristic post-ischemic congestion still worsens the relative hypoxia, leading to prolonged cellular injury and cell death in these predisposed tubule segments [46]. However, hypothermia resuscitation may stop the sustained hemodynamic alterations, consistent changes of plasma and urine biomarkers in our study supporting the speculation. Further large-sample clinical study on the impact of hypothermia resuscitation on type 1 CRS patients is necessary.
Furthermore, clinical urine parameters such as urine albumin creatinine ratio appear to be more sensitive than serum ones such as Scr and BUN which even declined in our subclinical AKI model. In the very early stage of type 1 CRS, the glomeruli are usually unimpressive and prominent morphologic features include effacement and loss of proximal tubule brush border, patchy loss of tubule cells, focal areas of proximal tubular dilation and distal tubular casts, and areas of cellular regeneration [47]. This phase in which ischemic insult occurs before kidney function begins to fall, probably represents the most optimal window of opportunity for early diagnosis and active therapeutic intervention. Urinalysis has been accepted as an essential diagnostic tool in the approach to ARF for years [48]. A higher number of urinary casts was reported to present in patients with severe ATN and those who required dialytic support for their ATN [49]. Besides, numerous urinary markers of proximal tubular injury have been studied and correlated with the clinical course of ARF. In our study, urine AC level didn’t extremely elevated until 10-min resuscitation in group 2 and 3, and in 1, 3, 5-min resuscitation, values in group 2 and 3 were significantly lower than group 1, indicating hypothermia resuscitation within 5 minutes may stop ischemic renal injury. However, urine biomarkers which were interfered by alterations of hemodynamics didn’t show the similar tendency. On the contrary, plasma NAGL and L-FABP in the 10-min resuscitation of group 3 were significantly lower than other 2 groups, may suggesting hypothermia resuscitation effective and useful even within 10 minutes. Scr and BUN levels in the 3, 5, 10-min resuscitation of group 3 decreased significantly may be the result of stopping of sustained renal damage under resuscitation with 4°C 500 ml saline, which also indicates the importance of hypothermia resuscitation.
To our knowledge, there are scant even no literatures of animal models in type 1 CRS. Our ischemia-reperfusion swine model being closer to the human physiological status, less trauma, being able for repeated coronary angiography and electrical physiological examination, gives profound understanding of subclinical AKI in type 1 CRS. Moreover, the parameters measured in this model are not monitored time-dependently. That means they are different from those observed of 2-hour or 4-hour, 1 day etc post cardiac surgery in previous literature. Our finding implies subclinical AKI could occur in a very early stage in type 1 CRS, which are much earlier than those have been reported. In return, it proves the undisputed importance of those biomarkers in ischemia-reperfusion injury.
In conclusion, our study on the basis of the type 1 CRS swine model may give some hints in clinical assessments and therapeutic decision-making. Firstly, monitoring tubular damage biomarker status even in the absence of functional impairment, both in plasma and urine samples, helps alertness of renal risk and makes effective salvage timely to improve patients’ prognosis. Secondly, urine output continues to represent a useful parameter for initiating adjustments to fluid balance and the commencement or ending of RRT (renal replacement treatment), likewise, glomerular filtration markers, such as levels of serum creatinine or serum cystatin C, still are needed for the diagnosis and quantification of excretory function loss. It is important to combine biomarker-driven approach with those traditional parameters in the diagnosis and treatment of Type 1 CRS. Thirdly, hypothermia resuscitation with sufficient fluid supply in a very early stage of subclinical AKI in Type 1 CRS may preserve renal function and reduce cardiovascular mortality.
Acknowledgements
This work was supported by grants from The National Natural Science Foundation of China (81372035, 81100512/H0510, 81170660/H0509, 81370815/H0509), The Foundation of the Health Department of Jiangsu Province (NO. H201301), The Open Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. SKLNMKF201311), The project of Jiangsu provincial Six Talent Peaks (NO. 2013WSN035), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, Jx10231081), The Ph.D. Programs Foundation of the Ministry of Education of China (HA10), The Natural Science Foundation of Jiangsu (BK2011849).
Disclosure of conflict of interest
None.
References
- 1.Thiele H, Hildebrand L, Schirdewahn C, Eitel I, Adams V, Fuernau G, Erbs S, Linke A, Diederich KW, Nowak M, Desch S, Gutberlet M, Schuler G. Impact of high-dose Nacetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Am Coll Cardiol. 2010;55:2201–09. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- 2.Marenzi G, Assanelli E, Campodonico J, De Metrio M, Lauri G, Marana I, Moltrasio M, Rubino M, Veglia F, Montorsi P, Bartorelli A. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med. 2010;38:438–44. doi: 10.1097/CCM.0b013e3181b9eb3b. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg A, Kogan E, Hammerman H, Markiewicz W, Aronson D. The impact of transient and persistent acute kidney injury on longterm outcomes after acute myocardial infarction. Kidney Int. 2009;76:900–06. doi: 10.1038/ki.2009.295. [DOI] [PubMed] [Google Scholar]
- 4.Marenzi G, Moltrasio M, Assanelli E, Lauri G, Marana I, Grazi M, Rubino M, Metrio MD, Veglia F, Bartorelli AL. Impact of cardiac and renal dysfunction on inhospital morbidity and mortality of patients with acute myocardial infarction undergoing primary angioplasty. Am Heart J. 2007;153:755–62. doi: 10.1016/j.ahj.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR. The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta-analysis. Am J Kidney Dis. 2007;50:712–20. doi: 10.1053/j.ajkd.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–73. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, O’Connor CM, Rich MW, Stevenson LW, Wang YF, Young JB, Krumholz HM. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004;147:331–38. doi: 10.1016/j.ahj.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–58. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 9.Ronco C, Cruz DN, Ronco F. Cardiorenal syndromes. Curr Opin Crit Care. 2009;15:384–91. doi: 10.1097/MCC.0b013e32832e971b. [DOI] [PubMed] [Google Scholar]
- 10.Fabbian F, Pala M, De Giorgi A, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Scalone SA, Molino C, Portaluppi F, Mikhailidis DP, Manfredini R. Clinical features of cardio-renal syndrome in a cohort of consecutive patients admitted to an internal medicine ward. Open Cardiovasc Med J. 2001;5:220–25. doi: 10.2174/1874192401105010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane BR, Demirjian S, Weight CJ, Larson BT, Poggio ED, Campbell SC. Performance of the chronic kidney diseaseepidemiology study equations for estimating glomerular filtration rate before and after nephrectomy. J Urol. 2010;183:896–901. doi: 10.1016/j.juro.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Prosek J, Agarwal A, Parikh SV. Cardiorenal syndrome and the role of ultrafiltration in heart failure. Curr Heart Fail Rep. 2013;10:81–8. doi: 10.1007/s11897-012-0129-1. [DOI] [PubMed] [Google Scholar]
- 13.Lauschke A, Teichgräber UK, Frei U, Eckardt KU. ‘Low-dose’ dopamine worsens renal perfusion in patients with acute renal failure. Kidney Int. 2006;69:1669–74. doi: 10.1038/sj.ki.5000310. [DOI] [PubMed] [Google Scholar]
- 14.Allgren RL, Marbury TC, Rahman SN, Weisberg LS, Fenves AZ, Lafayette RA, Sweet RM, Genter FC, Kurnik BRC, Conger JD, Sayegh MH. Anaritide in acute tubular necrosis. N Engl J Med. 1997;336:828–34. doi: 10.1056/NEJM199703203361203. [DOI] [PubMed] [Google Scholar]
- 15.Malyszko J. Biomarkers of acute kidney injury in different clinical settings: a time to change the paradigm? Kidney Blood Press Res. 2010;33:368–82. doi: 10.1159/000319505. [DOI] [PubMed] [Google Scholar]
- 16.Haase M, Kellum JA, Ronco C. Subclinical AKI-an emerging syndrome with important consequences. Nat Rev Nephrol. 2012;8:735–9. doi: 10.1038/nrneph.2012.197. [DOI] [PubMed] [Google Scholar]
- 17.Ronco C, Stacul F, McCullough PA. Subclinical acute kidney injury (AKI) due to iodine-based contrast media. Eur Radiol. 2013;23:319–23. doi: 10.1007/s00330-012-2607-y. [DOI] [PubMed] [Google Scholar]
- 18.Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2013;16:313. doi: 10.1186/cc11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Shao DB, Zhang FX, Zhang JW, Man YH, Du W, Liu BX, Wang DW, Li XR, Cao KJ. Establishment and evaluation of a swine model of acute myocardial infarction and reperfusion-ventricular fibrillation-cardiac arrest using the interventional technique. J Chin Med Assoc. 2013;76:491–6. doi: 10.1016/j.jcma.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1:pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–42. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 21.Logeart D, Tabet JY, Hittinger L, Thabutc G, Jourdaind P, Maisonb P, Tartierea JM, Solal AC. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int J Cardiol. 2008;127:228–32. doi: 10.1016/j.ijcard.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L. Worsening renal function in patients hospitalised for acute heart failure: Clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–95. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Latchamsetty R, Fang J, Kline-Rogers E, Mukherjee D, Otten RF, LaBounty TM, Emery MS, Eagle KA, Froehlich JB. Prognostic value of transient and sustained increase in in-hospital creatinine on outcomes of patients admitted with acute coronary syndrome. Am J Cardiol. 2007;99:939–42. doi: 10.1016/j.amjcard.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 24.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–16. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 25.Marenzi G, Assanelli E, Campodonico J, De Metrio M, Lauri G, Marana I, Moltrasio M, Rubino M, Veglia F, Montorsi P, Bartorelli AL. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med. 2010;38:438–44. doi: 10.1097/CCM.0b013e3181b9eb3b. [DOI] [PubMed] [Google Scholar]
- 26.Amin AP, Spertus JA, Reid KJ, Lan X, Buchanan DM, Decker C, Masoudi FA. The prognostic importance of worsening renal function during an acute myocardial infarction on long-term mortality. Am Heart J. 2010;160:1065–71. doi: 10.1016/j.ahj.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Cruz DN. Cardiorenal syndrome in critical care: the acute cardiorenal and renocardiac syndromes. Adv Chronic Kidney Dis. 2013;20:56–66. doi: 10.1053/j.ackd.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Cho E, Kim M, Ko YS, Lee HY, Song M, Kim MG, Kim HK, Cho WY, Jo SK. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant. 2013;28:2766–78. doi: 10.1093/ndt/gft376. [DOI] [PubMed] [Google Scholar]
- 29.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–22. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 30.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 31.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/ reperfusion. Kidney Int. 2003;63:1714–24. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 32.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–73. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery-a prospective cohort study. Crit Care Med. 2009;37:553–60. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- 34.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Goldstein SL. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–14. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 37.Pomerantz BJ, Reznikov LL, Harken AH, Alouani S, Scoazec A, Beaufils P, Tedgui A. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci U S A. 2001;98:2871–76. doi: 10.1073/pnas.041611398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seta Y, Kanda T, Tanaka T, Arai M, Sekiguchi K, Yokoyama T, Kurimoto M, Tamura J, Kurabayashi M. Interleukin 18 in acute myocardial infarction. Heart. 2000;84:668–9. doi: 10.1136/heart.84.6.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–52. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melnikov VY, Faubel SG, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002;110:1083–91. doi: 10.1172/JCI15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Guo W, Zhang J, Xu C, Yu S, Mao Z, Wu J, Ye C, Mei C, Dai B. Urinary Interleukin 18 for Detection of Acute Kidney Injury: A Meta-analysis. Am J Kidney Dis. 2013;62:1058–67. doi: 10.1053/j.ajkd.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 43.Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2007;66:480–5. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 44.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–90. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 45.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: Role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–9. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 46.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–20. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 47.Racusen LC. The morphologic basis of acute renal failure. Acute Renal Failure. 2001:1–12. [Google Scholar]
- 48.Rabb H. Evaluation of urinary markers in acute renal failure. Curr Opin Nephrol Hypertens. 1998;7:681–5. doi: 10.1097/00041552-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Marcussen N, Schumann J, Campbell P, Kjellstrand C. Cytodiagnostic urinalysis is very useful in the differential diagnosis of acute renal failure and can predict the severity. Ren Fail. 1995;17:721–9. doi: 10.3109/08860229509037640. [DOI] [PubMed] [Google Scholar]


