Abstract
Purpose: The aim of this study was to identify the correlations of IFN-γ-inducible protein-10 (IP-10) with the risk of chronic hepatitis B (CHB) and the efficacy of interferon therapy in Asians. Method: Serum IP-10 levels were assayed using enzyme linked immunosorbent assay (ELISA) in both CHB and control group. CHB group received interferon-α2b treatment to compare the pre-treatment and post-treatment serum IP-10 levels. Relevant studies met predefined inclusion and exclusion criteria were enrolled into further meta-analysis. Stata 12.0 software was applied for data analysis. Result: Our case-control study demonstrated that CHB group had evaluated serum IP-10 levels compared with control group (285.7 ± 41.6 pg/mL vs. 79.1 ± 33.8 pg/mL, t = 21.85, P < 0.001. After treatment for 12 weeks, CHB group had remarkably decreased post-treatment serum IP-10 levels than pre-treatment (78.5 ± 20.4 pg/mL vs. 285.7 ± 41.6 pg/mL, t = 33.76, P < 0.001). No significance was observed on post-treatment serum IP-10 levels between CHB and control group (78.5 ± 20.4 pg/mL vs. 78.1 ± 33.8 pg/mL, t = 0.07, P = 0.947). Meta-analysis results demonstrated that serum IP-10 levels in CHB group were obviously higher than healthy controls (SMD = 2.21, 95% CI = 1.55~2.87, P < 0.001). A subgroup based on the HBeAg states revealed that serum IP-10 levels in both HBeAg-positive and HBeAg-negative CHB patients were notably higher than healthy controls (HBeAg-positive: SMD = 2.00, 95% CI = 1.13-2.87, P < 0.001; HBeAg-negative: SMD = 1.34, 95% CI = 0.97-1.72, P < 0.001). Conclusion: Serum IP-10 may be correlated with the risk of CHB and the efficiency of interferon therapy, thus IP-10 may be a good biomarker for the diagnosis and treatment of CHB.
Keywords: Chronic hepatitis B, hepatitis B virus, interferon inducible protein 10, interferon-α2b
Introduction
Chronic hepatitis B (CHB) is one of the leading causes of liver-related morbidity and mortality worldwide, affecting 350 to 400 million people globally with 75% infectors in the Asia-Pacific region [1]. CHB is an immune-mediated infectious disease resulted from complicated interplay among hepatitis B virus (HBV), hepatocytes and the host immune system [2]. The CHB patients were characterized by the infiltration of inflammatory cells including neutrophils, monocytes, natural killer cells and lymphocytes [3]. There are four stages of this disease, namely the immune-tolerant, the immune-active, inactive hepatitis B surface antigen (HBsAg) positive, and the hepatitis B e antigen (HBeAg)-negative CHB carrier phase [4]. Evidence has been suggested that the active HBV replication is the key factor involved in liver injury and CHB progression, thus the primary aim of CHB treatment is to permanently suppress HBV replication [5]. However, the exact mechanism of HBV activity was not known while evidence demonstrated that the interplay between viral factors and host genetic factors was involved [6]. Currently, indications for CHB treatment initiation were serum aminotransferase levels including Alanine aminotransferase (ALT) levels, serum HBV DNA levels and histological grade [7]. It has been recognized that effective anti-HBV therapy arrests or slows the progression of CHB, especially in patients who achieve HBeAg (or further HBsAg) clearance or seroconversion [8]. Many chemokines may implicate in immune reactions in response to HBV infection while chemokine expression and its correlation with virological response in patients with CHB were still unclear [9].
The interferon-gamma inducible protein 10 (IP-10), also known as CXCL10, is a chemokine involved in both innate and acquired immune responses that direct T cells to sites of inflammation [10]. IP-10 was secreted by microglia, macrophages and astrocytes in response to stimulus, such as interferon (IFN)-a, IFN-b, IFN-c or viruses [11]. A higher pre-treatment serum IP-10 level, which was correlated with a higher HBV DNA loader and greater HBeAg and HBsAg decline, might predict HBeAg loss in HBeAg-positive CHB patients [8]. Increasing evidence showed that serum IP-10 levels were notably correlated with CHB patients [12,13]. Nevertheless, whether IP-10 expression level is related with the progression of disease caused by HBV infection and with the biochemical and virological factors in patients with CHB remains unclear [8,13].
To address the correlation of serum IP-10 levels with CHB and to further investigate the efficiency of interferon therapy, this study was performed by investigating the pre-treatment and post-treatment serum ALT levels, one of the widely used biomarker for HBV-related liver cell injury [14], and serum IP-10 levels in a case- control study and a meta-analysis.
Materials and methods
Subjects
From Jan 2012 to Dec 2014, 57 patients (41 male and 16 female) with CHB hospitalized in the Zhongnan Hospital Wuhan University were selected as CHB group. The mean age of CHB group ranged from 25~51 year (mean, 35.8 ± 10.5 years). All patients were diagnosed with CHB based on criteria from the guideline of prevention and treatment for chronic hepatitis. All patients in CHB group were presented with increased serum ALT levels and were arranged for antiviral therapy. Based on the HBeAg states, 23 patients in case group were HBeAg-positive CHB and 25 patients had HBeAg-negative CHB. Furthermore, 25 healthy controls (21 male and 4 female) were included in current case and control study with mean age of 31.2 ± 8.5 years (ranging from 25~50 years). This study was approved by the Ethics committee of Zhongnan Hospital Wuhan University and written informed consents were obtained from each patient prior to study.
Sample collection
After an overnight fast, venous blood (3 mL) were collected and maintained in room temperature for 30 min. After centrifugation at 1500 r/min for 15 min, the sample were removed the supernatant and stored at -20°C for further usage.
Detection for ALT and IP-10
The serum ALT level was measured using Beckman LX20 automated analyzer (Beckman Corp., Brea, CA, USA) and Backman kit. The normal serum ALT level was 0~40 U/L. Moreover, the serum IP-10 level was assayed using enzyme linked immunosorbent assay (ELISA) kit (R & D system) and KHB ST-360 ELISA analyzer. All procedures were conducted based on the manufacturer’s instructions. Real-time Fluorescence Quantitative PCR (RTFQ-PCR) was conducted to detect the HBV DNA with detection limit of 500 IU/mL by reagent (Da An Gene Co., Ltd. of Sun Yat-Sen University) and PE-5700 thermal cycler. The primers for PCR were as follows: the upstream primer HBxF: 5’-CACTTCGCTTCACCTCTGCA-3’, downstream primer HBxR: TGCCTCAAGGTCGGTCGT-3’; Tap-man probe HBxP: 5’-FAM-ACCACCGTGAACGCCCACC-TAMRA-3’. The amplified fragments: 121 bp.
Interferon-α2b therapy
CHB group (n = 57) were arranged for interferon-α2b therapy once every other day through subcutaneous injection (6000, 000 IU). The pre-treatment and 12 weeks post-treatment serum samples were respectively obtained for further comparison. According to the virological response in 12 weeks, 48 CHB patients were grouped into responding group with post-treatment serum HBV DNA decreased ≥ 2log10 IU/mL compared with pre-treatment [15] and the remaining 9 patients were included in nonresponse group with post-treatment serum HBV DNA decreased < 2log10 IU/mL compared with pre-treatment.
Statistical analysis
Data analysis in current case-control study was conducted utilizing Stata statistical software (Version 12.0, Stata Corporation, College Station, TX, USA). Categorical data were presented with percentages and frequency counts and a x 2 test was employed to compare the percentages and frequencies, while continuous data were expressed as mean ± standard deviation (mean ± SD) and were tested using student t test. Computerized bibliographic databases and manual search were combined to identify relevant articles published prior to Sept. 2014 with language restricted to English and Chinese. Keywords applied in our initial literature search include (“Hepatitis B” or “Hepatitis B, Chronic” or “Hepatitis B virus” or “Chronic Hepatitis B”) and (“Chemokine CXCL10” or “CXCL10 protein, human” or “CXCL10” or “Chemokine CXCL10” or “IP 10” or “IP-10” or “gammaIP-10” or “gammaIP 10” or “Interferon-Inducible Protein 10” or “Interferon Inducible Protein 10” or “Small Inducible Cytokine B10” or “CXC Chemokine IP-10” or “CXC Chemokine IP 10” or “IFN-gamma-Inducible Protein” or “interferon-gamma induced protein 10” or “interferon-gamma-inducible protein 10”). The inclusions for current meta-analysis were: (1) Only those studies conducted to clarify the correlation between serum IP-10 levels and CHB were incorporated; (2) The CHB patients from included studies were diagnosed with CHB based on clear and specific diagnostic criteria; (3) The articles provided with sufficient information on serum IP-10 levels; (4) Studies with overlapped publication were only enrolled for once with the largest sample size. Ineligibles papers were excluded based on the following exclusion criteria: (1) insufficient data information; (2) significant differences in baseline characteristics between cases and controls; (3) duplicated publications or studies with overlapped data; (4) no clear diagnostic criteria. Two independent investigators were employed for data collection and following descriptive information were recorded: authors, country, years, ethnicity, language, HBeAg status, diagnostic criteria, sex, age, detection method and serum IP-10 levels. The disagreements during data collection were dissolved by discussion with a third investigator. The data analysis in meta-analysis was conducted by STATA 12.0 (Stata Corporation, College Station, TX, USA). The difference on the serum IP-10 levels was calculated by standardized mean difference (SMD) with 95% confidence intervals (95% CI) with Z test to evaluate the combined effect size. Cochran Q-statistic (P < 0.05 was considered significant) and I 2 tests to quantify heterogeneity among studies with P < 0.05 or I 2 test exhibited > 50% considered as indicator of presence of heterogeneity. Random effects model was applied for the evidence of significant heterogeneity, whereas fixed-effects model was employed based on absence of heterogeneity.
Results
Basic characteristics
The comparisons on the basic characteristics between two groups were presented in Table 1. No significances on sex, year, body mass index (BMI) and drink history were found between case and control group (all P > 0.05). Prior to treatment, CHB group presented with a remarkable increased serum ALT and IP-10 levels compared with control group (both P < 0.05).
Table 1.
The comparisons conducted on the baseline characteristic in subjects included in both chronic hepatitis B (CHB) group and control group
| Group | CHB group (n = 57) | Control group (n = 25) | P |
|---|---|---|---|
| Sex (M/F) | 41/16 | 21/4 | 0.241 |
| Age (year) | 35.8 ± 10.5 | 31.2 ± 8.5 | 0.057 |
| BMI (kg/cm2) | 24.9 ± 2.5 | 25.3 ± 2.8 | 0.522 |
| Drink history [case (%)] | 31 (54.4%) | 14 (56%) | 0.892 |
| ALT (U/L) | 40.6 ± 5.2 | 32.8 ± 3.5 | < 0.001 |
| IP-10 (pg/mL) | 285.7 ± 41.6 | 79.1 ± 33.8 | < 0.001 |
M, male; F, female; BMI, body max index; ALT, Alanine aminotransferase; IP-10, interferon-gamma inducible protein 10; CHB, Chronic hepatitis B.
Serum ALT and IP-10 levels before and after interferon-α2b treatment
The post-treatment serum ALT and IP-10 levels of CHB patients was notably decreased compared to pre-treatment (t = 8.71, P < 0.001; t = 33.76, P < 0.001), despite no statistical significance was found between CHB group and control group on post-treatment serum ALT and IP-10 levels (t = 0.33, P = 0.742; t = 0.07, P = 0.947). The post-treatment serum ALT and IP-10 levels in both HBeAg-positive and HBeAg-negative CHB patients were reduced compared to pre-treatment (all P < 0.05). Based on virological response, CHB patients in both responding group and nonresponse group had a lower post-treatment serum ALT and IP-10 levels than pre-treatment (both P < 0.05). After treatment for 12 weeks, the serum ALT and IP-10 levels in HBeAg-negative CHB patients were evidently decreased compared with HBeAg-positive CHB patients (32.6 ± 5.1 U/L vs. 33.7 ± 2.4 U/L, t = 2.17, P = 0.034; 77.5 ± 19.6 pg/mL vs. 80.1 ± 21.4 pg/mL, t = 2.13, P = 0.038). In addition, responding group had declined post-treatment serum ALT and IP-10 levels compared with nonresponse group (32.9 ± 4.2 U/L vs. 36.2 ± 4.4 U/L, t = 2.15, P = 0.036; 76.1 ± 19.7 pg/mL vs. 91.2 ± 24.1 pg/mL, t = 2.04, P = 0.046) (Table 2).
Table 2.
The comparisons between pre-treatment and post-treatment serum Alanine aminotransferase (ALT) and IFN-γ-inducible protein-10 (IP-10) levels were conducted in chronic hepatitis B (CHB) patients
| Group | Case | ALT (U/L) | IP-10 (pg/mL) | ||
|---|---|---|---|---|---|
|
| |||||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||
| Control group | 25 | 32.8 ± 3.5 | 78.1 ± 33.8 | ||
| CHB group | 57 | 40.6 ± 5.2 | 33.1 ± 3.9* | 285.7 ± 41.6 | 78.5 ± 20.4* |
| HBeAg-negative | 32 | 36.1 ± 4.9 | 32.6 ± 5.1* | 208.6 ± 35.2 | 77.5 ± 19.6* |
| HBeAg-positive | 25 | 46.4 ± 5.6 | 35.0 ± 2.4* | 384.4 ± 49.8 | 89.1 ± 21.4* |
| Responding group | 48 | 39.6 ± 5.4 | 32.9 ± 4.2* | 278.5 ± 43.1 | 76.1 ± 19.7* |
| Nonresponse group | 9 | 42.1 ± 5.9 | 36.2 ± 4.4* | 324.1 ± 33.7 | 91.2 ± 24.1* |
ALT, Alanine aminotransferase; IP-10, interferon-gamma inducible protein 10; CHB, Chronic hepatitis B;
Compared with pre-treatment serum ALT and IP-10 levels.
Meta-analysis
Based on our predefined search strategy, 68 studies were identified and after applying our eligibility criteria, 11 studies were fulfilled the inclusion criteria for our meta-analysis with publication year ranging from 2001 and 2014 [3,8,16-24]. A total of 482 CHB patients and 205 healthy controls were incorporated in current meta-analysis (Table 3). Random effect model was applied with the presence of heterogeneity (I 2 = 90.3%, Ph < 0.001). Our meta-analysis demonstrated that CHB patients presented with a remarkably increased IP-10 serum levels compared with healthy controls (SMD = 2.21, 95% CI = 1.55~2.87, P < 0.001) (Figure 1). A subgroup based on HBeAg statue revealed that both HBeAg-positive and HBeAg-negative patients had higher serum IP-10 levels than healthy controls (HBeAg-positive: SMD = 2.00, 95% CI = 1.13~2.87, P < 0.001; HBeAg-negative: SMD = 1.34, 95% CI = 0.97~1.72, P < 0.001) (Figure 2).
Table 3.
Basic Characteristics for the eleven eligible studies included in current met-analysis
| First author | Year | Country | HBeAg status | Diagnostic Criteria | Detecting Method | Number | Gender (M/F) | Age (years) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| CHB | Control | CHB | Control | CHB | Control | ||||||
| Nishioji K [16] | 2001 | Japan | Positive | NR | ELISA | 20 | 20 | 13/7 | 10/10 | 41.9 ± 11.5 | 46.4 ± 14.4 |
| Shi LY [17] | 2003 | China | Mixed | I | ELISA | 58 | 20 | 41/17 | 12/8 | 32.7 (17-51) | 37.1 |
| Wang J [3] | 2008 | China | Positive | II | ELISA | 45 | 24 | 25/20 | 14/10 | 30 ± 9 (18-50) | 25 ± 5 (20-48) |
| Ding HH [18] | 2009 | China | Mixed | II | ELISA | 19 | 5 | 17/2 | 5/0 | 36.7 ± 9.0 | 24.4 ± 3.4 |
| Liu SC-a [19] | 2011 | China | Positive | III | ELISA | 16 | 28 | 27/13 | 24/4 | 44.2 ± 12.6 (21-72) | 36.5 ± 10.9 (17-58) |
| Liu SC-b [19] | 2011 | China | Negative | III | ELISA | 24 | 28 | ||||
| Zhu XJ-a [20] | 2011 | China | Positive | III | ELISA | 33 | 20 | 46/20 | 11/9 | 38 ± 11(21-61) | 39 ± 13 (21-56) |
| Zhu XJ-b [20] | 2011 | China | Negative | III | ELISA | 33 | 20 | ||||
| Li WL [21] | 2012 | China | Positive | IV | ELISA | 50 | 30 | 35/15 | 21/9 | 27.8 (20-38) | 27.1 (20-40) |
| Jiao YQ [22] | 2013 | China | Mixed | II | ELISA | 40 | 23 | 41/22 | 34.4 ± 9.1 (19-47) | ||
| Okuhara S [23] | 2013 | Japan | Positive | NR | ELISA | 48 | 10 | 33/15 | NR | 55 (24-81) | NR |
| Lian JQ [24] | 2014 | China | Mixed | NR | Luminex200 | 36 | 10 | 29/7 | 7/3 | 31.3 ± 8.7 | 28.4 ± 7.3 |
| Wang Y-a [8] | 2014 | China | Positive | V | ELISA | 38 | 15 | 22/16 | 8/7 | 35.5 ± 10 | 38 ± 12 |
| Wang Y-b [8] | 2014 | China | Negative | V | ELISA | 22 | 15 | 14/8 | 8/7 | 40 ± 15 | 38 ± 12 |
Liu SC-a: HBeAg positive on subgroup; Liu SC-b: HBeAg negative on subgroup; Zhu XJ-a: HBeAg positive on subgroup; Zhu XJ-b: HBeAg negative on subgroup; Wang Y-a: HBeAg positive on subgroup; Wang Y-b: HBeAg negative on subgroup; NR, Not Reported; I: GB 15990-1995; II: Prevention Guideline for Viral Hepatitis (2000); III: Prevention Guideline for Chronic Hepatitis B (2005); IV: Prevention Guideline for Chronic Hepatitis B (2010); V: APASL, Asian Pacific Association for the Study of the Liver; ELISA, enzyme-linked immunosorbent assay; CHB, Chronic hepatitis B.
Figure 1.
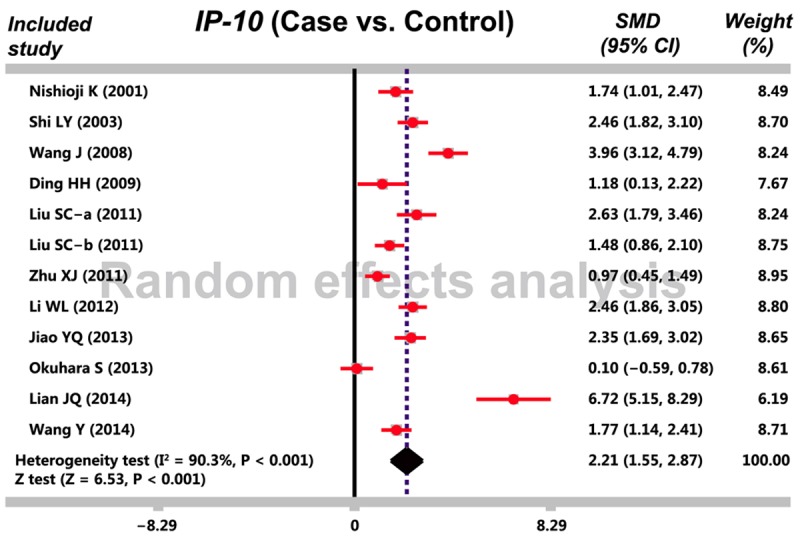
The forest plot in meta-analysis investigates the correlation between serum IFN-γ-inducible protein-10 (IP-10) levels and chronic hepatitis B (CHB).
Figure 2.
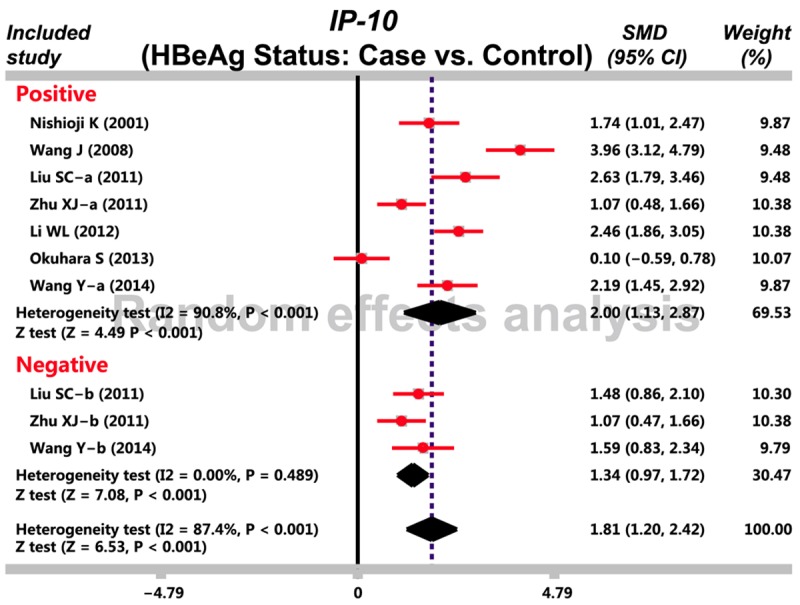
Subgroup analysis based on HBeAg investigates the correlation between serum IFN-γ-inducible protein-10 (IP-10) levels and chronic hepatitis B (CHB).
Discussion
To clarify the correlation between serum IP-10 levels and CHB, a case-control study and a meta-analysis was conducted. The main results of case-control study and meta-analysis demonstrated that serum IP-10 levels in CHB patients were evidently higher than that in healthy controls, implying that IP-10 may be an important indicator for CHB. Recent reports have also verified that IP-10 was expressed in liver with CHB patients, and this expression varied among patients with different liver diseases [9,13]. Furthermore, we also found that CHB patients had declined serum IP-10 and ALT levels after treatment and responding group presented with reduced serum IP-10 and ALT levels compared with nonresponse group. Those results suggested the positive correlation between serum IP-10 and ALT levels and serum HBV DNA. Chronic HBV infection reflected a complex interplay between humoral immune response and host immune activities [25]. Serum ALT levels should reflect the degree of liver damage, whereas IP-10 levels may reflect immune activity [26,27].
It has been recognized that higher ALT levels was a risk predictor for more active immune response against HBV and more extensive hepatocyte damage [5,28]. Given the strong association of IP-10 with ALT, high levels of IP-10 may predict active host immune response, consequently lead to a more active liver inflammation [13]. Generally, IP-10 activated T-lymphocytes and natural killer cells, and then recruited Th cells and monocytes cells to take part in inflammation and immune response through interaction with its CXCR3 receptor, thus facilitating the hepatic necroinfammatory damage in chronic HBV infection [24,29]. Increasing evidence elucidated that serum IP-10 level may be an important factor in regulation of antiviral immunity and may affect disease progression [8], which was consistent with our results that post-treatment serum IP-10 levels were reduced compared with pre-treatment. A study by Lagging M confirmed that the serum IP-10 and ALT levels are significantly associated with the severity of hepatic inflammation [30]. With respect to this, the serum IP-10 levels were correlated with the CHB and may relate to the disease progression.
In consideration of other related factors may influence the correlation between the serum IP-10 levels and CHB, a further subgroup analysis based on the HBeAg states was performed. The HBeAg-stratified found that CHB patients with HBeAg positive or HBeAg negative both had higher serum IP-10 levels than healthy controls, which was in agreement with previous studies [31,32]. Moreover, the case and control study also elucidated that after treatment for 12 weeks, the HBeAg-negative CHB patients had decreased serum IP-10 and ALT levels compared with HBeAg-positive CHB patients, which suggested that serum IP-10 and ALT expression levels responds to antiviral therapy.
In summary, our case-control study and meta-analysis revealed that serum IP-10 levels were correlated with the risk of CHB and the efficacy of interferon therapy in Asians, which suggesting that serum IP-10 level may be an important biomarker for the diagnose and treatment of CHB. However, due to the limited sample size of this study, this conclusion needs to be double investigated and validated with a large sample size.
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
Disclosure of conflict of interest
None.
References
- 1.Huang Y, Lok AS. Viral factors and outcomes of chronic HBV infection. Am J Gastroenterol. 2011;106:93–95. doi: 10.1038/ajg.2010.404. [DOI] [PubMed] [Google Scholar]
- 2.Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, Liaw YF, Xie Q, Heathcote EJ, Chan HL, Janssen HL. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology. 2013;58:872–880. doi: 10.1002/hep.26436. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Zhao JH, Wang PP, Xiang GJ. Expression of CXC chemokine IP-10 in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2008;7:45–50. [PubMed] [Google Scholar]
- 4.Coffin CS, Osiowy C, Gao S, Nishikawa S, van der Meer F, van Marle G. Hepatitis B virus (HBV) variants fluctuate in paired plasma and peripheral blood mononuclear cells among patient cohorts during different chronic hepatitis B (CHB) disease phases. J Viral Hepat. 2015;22:416–426. doi: 10.1111/jvh.12308. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S Chronic Hepatitis B Guideline Working Party of the Asian-Pacific Association for the Study of the Liver. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam YF, Wong DK, Seto WK, To KK, Hung IF, Fung J, Lai CL, Yuen MF. HLA-DP and gamma-interferon receptor-2 gene variants and their association with viral hepatitis activity in chronic hepatitis B infection. J Gastroenterol Hepatol. 2014;29:533–539. doi: 10.1111/jgh.12378. [DOI] [PubMed] [Google Scholar]
- 7.Ahn SH, Chan HL, Chen PJ, Cheng J, Goenka MK, Hou J, Lim SG, Omata M, Piratvisuth T, Xie Q, Yim HJ, Yuen MF APPROACH Working Group. Chronic hepatitis B: whom to treat and for how long? Propositions, challenges, and future directions. Hepatol Int. 2010;4:386–395. doi: 10.1007/s12072-010-9163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Zhao C, Zhang L, Yu W, Shen C, Wang W, Zhen Z, Zhou J. Predictive value of interferon-gamma inducible protein 10 kD for hepatitis B e antigen clearance and hepatitis B surface antigen decline during pegylated interferon alpha therapy in chronic hepatitis B patients. Antiviral Res. 2014;103:51–59. doi: 10.1016/j.antiviral.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Hou FQ, Wu XJ, Wang Y, Chen J, Liu YZ, Ren YY, Song G, Ding YP, Yu M, Wang GQ. Rapid downregulation of programmed death-1 and interferon- gamma-inducible protein-10 expression is associated with favourable outcome during antiviral treatment of chronic hepatitis B. J Viral Hepat. 2013;20(Suppl 1):18–26. doi: 10.1111/jvh.12060. [DOI] [PubMed] [Google Scholar]
- 10.Liovat AS, Rey-Cuille MA, Lecuroux C, Jacquelin B, Girault I, Petitjean G, Zitoun Y, Venet A, Barre-Sinoussi F, Lebon P, Meyer L, Sinet M, Muller-Trutwin M. Acute plasma biomarkers of T cell activation set-point levels and of disease progression in HIV-1 infection. PLoS One. 2012;7:e46143. doi: 10.1371/journal.pone.0046143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen FH, Tsai CC, Wang LC, Chang KC, Tung YY, Su IJ, Chen SH. Enterovirus 71 infection increases expression of interferon-gamma-inducible protein 10 which protects mice by reducing viral burden in multiple tissues. J Gen Virol. 2013;94:1019–1027. doi: 10.1099/vir.0.046383-0. [DOI] [PubMed] [Google Scholar]
- 12.Xu Z, Liu Y, Liu L, Li X, Bai S, Rong Y, Wang H, Mao Y, Xin S, Xu D. Association of interferon-gamma induced protein 10 promoter polymorphisms with the disease progression of hepatitis B virus infection in Chinese Han population. PLoS One. 2013;8:e72799. doi: 10.1371/journal.pone.0072799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonneveld MJ, Arends P, Boonstra A, Hansen BE, Janssen HL. Serum levels of interferon-gamma-inducible protein 10 and response to peginterferon therapy in HBeAg-positive chronic hepatitis B. J Hepatol. 2013;58:898–903. doi: 10.1016/j.jhep.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582–592. doi: 10.1016/S0140-6736(09)60207-5. [DOI] [PubMed] [Google Scholar]
- 15.Koh C, Zhao X, Samala N, Sakiani S, Liang TJ, Talwalkar JA. AASLD clinical practice guidelines: a critical review of scientific evidence and evolving recommendations. Hepatology. 2013;58:2142–2152. doi: 10.1002/hep.26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishioji K, Okanoue T, Itoh Y, Narumi S, Sakamoto M, Nakamura H, Morita A, Kashima K. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin Exp Immunol. 2001;123:271–279. doi: 10.1046/j.1365-2249.2001.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi LY, He JQ, Chen Z, Wu LJ. The expression and significance of chemokine interferon-inducible protein-10 (IP-10) in chronic hepatitis B patients. Chin J Clini Hepatol. 2003;19:220–221. [Google Scholar]
- 18.Ding HH, Hao YH, Yang XX, Yang DL. The elevated serum chemokine IP-10 and RANTES levels in individuals with HBV infection. Practical Journal of Hepatobiliary Disease. 2009;12:176–178. [Google Scholar]
- 19.Liu SC, Chen SL. Expression and significance of TLR4 in peripheral blood mononuclear cell and serum IP-10 in chronic hepatitis B. Journal of Guangxi Medical University. 2011;28:73–74. [Google Scholar]
- 20.Zhu XJ, Song YF, Cao YP, Xu WQ, Zhang CQ. The expression and significance of CXCL10 and CXCL11 in chronic hepatitis B patients. Journal of Clinical Hepatology. 2011;27:804–806. [Google Scholar]
- 21.Li WL, Wu MS, Huang YB, Zhu JF, Liu XL, Xu RR. Relationship between the serum chemokine IP-10 and RANTES levels and Interferon therapeutic early response in patients with chronic hepatitis B. Chinese Journal of Primary Medicine and Pharmacy. 2012;19:1283–1285. [Google Scholar]
- 22.Jiao YQ, Zhang H, Wang L. To investigate the relationship between factor IP-10, Rantes and oxidative damage in patients with chronic viral hepatitis. China Practical Medical. 2013;08:148–149. [Google Scholar]
- 23.Okuhara S, Umemura T, Joshita S, Shibata S, Kimura T, Morita S, Komatsu M, Matsumoto A, Yoshizawa K, Katsuyama Y, Ota M, Tanaka E. Serum levels of interleukin-22 and hepatitis B core-related antigen are associated with treatment response to entecavir therapy in chronic hepatitis B. Hepatol Res. 2014;44:E172–80. doi: 10.1111/hepr.12287. [DOI] [PubMed] [Google Scholar]
- 24.Lian JQ, Yang XF, Zhao RR, Zhao YY, Li Y, Zhang Y, Huang CX. Expression profiles of circulating cytokines, chemokines and immune cells in patients with hepatitis B virus infection. Hepat Mon. 2014;14:e18892. doi: 10.5812/hepatmon.18892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loggi E, Bihl FK, Cursaro C, Granieri C, Galli S, Brodosi L, Furlini G, Bernardi M, Brander C, Andreone P. Virus-specific immune response in HBeAg-negative chronic hepatitis B: relationship with clinical profile and HBsAg serum levels. PLoS One. 2013;8:e65327. doi: 10.1371/journal.pone.0065327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 27.Deng G, Zhou G, Zhang R, Zhai Y, Zhao W, Yan Z, Deng C, Yuan X, Xu B, Dong X, Zhang X, Zhang X, Yao Z, Shen Y, Qiang B, Wang Y, He F. Regulatory polymorphisms in the promoter of CXCL10 gene and disease progression in male hepatitis B virus carriers. Gastroenterology. 2008;134:716–726. doi: 10.1053/j.gastro.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 28.Yang HI, Yeh SH, Chen PJ, Iloeje UH, Jen CL, Su J, Wang LY, Lu SN, You SL, Chen DS, Liaw YF, Chen CJ REVEAL-HBV Study Group. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134–1143. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maini MK, Schurich A. The molecular basis of the failed immune response in chronic HBV: therapeutic implications. J Hepatol. 2010;52:616–619. doi: 10.1016/j.jhep.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Lagging M, Askarieh G, Negro F, Bibert S, Söderholm J, Westin J, Lindh M, Romero A, Missale G, Ferrari C, Neumann AU, Pawlotsky JM, Haagmans BL, Zeuzem S, Bochud PY, Hellstrand K DITTO-HCV Study Group. Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One. 2011;6:e17232. doi: 10.1371/journal.pone.0017232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan AT, Koh S, Goh W, Zhe HY, Gehring AJ, Lim SG, Bertoletti A. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol. 2010;52:330–339. doi: 10.1016/j.jhep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Okuhara S, Umemura T, Joshita S, Shibata S, Kimura T, Morita S, Komatsu M, Matsumoto A, Yoshizawa K, Katsuyama Y, Ota M, Tanaka E. Serum levels of interleukin-22 and hepatitis B core-related antigen are associated with treatment response to entecavir therapy in chronic hepatitis B. Hepatol Res. 2014;44:E172–180. doi: 10.1111/hepr.12287. [DOI] [PubMed] [Google Scholar]


