Abstract
Background and purpose: MicroRNA-128 (miR-128) has been identified as a negative regulator of malignant phenotypes of prostate cancer (PCa) cells. The aim of this study was to evaluate the prognostic implications of both tissue and serum levels of miR-128 expression in PCa patients undergoing radical prostatectomy. Methods: A series of 128 cases with PCa were evaluated for both tissue and serum levels of miR-128 expression by quantitative reverse-transcription PCR. Results: Compared with non-cancerous prostate tissues and normal sera, both tissue and serum levels of miR-128 expression were significantly decreased in PCa patients (both P<0.001). Importantly, there was a close correlation between tissue and serum levels of miR-128 expression in PCa patients (rs=0.808, P<0.001). Then, low miR-128 expression in both PCa tissues and patients’ sera were dramatically associated with aggressive clinicopathological features, including advanced pathological stage (both P=0.001), positive lymph node metastasis (P=0.006 and 0.01, respectively), high preoperative PSA (both P=0.01) and positive angiolymphatic invasion (both P=0.02). Moreover, Kaplan-Meier survival analysis showed that low miR-128 expression in both PCa tissues and patients’ sera were significantly associated with short biochemical recurrence (BCR)-free survival. Furthermore, multivariate analysis indicated that both tissue and serum levels of miR-128 expression were independent prognostic factors for BCR-free survival of PCa patients. Conclusion: Our data suggest that the decreased expression of miR-128 in both tissue and serum samples of PCa patients may be associated with tumor malignant progression and BCR-free survival. Particularly, serum miR-128 may be developed as a novel noninvasive biomarker for PCa diagnosis and prognosis.
Keywords: Prostate cancer, microRNA-128, clinical pathology, serum, prognosis
Introduction
Prostate cancer (PCa) represents the most common type of cancer and the third leading cause of cancer death among men in the developed world [1]. About one in three men over the age of 50 years shows histological evidence of this cancer [2]. Despite the advancement of therapeutic strategies, there are few effective therapeutic options for advanced PCa. The annual morbidity rate of this cancer has increased by 14% since 1990 [3]. PCa is a clinically heterogeneous-multifocal and aggressive disease, the course of which is highly variable and difficult to predict because a significant proportion of cases are indolent and do not require curative treatment [4]. Thus, better understanding of factors that modify the risk of PCa and its preventive measures that include pharmacological intervention will help us reduce the burden of this disease. Several clinicopathological features, including tumor volume, pathological grade, status of lymph node metastasis, preoperative PSA, Gleason score, have been used for diagnosis and prognosis. However, there is no widely accepted method for quantifying tumor volume [5]; pathological grade scoring methods can result in significant inter-observer variations, particularly when defining intermediate tumor grades [6]; accumulating studies have found that PCa patients with the equivalent PSA level could have various clinical outcomes because of the molecularly heterogeneous subtypes [7]. To overcome these limitations, it appears that novel and efficient biomarkers are needed for the diagnosis and prognosis of patients with PCa.
MicroRNAs (miRNAs) are small (18-28 nt), non-coding, well-conserved RNA molecules, which play crucial roles in the regulation of basic biological processes, including cell growth, apoptosis and differentiation, by negatively regulating gene expression via binding to imperfect complementary sites within the 3’ untranslated region (UTR) of their mRNA target at the post-transcriptional level [8]. Until now, there have been more than 1,400 human miRNA sequences identified and many of them have been reported to be implicated into cancer pathogenesis [9]. Functionally, miRNAs can act either as oncogenes or tumor suppressors according to the roles of their target genes. Growing evidence also show the potential of miRNAs for biomarker applications since various cancer-specific miRNAs have been detectable in cell-free blood fluids such as serum and plasma. In relation to PCa, accumulating studies have reported circulating miRNAs that can assist in diagnosis or the stratification of metastatic from localized disease. For example, Srivastava et al. [10] reported that circulatory miR-628-5p was downregulated in PCa patients, and might be further developed as a biomarker, which can serve as novel noninvasive biomarker for PCa diagnosis and prognosis; Selth et al. [11] indicated that circulating miR-146b-3p and miR-194 were associated with disease progression in PCa, and multivariate analysis revealed that miR-146b-3p possessed prognostic information beyond standard clinicopathological parameters.
MiR-128 is encoded by two distinct genes, miR-128-1 and miR-128-2, which are both intronic and present on two different chromosomes. miR-128-1 is embedded in the R3HDM1 (R3H domain containing 1) gene on chromosome 2q21.3 and miR-128-2 is in the ARPP21 (cyclic AMP-regulated phosphoprotein, 21 kDa) on chromosome 3p22.3, respectively. MiR-128-1 and miR-128-2 are processed into an identical mature sequence [12]. As a brain-enriched miRNA, most of the studies of miR-128 in carcinogenesis are focusing on glioma. The tumor suppressive roles of miR-128 have been found in glioma, neuroblastoma, and PCa [13-16]. Especially, Medina-Villaamil et al. [15] identified a candidate circulating biomarker to screen risk groups in PCa; Khan et al. [16] also revealed miR-128 as a potentially important negative regulator of PCa cell invasion. The aim of the current study was to assess the role of both tissue and serum expression of miR-128 in PCa patients with focus on diagnosis and prognosis. Toward this aim, we detected the expression levels of miR-128 in PCa tissues and patients’ sera by quantitative reverse-transcription PCR. Then, we evaluated the possible prognostic implications of both tissue and serum levels of miR-128 expression in PCa patients undergoing radical prostatectomy.
Materials and methods
Patients and samples
The study was approved by the Research Ethics Committee of Xi’an Hong Hui Hospital and Xi’an Electricity Power Center Hospital. Written informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
One hundred and twenty-eight primary PCa tissues samples, patients’ sera and corresponding noncancerous prostate tissue samples from the same specimens (n=128) were collected from Xi’an Hong Hui Hospital and Xi’an Electricity Power Center Hospital, from 1996 to 2008. None of the patients received androgen deprivation treatment, chemotherapy, or radiation therapy prior to radical prostatectomy. All 128 patients with PCa received radical prostatectomy. The complete records of the cases pre- and post-operation, and samples of the primary tissue, had been preserved. The following clinicopathological parameters, including preoperative PSA, Gleason score, pathological stage, lymph node status, angiolymphatic invasion, margin status, and biochemical relapse, were recorded. The clinicopathological information of the patients is shown in Table 1. In addition, age-matched serum samples from 100 healthy individuals were obtained from Xi’an Hong Hui Hospital and Xi’an Electricity Power Center Hospital.
Table 1.
Correlation of miR-128 expression with clinicopathological features of PCa patients
| Clinicopathological features | Cases No (n, %) | miR-128 expression | |||
|---|---|---|---|---|---|
|
| |||||
| Low-tissues (n, %) | P | Low-sera (n, %) | P | ||
| Age | |||||
| <70 | 70 (54.69) | 36 (51.43) | NS | 35 (50.00) | NS |
| ≥70 | 58 (45.31) | 32 (55.17) | 30 (51.72) | ||
| Preoperative PSA | |||||
| <4 ng/mL | 3 (2.34) | 0 (0) | 0.01 | 0 (0) | 0.01 |
| 4-10 ng/mL | 35 (27.34) | 12 (34.29) | 10 (28.57) | ||
| >10 ng/mL | 90 (70.31) | 56 (62.22) | 55 (61.11) | ||
| Gleason score | |||||
| 4-6 | 62 (48.44) | 31 (50.00) | NS | 31 (50.00) | NS |
| 7 | 30 (23.44) | 19 (63.33) | 16 (53.33) | ||
| 8-10 | 36 (28.13) | 18 (50.00) | 18 (50.00) | ||
| Pathological stage | |||||
| T1 | 72 (56.25) | 20 (27.78) | 0.001 | 20 (27.78) | 0.001 |
| T2/T3 | 56 (43.75) | 48 (85.71) | 45 (80.36) | ||
| Lymph node metastasis | |||||
| Negative | 106 (82.81) | 48 (45.28) | 0.006 | 50 (47.17) | 0.01 |
| Positive | 22 (17.19) | 20 (90.91) | 15 (68.18) | ||
| Angiolymphatic invasion | |||||
| Negative | 110 (85.94) | 55 (50.00) | 0.02 | 52 (47.27) | 0.02 |
| Positive | 18 (14.06) | 13 (72.22) | 13 (72.22) | ||
| Surgical margin status | |||||
| Negative | 108 (84.38) | 56 (51.85) | NS | 53 (49.07) | NS |
| Positive | 20 (15.62) | 12 (60.00) | 12 (60.00) | ||
Note: ‘NS’ refers to the difference without statistic significance.
All 128 patients with PCa were given a follow-up exam ranging from three to ten years. All the patients who died from diseases other than PCa or from unexpected events were excluded from the case collection. For the analysis of biochemical recurrence-free survival, the date of prostatectomy was used to represent the beginning of the follow-up period. The endpoint was the time to biochemical relapse which was defined as the period between surgical treatment and the measurement of two successive values of serum PSA level ≥ 0.2 ng/ml.
Quantitative reverse-transcription PCR
RNA was extracted from fresh tissues or sera of patients with PCa using Trizol Reagent (Invitrogen, USA) according to the manufacturer’s protocol. One microgram of total RNA was reverse-transcribed using SuperScrip III (Invitrogen, USA). After cDNA was synthesized with a miRNA-specific stem-loop primer, the quantitative PCR was performed with the specific primers as follows: miR-128_F, 5’-GCC GGC GCC CGA GCT CTG GCT C-3’; miR-128_R, 5’-TCA CAG TGA ACC GGT CTC TTT-3’; U6_F, 5’-CTC GCT TCG GCA GCA CA-3’; U6_R, 5’-AAC GCT TCA CGA ATT TGC GT-3’. Real-time PCR was performed using an Applied Biosystems 7500 real-time PCR system using 1 μl reverse transcriptase samples in a 20 μl final reaction mixture. 1X TaqMan Universal PCR master mix (Takara, Japan) was used for general PCR. RNU6B was used as an internal control. Relative quantification of target miRNA expression was evaluated using the comparative cycle threshold (CT) method. Each sample was examined in triplicate and the amounts of the PCR products produced were nonneoplasticized to RNU6B.
Statistical analysis
Statistical analysis was performed using the software of SPSS version12.0 for Windows (SPSS Inc, IL, USA). Continuous variables were expressed as mean ± S.D. Fisher’s exact test and Pearson χ2 test were respectively used to analyze 2×2 tables and non-2×2 tables. Kaplan-Meier and Cox Regression methods were used for the question of survival analysis. Differences were considered statistically significant when P was less than 0.05.
Results
Decreased expression of miR-128 in PCa tissues and patients’ sera
Compared with non-cancerous prostate tissues and normal sera, both tissue and serum levels of miR-128 expression were significantly decreased in PCa patients (both P<0.001, Figure 1A and 1B). Importantly, there was a close correlation between tissue and serum levels of miR-128 expression in PCa patients (rs=0.808, P<0.001, Figure 1C).
Figure 1.
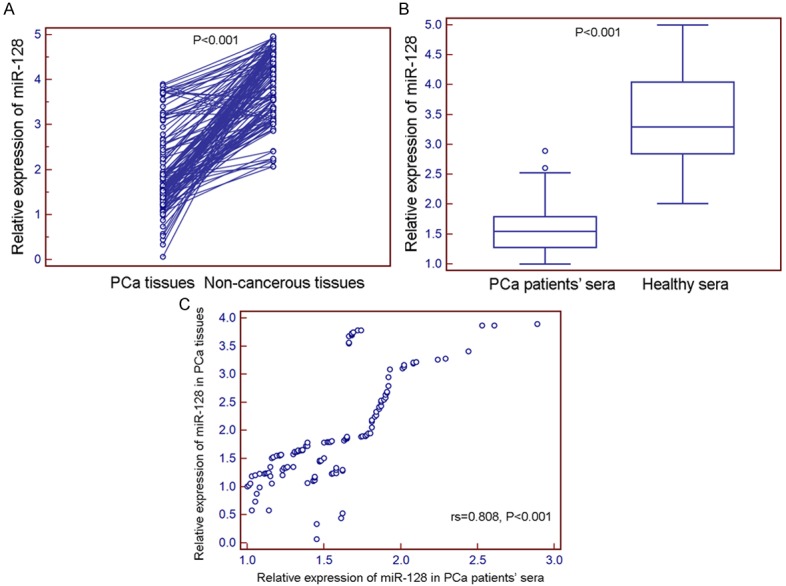
microRNA-128 (miR-128) expression in 128 prostate cancer (PCa) tissues and matched non-cancerous prostate tissues normalized to RNU6B detected by quantitative reverse-transcription PCR (qRT-PCR) assay. The expression levels of miR-128 were detected and analyzed in 128 pairs of PCa and adjacent non-cancerous prostate tissues by qRT-PCR analysis. The results showed that miR-128 expression level was significantly lower in PCa tissues compared to that in adjacent non-cancerous prostate tissues (1.05±0.63 vs. 2.92±0.98, P<0.001).
In addition, the median values of miR-128 expression levels in all PCa tissues and patients’ sera were respectively 1.66 and 1.55, which were used as cutoff points for the patients’ grouping. Thus, all 128 PCa patients were divided into miR-128-low-PCa-tissue group (n=68, based on a relative expression level in PCa tissues lower than 1.66), miR-128-high-PCa-tissue group (n=60, based on a relative expression level in PCa tissues higher than 1.66), miR-128-low-PCa-serum group (n=65, based on a relative expression level in PCa patients’ sera lower than 1.55), miR-128-high-PCa-serum group (n=63, based on a relative expression level in PCa patients’ sera higher than 1.55).
Decreased expression of miR-128 in PCa tissues and patients’ sera both correlate with aggressive clinicopathological features
Table 1 summarized the correlation between miR-128 expression and clinicopathological features of patients with PCa. Our data showed that low miR-128 expression in both PCa tissues and patients’ sera were dramatically associated with aggressive clinicopathological features, including advanced pathological stage (both P=0.001), positive lymph node metastasis (P=0.006 and 0.01, respectively), high preoperative PSA (both P=0.01) and positive angiolymphatic invasion (both P=0.02). However, the expression status of miR-128 was not associated with patients’ age, Gleason score, and surgical margin status.
Decreased expression of miR-128 in PCa tissues and patients’ sera both predict poor prognosis
To evaluate the possible prognostic value of miR-128, we performed BCR-free survival analysis for all 128 PCa patients undergoing radical prostatectomy. As shown in Figure 2, Kaplan-Meier curves were plotted between high or low miR-128 expression (tissues and sera) and BCR-free survival. Notably, patients with low miR-128 expression in both PCa tissues and patients’ sera had significantly shorter BCR-free survival after radical prostatectomy than patients with high miR-128 expression did (both P<0.001; Figure 2). In addition, the univariate analysis with Cox proportional hazards model found that miR-128 expression status in both PCa tissues and patients’ sera (both P<0.001), pathological stage (P<0.001), lymph node metastasis status (P=0.002), and positive angiolymphatic invasion (P<0.001) were significantly associated with BCR-free survival, while patients’ age, preoperative PSA, Gleason score, and surgical margin status were not significant factors (all P>0.05, Table 2).
Figure 2.
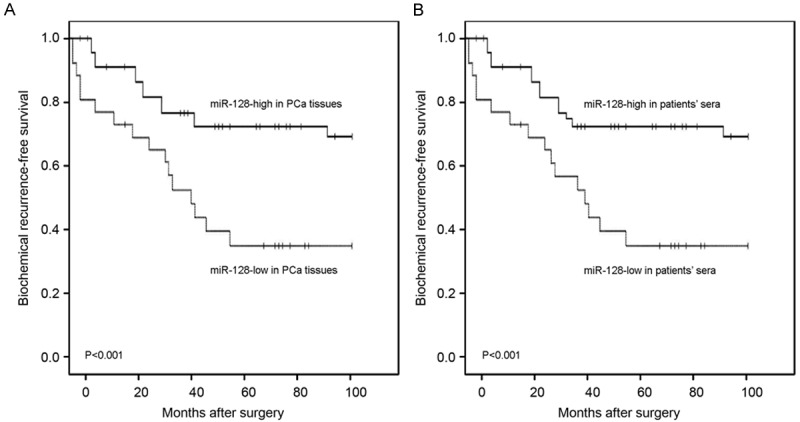
Biochemical recurrence (BCR)-free survival curves for two groups defined by low and high expression of miR-128 in PCa tissues (A) and patients’ sera (B). The patients with low miR-128 expression in both PCa tissues and patients’ sera had significantly shorter BCR-free survival after radical prostatectomy than patients with high miR-128 expression did (both P<0.001).
Table 2.
Univariate survival analysis of biochemical recurrence (BCR)-free survival in 128 patients with PCa
| Variables | BCR-free survival | ||
|---|---|---|---|
|
| |||
| Exp (B) | 95% CI | P | |
| Age (<70 vs. ≥70) | 2.68 | 0.17-5.03 | NS |
| Preoperative PSA (<10 ng/mL vs. ≥10 ng/mL) | 2.34 | 0.11-4.48 | NS |
| Gleason score (4-6 vs. 7-10) | 2.26 | 0.10-4.32 | NS |
| Pathological stage (T1-2 vs. T3-4) | 5.61 | 1.12-11.28 | <0.001 |
| Lymph node metastasis (Negative vs. Positive) | 4.13 | 0.82-8.22 | 0.002 |
| Angiolymphatic invasion (Negative vs. Positive) | 5.62 | 1.12-11.39 | <0.001 |
| Surgical margin status (Negative vs. Positive) | 2.27 | 0.10-4.82 | NS |
| miR-128 expression in PCa tissues (High vs. Low) | 6.06 | 1.22-13.09 | <0.001 |
| miR-128 expression in patients’ sera (High vs. Low) | 5.62 | 1.18-11.86 | <0.001 |
Note: ‘NS’ refers to the difference without statistic significance.
Furthermore, the Cox multivariate analysis demonstrated the value of miR-128 expression, and other clinicopathologic features for predicting BCR-free survival of patients with PCa. As shown in Table 3, miR-128 expression status in both PCa tissues and patients’ sera (both P=0.01), pathological stage (P=0.001), lymph node metastasis status (P=0.03), and positive angiolymphatic invasion (P=0.001) were all independent prognostic factors for predicting BCR-free survival of patients with PCa.
Table 3.
Multivariate survival analysis of biochemical recurrence (BCR)-free survival in 128 patients with PCa
| Variables | BCR-free survival | ||
|---|---|---|---|
|
| |||
| Exp (B) | 95% CI | P | |
| Pathological stage | 4.11 | 1.02-8.08 | 0.001 |
| Lymph node metastasis | 3.13 | 0.81-4.20 | 0.03 |
| Angiolymphatic invasion | 4.62 | 1.13-9.09 | 0.001 |
| miR-128 expression in PCa tissues | 3.96 | 1.02-8.12 | 0.01 |
| miR-128 expression in patients’ sera | 3.32 | 0.92-6.91 | 0.01 |
Note: ‘NS’ refers to the difference without statistic significance.
Discussion
The identification of diagnostic and prognostic biomarkers for human PCa is needed for optimizing management and treatment strategies. The accessibility and high stability of miRNAs in the circulation make them perfect biomarkers, especially for surveillance of early stage, presymptomatic diseases in at-risk patients [17,18]. In the current study, we found significantly decreased miR-128 levels in both tissue and serum samples of PCa patients comparing to those of adjacent non-cancerous prostate tissues and healthy sera, respectively. Low miR-128 serum concentrations and tissue expressions were both associated with aggressive clinicopathological features, including advanced pathological stage, positive lymph node metastasis, high preoperative PSA and positive angiolymphatic invasion, but not with patients’ age, Gleason score, and surgical margin status. Furthermore, analyzing the circulating miR-128 levels for the first time in the literature, we identified low miR-128 serum level as an independent, unfavorable prognostic factor for BCR-free survival in patients with PCa.
Recent studies have revealed that miR-128 could be implicated into malignant diseases and function as a tumor suppressor or an oncogene in different cancers, which may be dependent on cellular context [13-16,19-24]. It has been demonstrated that the abnormal expression of miR-128 might contribute to the malignant phenotypes of cancer cells, such as proliferation, cell motility, invasion, apoptosis, and self-renewal. The decreased level of miR-128 was originally identified in glioblastoma. Papagiannakopoulos et al. [19] identified miR-128 as a glioma tumor suppressor that targets receptor tyrosine kinases signaling to repress glioma-initiating neural stem cells self-renewal and enhance differentiation. Enforced expression of miR-128 could inhibit cell proliferation by targeting E2F3a and Bmi-1, and reduce neuroblastoma cell motility and invasiveness through inhibiting Reelin and DCX [20]. Hu et al. [21] indicated that the expression level of miR-128 was significantly downregulated in non-small cell lung cancer (NSCLC) tissues and cancer cells, and was significantly correlated with NSCLC differentiation, pathological stage and lymph node metastasis. In contrast, miR-128 expression levels in osteosarcoma tissues were significantly higher than those in noncancerous bone tissues, and its overexpression was associated with aggressive progression and poor prognosis of human osteosarcoma [22]; miR-128 has been found to be more highly expressed in drug-resistant breast cancer samples than in drug-sensitive samples [23]. Downregulation of miRNA-128 could sensitise breast cancer cell to chemodrugs by targeting Bax [23]. Although previous studies also reported that miR-128 might be act as a potentially important negative regulator of malignant phenotypes of PCa cells and a candidate circulating biomarker to screen risk groups in PCa [15,16], its prognostic implications in this cancer remain unexplored. Supporting these previous studies, we in the current study observed the decreased expression of miR-128 in both PCa tissues and patients’ sera, which were dramatically associated with malignant phenotypes and short BCR-free survival of patients with this cancer.
This study has some potential limitations regarding the low number of patients with lymph node metastasis. Furthermore, we did not investigate the mechanism of miR-128 acting on PCa. Since miRNAs exert functions via regulating the expression levels of their target genes, it is of great significance to identify the target genes of miR-128 in PCa and to investigate the involvement of their interactions in this malignancy. According to miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/), which has accumulated more than fifty thousand miRNA-target interactions (MTIs) validated experimentally by reporter assay, western blot, microarray and next-generation sequencing experiments, there are 13 potential target genes, including BMI1, CTDSP1, DCX, E2F3, EGFR, FBXW7, KLF4, NTRK3, POU5F1, RELN, SIRT1, SOX2 and TGFBR1, for miR-128. Among them, BMI1 was identified as a direct target of miR-128 in glioma cells and miR-128/BMI1 axis might play a crucial role in gliomagenesis [24]; MiR-128 up-regulation was reported to inhibit DCX expression and to reduce neuroblastoma cell motility and invasiveness [20]; Overexpression of miR-128 could specifically inhibit the truncated isoform of NTRK3 in neuroblastoma cells and be involved in apoptosis, cell survival and proliferation [14]. Based on these previous studies, we speculate that miR-128 might be involved into prostate carcinogenesis through regulating expression levels of its candidate targets. Further studies are needed to validate the miR-128-target interactions in PCa cells and to confirm their roles in this cancer.
In conclusion, our data suggest that the decreased expression of miR-128 levels in both tissue and serum samples of PCa patients may be associated with tumor malignant progression and BCR-free survival. Particularly, serum miR-128 may be further developed as a novel noninvasive biomarker for PCa diagnosis and prognosis.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev. 2013;1:CD004720. doi: 10.1002/14651858.CD004720.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parnes HL, House MG, Tangrea JA. Prostate cancer prevention: strategies for agent development. Curr Opin Oncol. 2013;25:242–251. doi: 10.1097/CCO.0b013e32835fc8d4. [DOI] [PubMed] [Google Scholar]
- 4.Louie KS, Seigneurin A, Cathcart P, Sasieni P. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol. 2015;26:848–864. doi: 10.1093/annonc/mdu525. [DOI] [PubMed] [Google Scholar]
- 5.Humphrey PA, Vollmer RT. Percentage of carcinoma as a measure of prostatic tumor size in radical prostatectomy tissues. Mod Pathol. 1997;10:326–333. [PubMed] [Google Scholar]
- 6.Montironi R, Mazzuccheli R, Scarpelli M, Lopez-Beltran A, Fellegara G, Algaba F. Gleason grading of prostate cancer in needle biopsies or radical prostatectomy specimens: contemporary approach, current clinical significance and sources of pathology discrepancies. BJU Int. 2005;95:1146–1152. doi: 10.1111/j.1464-410X.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 7.Wilt TJ, Ahmed HU. Prostate cancer screening and the management of clinically localized disease. BMJ. 2013;346:f325. doi: 10.1136/bmj.f325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovat F, Valeri N, Croce CM. MicroRNAs in the pathogenesis of cancer. Semin Oncol. 2011;38:724–733. doi: 10.1053/j.seminoncol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Sempere LF. Integrating contextual miRNA and protein signatures for diagnostic and treatment decisions in cancer. Expert Rev Mol Diagn. 2011;11:813–827. doi: 10.1586/erm.11.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava A, Goldberger H, Dimtchev A, Marian C, Soldin O, Li X, Collins SP, Suy S, Kumar D. Circulatory miR-628-5p is downregulated in prostate cancer patients. Tumour Biol. 2014;35:4867–73. doi: 10.1007/s13277-014-1638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selth LA, Townley SL, Bert AG, Stricker PD, Sutherland PD, Horvath LG, Goodall GJ, Butler LM, Tilley WD. Circulating microRNAs predict biochemical recurrence in prostate cancer patients. Br J Cancer. 2013;109:641–50. doi: 10.1038/bjc.2013.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adlakha YK, Saini N. MicroRNA-128 downregulates Bax and induces apoptosis in human embryonic kidney cells. Cell Mol Life Sci. 2011;68:1415–28. doi: 10.1007/s00018-010-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Chao T, Li R, Liu W, Chen Y. MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J Mol Med (Berl) 2009;87:43–51. doi: 10.1007/s00109-008-0403-6. [DOI] [PubMed] [Google Scholar]
- 14.Guidi M, Muiños-Gimeno M, Kagerbauer B, Martí E, Estivill X, Espinosa-Parrilla Y. Overexpression of miR-128 specifically inhibits the truncated isoform of NTRK3 and upregulates BCL2 in SH-SY5Y neuroblastoma cells. BMC Mol Biol. 2010;11:95. doi: 10.1186/1471-2199-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina-Villaamil V, Martínez-Breijo S, Portela-Pereira P, Quindós-Varela M, Santamarina-Caínzos I, Antón-Aparicio LM, Gómez-Veiga F. Circulating microRNAs in blood of patients with prostate cancer. Actas Urol Esp. 2014;38:633–639. doi: 10.1016/j.acuro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Khan AP, Poisson LM, Bhat VB, Fermin D, Zhao R, Kalyana-Sundaram S, Michailidis G, Nesvizhskii AI, Omenn GS, Chinnaiyan AM, Sreekumar A. Quantitative proteomic profiling of prostate cancer reveals a role for miR-128 in prostate cancer. Mol Cell Proteomics. 2010;9:298–312. doi: 10.1074/mcp.M900159-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferracin M, Veronese A, Negrini M. Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2010;10:297–308. doi: 10.1586/erm.10.11. [DOI] [PubMed] [Google Scholar]
- 18.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papagiannakopoulos T, Friedmann-Morvinski D, Neveu P, Dugas JC, Gill RM, Huillard E, Liu C, Zong H, Rowitch DH, Barres BA, Verma IM, Kosik KS. Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene. 2012;31:1884–95. doi: 10.1038/onc.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evangelisti C, Florian MC, Massimi I, Dominici C, Giannini G, Galardi S, Buè MC, Massalini S, McDowell HP, Messi E, Gulino A, Farace MG, Ciafrè SA. MiR-128 up-regulation inhibits Reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J. 2009;23:4276–87. doi: 10.1096/fj.09-134965. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, Cheng Y, Li Y, Jin Z, Pan Y, Liu G, Fu S, Zhang Y, Feng K, Feng Y. microRNA-128 plays a critical role in human non-small cell lung cancer tumourigenesis, angiogenesis and lymphangiogenesis by directly targeting vascular endothelial growth factor-C. Eur J Cancer. 2014;50:2336–50. doi: 10.1016/j.ejca.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Tian Z, Guo B, Yu M, Wang C, Zhang H, Liang Q, Jiang K, Cao L. Upregulation of micro-ribonucleic acid-128 cooperating with downregulation of PTEN confers metastatic potential and unfavorable prognosis in patients with primary osteosarcoma. Onco Targets Ther. 2014;7:1601–8. doi: 10.2147/OTT.S67217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji S, Shao G, Lv X, Liu Y, Fan Y, Wu A, Hu H. Downregulation of miRNA-128 sensitizes breast cancer cell to chemodrugs by targeting Bax. Cell Biol Int. 2013;37:653–8. doi: 10.1002/cbin.10100. [DOI] [PubMed] [Google Scholar]
- 24.Peruzzi P, Bronisz A, Nowicki MO, Wang Y, Ogawa D, Price R, Nakano I, Kwon CH, Hayes J, Lawler SE, Ostrowski MC, Chiocca EA, Godlewski J. MicroRNA-128 coordinately targets polycomb repressor complexes in glioma stem cells. Neuro Oncol. 2013;15:1212–24. doi: 10.1093/neuonc/not055. [DOI] [PMC free article] [PubMed] [Google Scholar]


