Abstract
Aim: It is known that botulinum neurotoxin type A (BoNTA) improves some kinds of cancer (e.g. prostate) and that synaptic vesicle glycoprotein 2 (SV2) is the molecular target of this neurotoxin. Besides having potential therapeutic value, this glycoprotein has recently been proposed as a molecular marker for several types of cancer. Although the mechanisms of cancer development and the improvement found with botulinum treatment are not well understood, the formation of the botulinum-SV2 complex may influence the presence and distribution of SV2 and the function of vesicles. To date, there are no reports on the possible effect of botulinum on breast cancer of unknown causes, which have a great impact on women’s health. Thus we determined the presence of SV2 in three breast cancer cell lines and the alterations found with botulinum application. Materials and methods: With and without adding 10 units of botulinum, SV2 protein expression was determined by optical densitometry in T47D, MDA-MB-231 and MDA-MB-453 cell lines and the distribution of SV2 was observed with immunochemistry (hematoxylin staining). Results: The SV2 protein was abundant in the cancer cells herein tested, and maximally so in T47D. In all three cancer cell lines botulinum diminished SV2 expression, which was found mostly in the cell periphery. Conclusion: SV2 could be a molecular marker in breast cancer. Its expression and distribution is regulated by botulinum, suggesting an interesting control mechanism for SV2 expression and a possible alternative therapy. Further studies are needed in this sense.
Keywords: SV2, breast cancer, protein expression, BoNTA
Introduction
For women, breast cancer (BCa) is the most prevalent oncological disease worldwide. Of the mechanisms that lead to the development of cancer and its capacity to evade the immune response, the modification of molecular expression patterns is fundamental. In this sense, vesicle trafficking may play an important role in the molecular expression on the surface of cancer cells, and therefore could influence the regulation of transduction that results in the evasion of apoptosis [1-5].
The presence of secretory vesicles is characteristic of synaptic, gastric, pancreatic, adrenal and prostatic cells [6-13]. Synaptic vesicular receptors (SVs) play an important role in exocytosis and the secretory process in both synaptic and endocrine cells [5,8,14]. In particular, synaptic vesicle glycoprotein 2A (SV2A), the major glycoprotein with 12 transmembrane domains within the SV2 family, is known to be critical for regulating exocytosis [6].
In cancer cells this protein tends to be overexpressed [7,9,10,15]. Although the importance of the SV receptor is evidenced by its binding to neurotransmitters, drugs and neurotoxins [7], its role is not clear in breast or cancer cells. However, this receptor has been increasingly used over recent years as a molecular marker of cancer in organs other than the breast [15,16].
SV2 and synaptotagmin have been identified as the receptors for various serotypes of botulinum neurotoxins (BoNTs), which are highly poisonous two-chain bacterial protein toxins. SV2 and synaptotagmin have been identified as the receptors for various serotypes of botulinum neurotoxins (BoNTs), which are highly poisonous two-chain bacterial protein toxins [17].Botulinum neurotoxin type A (BoNTA) and B (BoNTB) cause the disease denominated botulism. These toxins act rapidly and in a highly specific manner to block neurotransmitter release by cleaving the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) complex at neuromuscular junctions [18].
SV2A is the receptor for BoNTA, the most important serotype of BoNTs [18-21]. BoNTA in therapeutic doses improves certain disorders such as epilepsy and cancer in a more specific manner than previous treatments. This neurotoxin is known to induce apoptosis [2,9,22-25]. BoNTA has been successfully used in the treatment of refractory detrusor overactivity [13]. It is internalized after binding to a high-affinity SV2 receptor, which is exposed on the cell membrane during exocytosis. In the cytoplasm, BoNTA cleaves specific sites of synaptosomal-associated protein 25 (SNAP-25), preventing the assembly of the synaptic fusion complex SNARE and blocking exocytosis [5,26-28]. In this way, BoNTA inhibits the growth of LNCaP human prostate cancer cells in vitro and in vivo [7].
Tumor formation involves the accumulation of genetic alterations, including mutations that either inactivate tumor suppressor genes or activate proto-onco-genes as well as epigenetic changes in gene expression. Although the exact underlying mechanism is still unknown, malignant transformation seems to be associated with a greater release of extracellular vesicles [29-37]. In general, the molecular composition of each extracellular vesicle closely mimics the parental cell or tissue and contains growth factors, receptors, proteases, adhesion molecules, proteins and lipids.
Since it is still not clear how the BoNTA/SV2 complex is formed, internalized and distributed in BCa cells, the aim of the present study was to evaluate SV2 expression and explore its location in three cell lines of this disease. Additionally, we sought to define possible mechanisms for the regulation of SV2 by BoNTA and assess the potential of this receptor as a molecular marker for BCa detection and as a target for therapy.
Materials and methods
Cell culture
MCF-10A, MDA-MB-231, MDA-MB-453 and T47D cell lines were donated by the Instituto Nacional de Cancerología, Secretaría de Salud in Mexico City. The MDA-MB-231 and MDA-MB-453 cell lines were cultured with DMEM (Sigma) and the T47D cell line with RPMI (Gibco). All culture media were supplemented with 5% bovine fetal serum and 1% antibiotic/antimycotic solution, and cells were incubated at 37°C with 5% CO2 atmosphere. Every four days the cells were washed with sterile PBS and fresh medium was added, a procedure that was followed until 90% confluence was reached.
Botulinum neurotoxin type A (BoNTA) and developmental treatment
An injectable therapeutic agent containing 4.8 ng of Clostridium botulinum neurotoxin complex A (BoNTA) per vial of 100 units (Allergan, Inc., Markham, Ontario, Canada) was freshly reconstituted in sterile 0.9% saline solution less than 2 h before each use (stored at 4°C). The confluent cells were further cultured in quadruplicate for 24 h in Lab Tech growth chambers. For the experimental group, the change of medium every four days contained 10 units of freshly reconstituted BoNTA. For the control cells, there was only a change of medium.
Immunocytochemistry
All cells were subsequently fixed with 2% paraformaldehyde and 0.1 M PBS (pH 7.4) during 2 h, followed by three PBS washings. Cells were permeabilized in blocking solution prior to incubation. For immunofluorescence, the primary anti-SV2 antibody (polyclonal antibody, Santa Cruz Biotechnology, Inc.) (1:100) was incubated overnight, and afterwards the complexes were revealed with avidin-biotin (1:300) for 30 minutes and then cells were washed three times with PBS. Later 0.02 mL of diaminobencyne was added as chromogen (DakoCytomation, CA, USA), cells were contrasted with hematoxylin, and Tissue-Tek O.C.T. 4583 compound (Sakura Finetek, Inc., USA) was added according to the manufacturer’s protocol. Images were captured with an inverted Olympus MIC-D digital microscope with phase contrast, a 40× objective lens, and an imaging acquisition system. The images were analyzed with Axion Vision LE software.
Statistics
Densitometric values of SV2 were determined and compared by two-way ANOVA based on a univariate general lineal model and then plotted. Statistical analysis was performed with SPSS v15 for Windows XP (SPSS, UK, Ltd, Woking, UK). P < 0.05 was regarded as significant.
Results
SV2 expression and location
SV2 protein expression, evaluated by immunocytochemistry and optical densitometry, was found in the cytoplasm of all three BCa cell lines (Figure 1A, control panel). Compared to the MCF-10A line, the TD47, MDA-MB-231 and MDA-MB-453 lines showed 29.9-, 9.4- and 63.7-fold greater (respectively) line-optical density for SV2 (Figure 1B). This difference was notable when comparing immunocytochemistry pictures (Figure 1A, dark field, below in control and BoNTA panels) with hematoxylin pictures (Figure 1A, light field, above in control and BoNTA panels). In this comparison it is clear that the SV2 protein is widely distributed in the cytoplasm and not in the nucleus (stained with hematoxylin). The effect of BoNTA in BCa cell lines was the depletion by 88.8, 64.6 and 63.7% of SV2 expression in the TD47, MDA-MB-231 and MDA-MB-453 lines, respectively (Figure 1A and 1B, BoNTA panel). Its distribution tended to be on the membrane and in the periphery of the cytoplasm.
Figure 1.
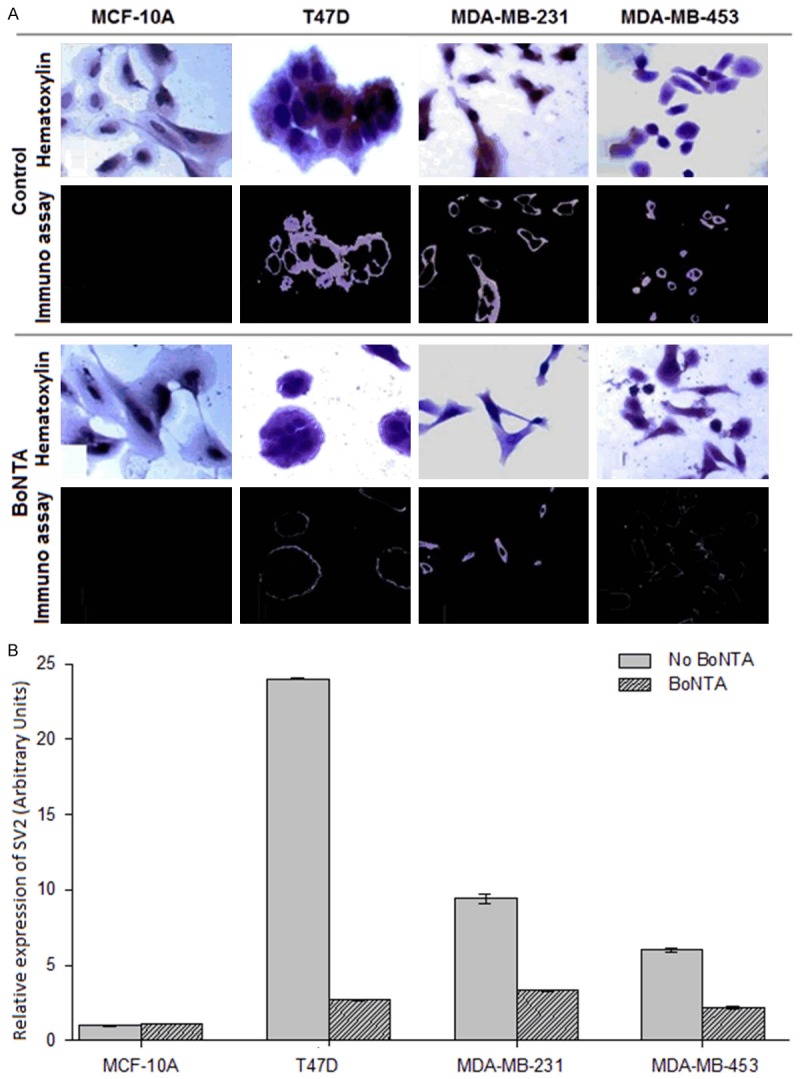
Effect of BoNTA on SV2 protein expression in BCA cells. A. Microcopy observations. The same fields were assayed by both hematoxylin and immunocytochemistry stains for control cells (light field) and BoNTA-treated cells (dark field). B. Relative optical densitometry of the presence of SV2. Two-way analysis of variance with P = 0.001 for treatments and P = 0.004 for cell lines. All pairwise multiple comparison procedures were carried out with the Holm-Sidak method (overall significance level = 0.05).
Protein network analysis
STRING network analysis of protein-protein interactions was performed to identify functionally linked proteins and determine the potential biological processes affected [PMID: 12519996]. The network is presented under confidence view, whereby stronger associations are represented by thicker lines or edges and vice versa, and proteins are represented as nodes. All gene symbols were derived from the HUGO Gene Nomenclature Committee (HGNC) (http://www.genenames.org). Figure 2 shows the main interactions and additional interactions between 33 identified proteins. There is a relationship between laminin proteins and SV2A.
Figure 2.
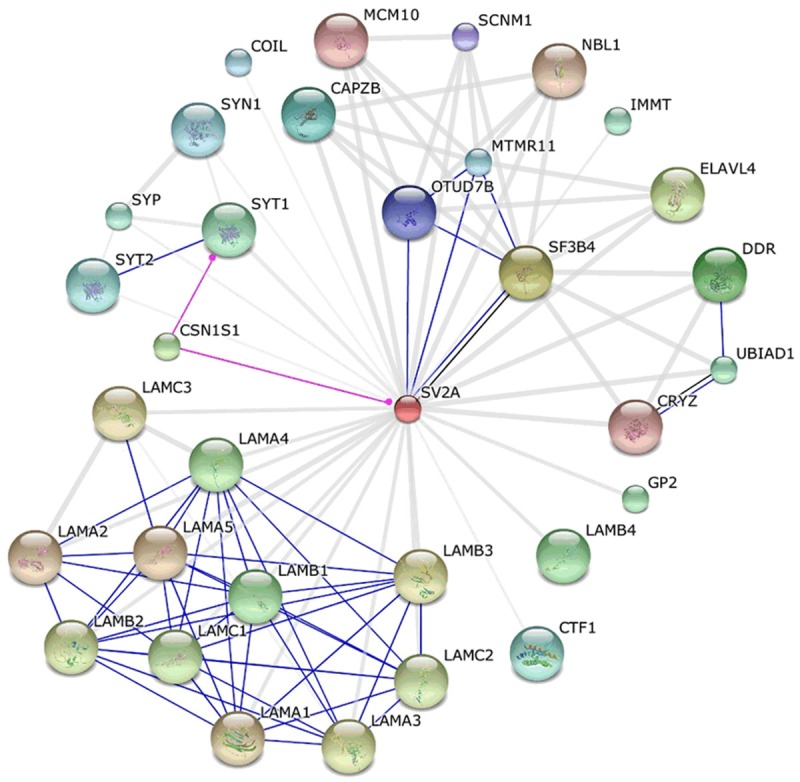
STRING interaction network showing the association between differentially expressed proteins. The interaction map was generated using default settings (medium confidence of 0.4 and 7 criteria for linkage: activation, inhibition, binding, phenotype, catalysis, post-translational modification, reaction, expression). Interactions are represented as follows: binding in blue, post-translational modification in pink, reaction in black, and other evidence (text mining, database and experimental) in light blue.
The extracellular matrix (ECM) consists of a complex mixture of structural and functional molecules such as laminin proteins. Specific interactions between cells and the ECM are mediated by transmembrane molecules, mainly integrins and proteoglycans, such as SV2 proteins or other components associated with the cell surface [PMID: 10617638]. Through these interactions there is direct or indirect control of cellular activities, including adhesion, migration, differentiation, proliferation and apoptosis. Regarding the formation of cancerous cells, the NBL1 protein plays an important role in preventing cells from entering the final stage (G1/S) of the transformation process [PMID: 11984879] and possibly acts as a tumor suppressor gene of neuroblastoma. Table 1 provides molecular function information.
Table 1.
Molecular function
| Term | P-value | Number |
|---|---|---|
| Structural molecular activity | 9.36e-5 | 10 |
| Glycosphingolipid | 6.50e-4 | 3 |
| Sphingolipid binding | 2.20e-3 | 3 |
| Glycolipid | 6.52e-3 | 3 |
| Extracellular matrix structural constituent | 7.44e-3 | 4 |
Discussion
BCa tumors are a cumulus of cells with modified ontogeny that show a certain degree of transdifferentiation. Some cells of BCa lines transdifferentiate into neural/glial-like cells and show neural-like molecular markers such as SV2, a protein characteristic of neurons. In breast cells SV2 could be associated with the secretory nature of mammary glands, and may interact with other vesicle proteins such as synaptobrevin, which is essential for secretion but not for the development of the synaptic process.
Little is known about the role of SV2 in BCa, although there are reports on its proposed role in other types of cancer [5,7-12,36] as a molecular and transdifferentiation marker of neural nature [36,37]. The SV2 membrane receptor binds to neurotransmitters, drugs and neurotoxins. One of the neurotoxins to which it binds is BoNTA, forming the BoNTA-SV2 complex. After translocation to the cytoplasm, BoNTA cleaves specific sites of synaptosomal-associated protein 25 (SNAP-25), preventing the assembly of the synaptic fusion complex SNARE and thus blocking exocytosis [5,8,26,27]. BoNTA in therapeutic doses is known to improve certain conditions such as epilepsy and cancer, and does so in a more specific manner than some other treatments [7-9,13,22-25], in part by inducing apoptosis [2].
In the present study, SV2 was not present in control breast cells (MCF-10A), but it was abundant in BCa cells (T47D, MDA-MB-231 and MDA-MB-453), mainly in the cytoplasm and membrane where SV2 plays an important role in exocytosis and the secretory process of synaptic and endocrine cells [5,8,14]. BoNTA treatment reduced the expression of the SV2 protein in BCa lines, especially in the interior of the cytoplasm. SV2 expression continued in the periphery of the cytoplasm and on the cell membrane. The fact that the SV2 signal tends to disappear discards the possibility of an accumulation of the BoNTA/SV2 complex. Thus, there is evidence of a continuing presence of the BoNTA/SV2 complex or SV2 alone in the membrane region, the blocking of the signaling cascade and the resulting inhibition of the translation of SV2 and the formation of vesicles. Further research is required in regard to these events.
The improvement in health resulting from therapeutic doses of BoNTA in certain disorders, including epilepsy and cancer [2,9,22-25], may be attributable to the disassembly of secretion vesicles mediated by a decrease in SV2. Since exocytosis plays an important role in regulating external messenger interactions and internal signaling, it is possible that in cancerous tumors SV2 promotes the cellular mechanism of antigen processing and presentation in order to evade the immune system [3,4,38,39]. In cancer SV2 may also be involved in the regulation of membrane markers, transdifferentiation [37] and tumor angiogenesis, as well as in the inhibition of apoptosis [1].
In conclusion, there are many reasons to continue studying the BoNTA-SV2 interaction in relation to BCa. SV2 is already used as a molecular marker in certain cases of cancer [37]. It is known that SV2 is overexpressed on some cancer cells, that BoNTA administration leads to an improvement in some kinds of cancer, and that SV2 is the receptor target for BoNTA. The possible anti-tumor effect of BoNTA in cases of malignancy may be due to its internalization by SV2 and the possible interruption of vesicle function. This may explain the known anti-proliferative, pro-apoptotic and chemodenervation functions of BoNTA in prostate cancer [2,7,14,22]. BoNTA could possibly have these same functions in BCa. Further studies on the expression patterns of SV2 could possibly allow for a fuller understanding of the BoNTA-SV2 complex and its effect on cancer cells.
Acknowledgements
This work was partly supported by operating research grants from the National Institute of Rehabilitation.
Disclosure of conflict of interest
None.
References
- 1.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007;26:333–9. doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- 2.Choi J, Hwang YK, Choi YJ, Yoo KE, Kim JH, Nam SJ, Yang JH, Lee SJ, Yoo KH, Sung KW, Koo HH, Im YH. Neuronal apoptosis inhibitory protein is overexpressed in patients with unfavorable prognostic factors in breast cancer. J Korean Med Sci. 2007;22(Suppl):S17–23. doi: 10.3346/jkms.2007.22.S.S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukashev D, Ohta A, Sitkovsky M. Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer Metastasis Rev. 2007;26:273–9. doi: 10.1007/s10555-007-9054-2. [DOI] [PubMed] [Google Scholar]
- 4.Lukashev D, Sitkovsky M, Ohta A. From “Hellstrom Paradox” to anti-adenosinergic cancer immunotherapy. Purinergic Signal. 2007;3:129–34. doi: 10.1007/s11302-006-9044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelho A, Dinis P, Pinto R, Gorgal T, Silva C, Silva A, Silva J, Cruz CD, Cruz F, Avelino A. Distribution of the high-affinity binding site and intracellular target of botulinum toxin type A in the human bladder. Eur Urol. 2010;57:884–890. doi: 10.1016/j.eururo.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Vogl C, Tanifuji S, Danis B, Daniels V, Foerch P, Wolff C, Whalley BJ, Mochida S, Stephens GJ. Synaptic vesicle glycoprotein 2A modulates vesicular release and calcium channel function at peripheral sympathetic synapses. Eur J Neurosci. 2015;41:398–409. doi: 10.1111/ejn.12799. [DOI] [PubMed] [Google Scholar]
- 7.Karsenty G, Rocha J, Chevalier S, Scarlata E, Andrieu C, Zouanat FZ, Rocchi P, Giusiano S, Elzayat EA, Corcos J. Botulinum toxin type A inhibits the growth of LNCaP human prostate cancer cells in vitro and in vivo. Prostate. 2009;69:1143–50. doi: 10.1002/pros.20958. [DOI] [PubMed] [Google Scholar]
- 8.de Groot M, Toering ST, Boer K, Spliet WG, Heimans JJ, Aronica E, Reijneveld JC. Expression of synaptic vesicle protein 2A in epilepsy-associated brain tumors and in the peritumoral cortex. Neuro Oncol. 2010;12:265–73. doi: 10.1093/neuonc/nop028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groot M, Aronica E, Heimans JJ, Reijneveld JC. Synaptic vesicle protein 2A predicts response to levetiracetam in patients with glioma. Neurology. 2011;77:532–9. doi: 10.1212/WNL.0b013e318228c110. [DOI] [PubMed] [Google Scholar]
- 10.Bümming P, Nilsson O, Ahlman H, Welbencer A, Andersson MK, Sjölund K, Nilsson B. Gastrointestinal stromal tumors regularly express synaptic vesicle proteins: evidence of a neuroendocrine phenotype. Endocr Relat Cancer. 2007;14:853–63. doi: 10.1677/ERC-06-0014. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsen AM, Ahlman H, Wängberg B, Kölby L, Bengtsson M, Nilsson O. Expression of synaptic vesicle protein 2 (SV2) in neuroendocrine tumours of the gastrointestinal tract and pancreas. J Pathol. 2002;196:44–50. doi: 10.1002/path.1002. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Johansson H, Grimelius L. Innervation of human adrenal gland and adrenal cortical lesions. Virchows Arch. 1999;435:580–9. doi: 10.1007/s004280050444. [DOI] [PubMed] [Google Scholar]
- 13.Bandala C, Miliar-García A, Mejía-Barradas CM, Anaya-Ruiz M, Luna-Arias JP, Bazán-Méndez CI, Gómez-López M, Juárez-Méndez S, Lara-Padilla E. Synaptic vesicle protein 2 (SV2) isoforms. Asian Pac J Cancer Prev. 2012;13:5063–7. doi: 10.7314/apjcp.2012.13.10.5063. [DOI] [PubMed] [Google Scholar]
- 14.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–6. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 15.Portela-Gomes GM, Lukinius A, Grimelius L. Synaptic vesicle protein 2, A new neuroendocrine cell marker. Am J Pathol. 2000;157:1299–309. doi: 10.1016/S0002-9440(10)64645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Fan H, Shen J, Hoffman RM, Xing HR. Human breast cancer cell lines co-express neuronal, epithelial, and melanocytic differentiation markers in vitro and in vivo. PLoS One. 2010;5:e9712. doi: 10.1371/journal.pone.0009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harper CB, Martin S, Nguyen TH, Daniels SJ, Lavidis NA, Popoff MR, Hadzic G, Mariana A, Chau N, McCluskey A, Robinson PJ, Meunier FA. Dynamin inhibition blocks botulinum neurotoxin type A endocytosis in neurons and delays botulism. J Biol Chem. 2011;286:35966–76. doi: 10.1074/jbc.M111.283879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strotmeier J, Mahrhold S, Krez N, Janzen C, Lou J, Marks JD, Binz T, Rummel A. Identification of the synaptic vesicle glycoprotein 2 receptor binding site in botulinum neurotoxin A. FEBS Lett. 2014;588:1087–93. doi: 10.1016/j.febslet.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldwin MR, Barbieri JT. Association of botulinum neurotoxins with synaptic vesicle protein complexes. Toxicon. 2009;54:570–4. doi: 10.1016/j.toxicon.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam KH, Yao G, Jin R. Diverse binding modes, same goal: The receptor recognition mechanism of botulinum neurotoxin. Prog Biophys Mol Biol. 2015;117:225–231. doi: 10.1016/j.pbiomolbio.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahrhold S, Strotmeier J, Garcia-Rodriguez C, Lou J, Marks JD, Rummel A, Binz T. Identification of the SV2 protein receptor-binding site of botulinum neurotoxin type E. Biochem J. 2013;453:37–47. doi: 10.1042/BJ20130391. [DOI] [PubMed] [Google Scholar]
- 22.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K Working Group on Civilian Biodefense. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059–70. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 23.Montal M. Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. [DOI] [PubMed] [Google Scholar]
- 24.Blum FC, Chen C, Kroken AR, Barbieri JT. Tetanus toxin and botulinum toxin a utilize unique mechanisms to enter neurons of the central nervous system. Infect Immun. 2012;80:1662–9. doi: 10.1128/IAI.00057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J, Anderson D, Lynch BA, Castaigne JG, Foerch P, Lebon F. Combining modelling and mutagenesis studies of synaptic vesicle protein 2A to identify a series of residues involved in racetam binding. Biochem Soc Trans. 2011;39:1341–7. doi: 10.1042/BST0391341. [DOI] [PubMed] [Google Scholar]
- 26.Chancellor MB, Fowler CJ, Apostolidis A, de Groat WC, Smith CP, Somogyi GT, Aoki KR. Drug Insight: biological effects of botulinum toxin A in the lower urinary tract. Nat Clin Pract Urol. 2008;5:319–28. doi: 10.1038/ncpuro1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson LL. Studies on the mechanism of action of botulinum toxin. Adv Cytopharmacol. 1979;3:27–34. [PubMed] [Google Scholar]
- 28.Aoki KR, Smith LA, Atassi MZ. Mode of action of botulinum neurotoxins: current vaccination strategies and molecular immune recognition. Crit Rev Immunol. 2010;30:167–87. doi: 10.1615/critrevimmunol.v30.i2.50. [DOI] [PubMed] [Google Scholar]
- 29.Vader P, Breakefield XO, Wood MJ. Extracellular Vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014;20:385–93. doi: 10.1016/j.molmed.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–64. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma M, Lam TK, Hebert E, Divi RL. Extracellular vesicles: potential applications in cancer diagnosis, prognosis, and epidemiology. BMC Clin Pathol. 2015;15:6. doi: 10.1186/s12907-015-0005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg R. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Ogorevc E, Kralj-Iglic V, Veranic P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol Oncol. 2013;47:197–205. doi: 10.2478/raon-2013-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverman JM, Reiner NE. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol. 2011;13:1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 35.Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol. 2014;306:C621–33. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson O, Jakobsen AM, Kölby L, Bernhardt P, Forssell-Aronsson E, Ahlman H. Importance of vesicle proteins in the diagnosis and treatment of neuroendocrine tumors. Ann N Y Acad Sci. 2004;1014:280–3. doi: 10.1196/annals.1294.032. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Fan H, Shen J, Hoffman RM, Xing HR. Human breast cancer cell lines co-express neuronal, epithelial, and melanocytic differentiation markers in vitro and in vivo. PLoS One. 2010;5:e9712. doi: 10.1371/journal.pone.0009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Książkiewicz M, Markiewicz A, Zaczek AJ. Epithelial-mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology. 2012;79:195–208. doi: 10.1159/000337106. [DOI] [PubMed] [Google Scholar]
- 39.Palafox M, Ferrer I, Pellegrini P, Vila S, Hernandez-Ortega S, Urruticoechea A, Climent F, Soler MT, Muñoz P, Viñals F, Tometsko M, Branstetter D, Dougall WC, González-Suárez E. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 2012;72:2879–88. doi: 10.1158/0008-5472.CAN-12-0044. [DOI] [PubMed] [Google Scholar]


