Abstract
Objective: To investigate the diagnostic values of soluble cluster of differentiation 163 (sCD163) in patients with liver failure or various inflammations. Methods: Serum samples were collected from patients admitted to the First Affiliated Hospital, Zhejiang University from October 2013 to January 2015 for treatment of with liver diseases, including liver failure (n=38), hepatitis B virus (HBV)-induced liver cancer (HBsAg positive) (n=40), HBV-induced hepatic cirrhosis (HBsAg positive) (n=40), chronic hepatitis B (n=38), HBV carrier (n=40), fatty liver patients without HBV infection (n=40), chronic glomerulonephritis (n=38), community acquired pneumonia (n=38) and acute pancreatitis (n=38). The CD163/sCD163 was determined using commercial ELISA kits according to the manufacturer’s instructions. Results: Significant decrease was noticed in the sCD163 in patients with fatty liver and HBV carrier compared with that of patients with chronic hepatitis B (P < 0.05). Compared with the healthy controls, the level of sCD163 was remarkably increased in the other groups (P < 0.05). The serum sCD163 in patients with HBV-induced liver cancer showed statistical difference compared with those of the patients with fatty liver, HBV carrier, as well as those with liver failure (P < 0.05). The expression of sCD163 was remarkably elevated in patients with liver failure compared with the patients with liver cancer, HBV-induced hepatic cirrhosis, chronic hepatitis B, fatty liver, or HBV carrier (P < 0.05). No significant difference was noticed in the sCD163 in patients with chronic hepatitis B, community acquired pneumonia, chronic glomerulonephritis, and acute pancreatitis (P > 0.05). Conclusions: sCD163 is a sensitive marker protein for liver failure. The elevation of sCD163 was closely related to the progression of the liver failure. No statistical difference was noticed in the sCD163 in patients with inflammatory disorders, indicating sCD163 showed no organ specificity.
Keywords: Liver failure, sCD163, inflammation
Introduction
Hepatitis B virus (HBV) hepatitis is a common disease induced by HBV infection worldwide. Patients may develop chronic hepatitis, and part of which may progress to liver failure due to severe acute exacerbation [1]. As severe liver damages caused by many factors, liver failure could cause severe dysfunction or decompensation in biological synthesis, detoxication, excretion and biotransformation, which finally resulted in principal clinical symptoms of blood coagulation disorder, jaundice, hepatic encephalopathy and ascites [2].
The major cause for liver failure is hepatitis virus in China, especially HBV, followed by drug and hepatotoxic substances (e.g. ethanol and chemical agents). In Europe and North America, drug is the major cause of acute and subacute liver failure while the alcohol-induced liver damage usually results in chronic liver failure or acute-on-chronic liver failure. According to the Diagnostic and Treatment Guidelines for Liver Failure (2012 version), liver failure was divided into acute liver failure (ALF), subacute liver failure (SALF), acute-on-chronic liver failure (ACLF), and chronic liver failure (CLF) [3]. At present, no sensitive and reliable criteria or system is available for clinical evaluation of liver failure. In clinical practice, multivariate prognostic models are used as guidance for the prognostic evaluation of liver failure including King’s College Hospital Criteria (KCHC), Model for End-stage Liver Disease (MELD), Sequential Organ Failure Assessment (SOFA) and Child-Pugh-Turcotte (CPT). In addition, univariate indices such as total bilirubin level, prothrombin time, serum creatinine level, cholinesterase level, blood fat level and serum sodium level are also considered as applicable means [4].
Macrophages are closely related to normal homeostasis and in various pathological conditions such as infection, (chronic) inflammation, atherosclerosis and cancer [5]. CD163, a highly expressed macrophage membrane protein, belongs to the scavenger receptor cysteine rich (SRCR) domain family with a short cytoplasmic tail, a transmembrane segment, and an extracellular domain containing 9 consecutive class B SRCR domains [6,7]. Previously, it was named as RM3/1, Ki-M8 and M130, and was firstly confirmed to remove the free hemoglobin by recognizing hemoglobin-haptoglobin complex (Hb-Hp complex) [8]. Soluble CD163 (sCD163) could be easily detected in serum and tissue fluid, which may reflect the activation of the mononuclear phagocytic cells. The expression of CD163 was up-regulated by glucocorticoid, macrophage colony-stimulating factor and anti-inflammatory factor IL-10 and IL-6, and was down-regulated by granulocyte-macrophage colony-stimulating factor, PMA and the proinflammatory factors TNF-α, IFN-r and LPS [9].
The most clearly described function of CD163 was to mediate macrophage to clear redundant free hemoglobin in human body as the receptor of Hb-Hp complex [8]. Such process could induce the high expression of related molecule to develop anti-inflammatory and antioxidative effects. In addition, it is reported that CD163 showed anti-inflammatory effect in patients with liver failure [10]. Fabriek et al founded that CD163 could enhance the host immunity as the innate immune receptor of gram-negative bacteria and gram-positive bacteria [11]. Moreover, Moreno et al demonstrated that CD163 prevented TNF-like weak inducer of apoptosis (TWEAK) from inducing apoptosis by endocytosis as TWEAK receptor [12]. Fabriek et al also found that CD163 induced the release of inflammatory mediators to promote inflammatory reaction as the direct receptors of bacteria and viruses [11]. CD163 was shed from the membrane surface and existed as soluble protein in serum [13]. Previous reports showed increased soluble CD163 (sCD163) in serum of patients with acute liver failure, and the level of sCD163 was higher in died patients than that of survivals [10,14]. Also, serum sCD163 could predict the prognosis in patients with purulent meningitis or malaria, in abortion patients or pancreatic cancer patients [15]. Considering the capacity of CD163, it is necessary to explore its role in liver failure. In this study, we investigated the relationship between sCD163 and liver failure, as well as the relationship between sCD163 and inflammation.
Materials and methods
Sample preparation
Serum samples used in this study were collected from patients with liver disease admitted to the First Affiliated Hospital, Zhejiang University from October 2013 to January 2015, including 38 liver failure patients (HBsAg positive), 40 HBV-induced liver cancer patients (HBsAg positive, HCC), 40 HBV-induced hepatic cirrhosis patients (HBsAg positive, LC), 38 chronic hepatitis B patients (CH), 40 HBV carrier (ASC), 40 fatty liver patients without HBV infection (SS), 38 chronic glomerulonephritis patients (CGN), 38 community acquired pneumonia patients (CAP) and 38 acute pancreatitis patients (AP). Samples collected from 40 healthy subjects served as control. The exclusion criteria were as follows: hepatitis C, hepatitis D, autoimmune hepatitis, and severe complications, such as thalassemia, chronic renal failure and severe heart failure. Patients with chronic glomerulonephritis, acute pancreatitis, community acquired pneumonia and fatty liver were excluded if hepatitis B was simultaneously present. Patients with hepatic cirrhosis (HBV positive) induced by drug, alcohol or schistosome were also excluded. None of the included patients with HBV-induced liver cancer had been treated previously. Fifteen minutes after collection, the whole blood was centrifugated for 10 min at 1,500 g at 2-8°C. Then, 100 μl serum of each sample was collected and stored at -80°C.
Measurement of sCD163
sCD163 was determined by using commercial ELISA Kit (RayBiotech Inc., Norcross, GA, USA) according to the manufacturer’s instruction. The absorbance of sCD163 was measured at 450 nm, and the concentration of sCD163 was calculated with Curveexpert 1.3 (Curve Expert, Mississippi, MS, USA).
Statistical analysis
Continuous data are expressed as the mean ± standard deviation and were analyzed using Student’s t-test. Categorical data were expressed as counts and percentages and analyzed by the chi-square test. Correlations among the study variables were tested by Pearson’s correlation coefficient. P < 0.05 demonstrated significant difference.
Results
Expression of sCD163 in patients with liver disorders
The expression of sCD163 was remarkably elevated in patients with liver failure (Table 1) compared with patients with HBV-induced liver cancer, HBV-induced hepatic cirrhosis, chronic hepatitis B, HBV carrier, and fatty liver, respectively (P < 0.05, Figure 1). Statistical difference was observed in the sCD163 levels in patients with chronic hepatitis B, HBV-induced liver cancer, HBV-induced hepatic cirrhosis, fatty liver and HBV carrier (P < 0.05). Compared with the healthy controls, the level of sCD163 showed difference in the patients with liver failure, liver cancer, HBV-induced cirrhosis, chronic hepatitis B, fatty liver and the HBV carrier (P < 0.05). The serum sCD163 in patients with HBV-induced liver cancer showed statistical difference compared with those of the patients with fatty liver and HBV carrier (P < 0.05). Meanwhile, serum sCD163 in patients with hepatitis B induced hepatic cirrhosis showed statistical difference compared with those of the patients with fatty liver and HBV carrier (P < 0.05). Statistical difference was noticed in the sCD163 in patients with fatty liver compared with those with HBV-induced cirrhosis and liver cancer (P < 0.05). The sCD163 level in HBV carrier was statistically different from that of the patients with HBV-induced cirrhosis and liver cancer (P < 0.05). No statistical difference was noticed in the sCD163 in patients with liver cancer and those with HBV-induced cirrhosis (P > 0.05, Table 2). The sCD163 concentration showed no difference in patients with fatty liver compared with that of the HBV carrier (P > 0.05). Taken together, sCD163 was higher in patients with liver failure compared with the patients with liver cancer, HBV-induced cirrhosis, chronic hepatitis B, fatty liver and HBV carrier. This indicated sCD163 may be a sensitive serum marker for liver failure. In addition, the sCD163 level was closely related to the severity of the patient, which may be used as an index for the evaluation of liver failure prognosis.
Table 1.
Biochemical analysis of patients with liver failure
| Age, yr | Gender (male/female) | ALT (U/L) | AST (U/L) | TBIL μmol/L | PT (sec) | INR (inr) | e-AG |
|---|---|---|---|---|---|---|---|
| 45.73±2.21 | 27/11 | 246.76±43.09 | 178.07±29.17 | 304.31±29.45 | 21.93±1.70 | 1.86±0.14 | 80.43±37.25 |
ALT: alanine transarninase; AST: glutamic-oxalacetic transaminase; PT: prothrombin time; INR: international normalized ratio, TBIL: total bilirubin; e-AG: e-antigen.
Figure 1.
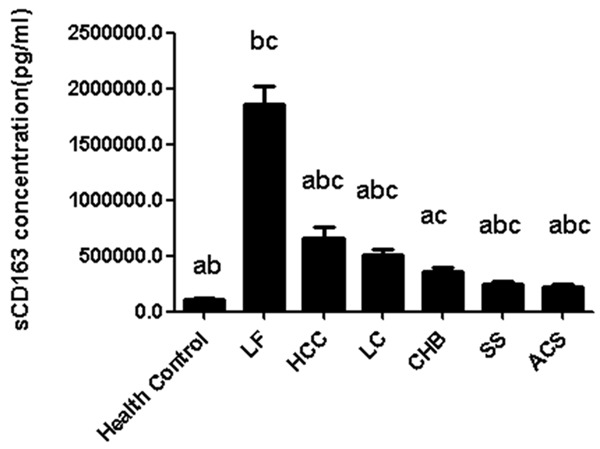
Expression of sCD163 in patients. a. P < 0.05, compared with those with liver failure; b. P < 0.05, compared with those with chronic hepatitis B; c. P < 0.05, compared with those with health control.
Table 2.
Demographic information of the patients
| Diseases | N | Age, yr | Gender (male/female) | ALT | AST | TBIL |
|---|---|---|---|---|---|---|
| Chronichepatitis B | 38 | 38.54±1.76 | 21/17 | 39.18±8.58 | 29.20±4.41 | 13.29±0.90 |
| Pneumonia | 38 | 57.52±2.92 | 22/16 | 35.47±6.76 | 29.60±3.18 | 8.78±0.84 |
| Pancreatitis | 40 | 49.85±2.27 | 21/19 | 42.40±13.45 | 36.37±7.95 | 21.95±4.11 |
| Nephritis | 38 | 43.44±3.04 | 27/11 | 29.73±8.04 | 37.02±11.60 | 10.72±1.50 |
| Liver cancer | 40 | 55.40±1.88 | 25/15 | 64.37±15.89 | 99.05±31.98 | 29.60±7.12 |
| Hepaticcirrhosis | 40 | 52.05±1.84 | 23/17 | 35.40±4.56 | 58.20±7.56 | 53.22±10.26 |
| Fatty liver | 40 | 51.17±2.08 | 21/19 | 38.23±3.34 | 29.53±1.85 | 15.61±1.35 |
| Hepatitis B carrier | 40 | 38.92±1.64 | 20/20 | 24.82±1.69 | 23.45±0.74 | 14.48±1.50 |
| Control | 40 | 32.32±1.97 | 18/22 | 15.37±1.56 | 18.10±0.69 | 11.50±0.88 |
Expression of sCD163 in various inflammations
As revealed in Figure 2, the expression of sCD163 was significantly decreased in the control group compared with those in patients with chronic hepatitis B, chronic glomerulonephritis, community acquired pneumonia, and acute pancreatitis (P < 0.05). However, no statistical differences were observed between the sCD163 expression in the patients with chronic hepatitis B, chronic glomerulonephritis, community acquired pneumonia, and acute pancreatitis (P > 0.05). On this basis, we speculated that the expression of sCD163 was comparatively lower in healthy individuals. Nevertheless, it was up-regulated in the presence of inflammatory reactions. The sCD163 was not specifically expressed in a certain organ.
Figure 2.
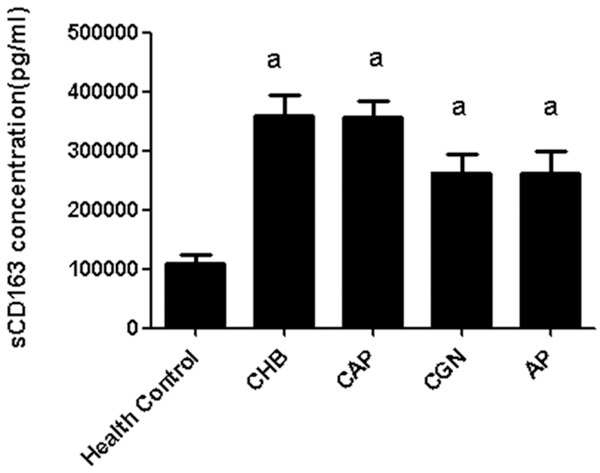
Expression of sCD163 in inflammations. a. P < 0.05, compared with the healthy control.
Correlation between sCD163 and clinical parameters
In this study, we also investigated the correlation between sCD163 and clinical parameters, including Alanine transarninase (ALT), glutamic-oxalacetic transaminase (AST), prothrombin time (PT), international normalized ratio (INR), and total bilirubin (TBIL), and e-antigen (e-AG). The results revealed no correlation was noticed between sCD163 and these parameters (Table 3). This indicated that those proteins showed no effects on the secretion of sCD163.
Table 3.
Correlation between sCD163 and the clinical parameters in patients with liver failure
| ALT | AST | PT | INR | TBIL | e-AG | ||
|---|---|---|---|---|---|---|---|
| sCD163 | Pearson correlation | -.110 | -.152 | -.121 | -.125 | -.096 | .306 |
| P value | .542 | .397 | .501 | .489 | .595 | .100 |
Discussion
Diagnostic values of sCD163 in patients with liver disorders liver failure is mainly developed from chronic hepatitis B infection due to infection, long-term fatigue, alcohol, virus replication or variation of nucleoside analog. To date, the pathogenesis of liver failure is commonly considered to be associated with the primary liver injury (PLI) and secondary liver injury (SLI) [16]. PLI was resulted from endotoxin (lipopolysaccharide, LPS) and the activation of Kupffer cells by various factors including drugs, alcohol and hepatotoxin, while the SLI was resulted from the endotoxemia induced liver injuries. As the first protective barrier, liver plays crucial roles in the clearance of enteropathogenic microorganism. Shifting of bacteria and virus was usually induced by injury of intestinal mucosa and elevation of portal vein pressure, which finally resulted in endotoxemia. LPS could further activate the Kupffer cells and lead to inflammatory reactions, circulation disorder and necrosis of hepatocytes [17]. On this occasion, secondary infection may be induced with the decrease of the immune function, together with the administration of the broad-spectrum antibiotics and immunosuppressants. In this process, the serum sCD163 levels may be alternated by various factors, including endotoxemia, excessive activation of macrophages and proinflammatory factors.
In this study, the serum sCD163 was remarkably increased in patients with liver failure compared with those in patients with hepatic cirrhosis, liver cancer, chronic hepatitis B, fatty liver, hepatitis B carrier and the healthy individuals. On this basis, we speculate that sCD163 could be considered as a biomarker for the liver failure. Previously, elevation of sCD163 was reported to be associated with the prognosis of patients with liver failure [10]. In addition, the sharp increase of sCD163 modulated by production of TNF-α and IL-1 [18] reflected the sudden deterioration of liver function, based on which to present the inflammatory reactions in patients with liver failure.
In the presence of HBV infection, the host may activate the cellular and humoral immune response, which resulted in generation of immunologic effector substance. Recent studies revealed the hepatitis was induced by antigen-specific cytotoxie T lymphocyte. CTL has the capacity to recognize and kill the HBV infected cells. In addition, several non-specific inflammatory factors were released by the activated CTL such as TNF-α and IFN-r, which could suppress the proliferation of virus [19]. Finally, such factors contributed to the up-regulation of sCD163 in vivo.
Patients with cancer may present metabolic disorders, which was initially manifested as increased metabolism, followed by depletion of energy, tissue injury and multiple organs failure and shock [20,21]. As previously described, patients with liver cancer showed remarkable increase of TNF compared with the normal individuals. The sudden elevation of TNF was speculated to be associated with the endotoxemia. Meanwhile, TNF could lead to up-regulation of CD163 as well as the serum sCD163 accordingly.
Hepatic cirrhosis refers to a disorder induced by extensive fibrosis and nodules secondary to long-term necrosis of hepatocytes. For patients with hepatic cirrhosis, the liver parenchyma was frequently affected by causative agents, which subsequently led to diffused degeneration and necrosis of hepatocytes as well as inflammation. Finally, the anatomical structure and the reconstruction of hepatic lobules were destroyed together with disorders in the blood circulation in liver. Therefore, patients with hepatic cirrhosis were in a long-term disorder, which may contribute to the elevation of sCD163.
In this study, the serum sCD163 was elevated in patients of hepatitis B carrier. Although the liver function was normal in these patients, the subpopulation of the T lymph nodes was changed, indicating the changes of immune system [22,23]. Moreover, with the proliferation of HBV, further immune disorders may be caused.
Fatty liver disease refers to the accumulation of hepatic steatosis due to degeneration of liver parenchymal cells. To date, various factors that could induce malmetabolism of lipid have been reported to be associated with the pathogenesis of fatty liver. To our knowledge, fatty tissues play important roles in the endocrine and immunity [24]. In addition, it could contribute to the release of TNF-α and IL-6 that were related to the inflammatory reactions in subjects with obesity [25]. Further, accumulation of lip in hepatocytes contributed to the activation of necrosis receptors, and increased sensitivity of hepatocytes to pro-apoptotic signals, increase of apoptosis and activation of stellate cells and Kuffer cells [25], which finally deteriorate the inflammatory reactions in liver.
A majority of patients with liver cancer showed hepatic cirrhosis. In this study, no statistical difference was noticed in the sCD163 in patients with liver cancer and hepatic cirrhosis, and the serum sCD163 was remarkably decreased compared with those with liver failure. On this basis, we considered sCD163 as a reliable biomarker for the liver failure, which could be used for the evaluation of prognosis in patients with liver failure. In addition, the level of sCD163 could be used to evaluate the severity of liver disorders.
Diagnostic values of sCD163 in inflammation
The pathogenesis of acute pancreatitis is still not well defined. It is considered as a type of inflammatory reaction that contributed to the cascade reaction through immune modulation [26]. Currently, inflammatory mediators play crucial roles in the local injury of pancreas and whole body inflammation. In this study, we proved sCD163 was involved in the inflammatory reaction in patients with acute pancreatitis.
Patients with lung inflammation were featured by aggregation of neutrophilic granulocytes mediated by pulmonary alveolar macrophage. Besides macrophages, the other inflammatory cells were also inhibited, which led to decrease of cytokines, and decreased activity of macrophages in pulmonary alveoli compared with the macrophages and Kupffer cells in the abdominal cavity [27]. In this study, the serum sCD163 was elevated in patients with pneumonia, however, the increase of the serum sCD163 was not significant compared with that of the patients with severe liver disease.
Chronic nephritis of different pathological types was mediated by immune inflammation that was depended on the activation of T cells. Immune and inflammatory reactions were the major causes for renal glomerular injuries, involved by several cytokines such as IL-1, IL-6, IL-8, TNF-Q and TGF-β. Resident cells were activated in the presence of inflammation including glomerular epithelial cells, endothelial cells, mesangial cells, fibroblasts in renal interstitum. In addition, TNF-α could affect the mesangial cells and stimulate the proliferation and sclerosis of the mesangial cells [28]. The level of sCD163 was elevated upon the stimulation of TNF-α. Thus, the sCD163 in patients with chronic glomerulonephritis was elevated, which was consistent with our results.
In conclusion, sCD163 could be used a biomarker for the diagnosis of liver failure. Also, it could be used for the evaluation of prognosis of liver failure, and the up-regulation of sCD163 was closely correlated with the severity of hepatitis B. In addition, sCD163 showed no organ-specificity in patients with hepatitis, chronic glomerulonephritis, pneumonia, and pancreatitis. sCD163 is a sensitive marker protein for liver failure. The elevation of sCD163 was closely related to the progression of the liver failure, demonstrating sCD163 was an important marker for the evaluation of liver disorder. No statistical difference was noticed in the sCD163 in patients with inflammatory disorders, indicating sCD163 showed no organ specificity.
No sensitive and reliable evaluation system is available for the prognosis of liver failure in China. The expression of sCD163 could display the activation of macrophages, which was closely related to the severity of liver failure. In the future, multi-center, large sample and prospective studies are needed to establish a standard for the evaluation of liver failure.
Acknowledgements
National Science and Technology Major project of 12th Five Year (2012ZX10002007, 2012ZX10002003, 2012ZX10002005).
Disclosure of conflict of interest
None.
References
- 1.Kitab B, El Feydi AE, Afifi R, Trepo C, Benazzouz M, Essamri W, Zoulim F, Chemin I, Alj HS, Ezzikouri S. Variability in the precore and core promoter regions of HBV strains in Morocco: characterization and impact on liver disease progression. PLoS One. 2012;7:e42891. doi: 10.1371/journal.pone.0042891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitzner SR, Stange JAN, Klammt S, Peszynski P, Schmidt R, NÖLdge-Schomburg G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J Am Soc Nephrol. 2001;12:S75–S82. [PubMed] [Google Scholar]
- 3.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 4.Fang Y. Guideline for diagnosis and treatment of liver failure. Chin J Clin Infe Dis. 2012 [Google Scholar]
- 5.Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Graversen JH, Madsen M, Moestrup SK. CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol. 2002;34:309–314. doi: 10.1016/s1357-2725(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 7.Madsen M, Møller HJ, Nielsen MJ, Jacobsen C, Graversen JH, van den Berg T, Moestrup SK. Molecular Characterization of the Haptoglobin. Hemoglobin Receptor CD163 Ligand Binding Properties Of The Scavenger Receptor Cysteine-Rich Domain Region. J Biol Chem. 2004;279:51561–51567. doi: 10.1074/jbc.M409629200. [DOI] [PubMed] [Google Scholar]
- 8.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SA, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 9.Møller HJ, Peterslund NA, Graversen JH, Moestrup SK. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood. 2002;99:378–380. doi: 10.1182/blood.v99.1.378. [DOI] [PubMed] [Google Scholar]
- 10.Møller HJ, Grønbaek H, Schiødt FV, Holland-Fischer P, Schilsky M, Munoz S, Hassanein T, Lee WM U.S. Acute Liver Failure Study Group. Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J Hepatol. 2007;47:671–676. doi: 10.1016/j.jhep.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, Vloet RP, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887–892. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 12.Moreno JA, Muñoz-García B, Martín-Ventura JL, Madrigal-Matute J, Orbe J, Páramo JA, Ortega L, Egido J, Blanco-Colio LM. The CD163-expressing macrophages recognize and internalize TWEAK: potential consequences in atherosclerosis. Atherosclerosis. 2009;207:103–110. doi: 10.1016/j.atherosclerosis.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Hiraoka A, Horiike N, Akbar SMF, Michitaka K, Matsuyama T, Onji M. Soluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failure. J Gastroenterol. 2005;40:52–56. doi: 10.1007/s00535-004-1493-8. [DOI] [PubMed] [Google Scholar]
- 14.Vogel I, Goepfert AR, Møller HJ, Cliver S, Thorsen P, Andrews WW. Early mid-trimester serum relaxin, soluble CD163, and cervical length in women at high risk for preterm delivery. Am J Obstet Gynecol. 2006;195:208–214. doi: 10.1016/j.ajog.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Shabo I, Stål O, Olsson H, Doré S, Svanvik J. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int J Cancer. 2008;123:780–786. doi: 10.1002/ijc.23527. [DOI] [PubMed] [Google Scholar]
- 16.Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961–965. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curran RD, Billiar TR, Stuehr DJ, Hofmann K, Simmons RL. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. The J Exp Med. 1989;170:1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suffredini AF, Hochstein HD, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O: 113 endotoxin. J Infect Dis. 1999;179:1278–1282. doi: 10.1086/314717. [DOI] [PubMed] [Google Scholar]
- 19.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 20.Nakazaki H. Preoperative and postoperative cytokines in patients with cancer. Cancer. 1992;70:709–713. doi: 10.1002/1097-0142(19920801)70:3<709::aid-cncr2820700328>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Gough DB, Winstanley FP, Fearon KCH, Carter DC. Regulation of tumor necrosis factor production in healthy humans and in patients with cancer. Arch Surg. 1992;127:713–717. doi: 10.1001/archsurg.1992.01420060089013. [DOI] [PubMed] [Google Scholar]
- 22.Yoffe B, Noonan CA, Melnick JL, Hollinger FB. Hepatitis B virus DNA in mononuclear cells and analysis of cell subsets for the presence of replicative intermediates of viral DNA. J Infect Dis. 1986;153:471–477. doi: 10.1093/infdis/153.3.471. [DOI] [PubMed] [Google Scholar]
- 23.Baroja ML, Sirit FL, Caldera DJ, Toro FI, Zabaleta ME, Colmenares CJ, Bianco NE, Machado IV. Anti-CD3-activated T cells from chronic nonviremic HBV carriers are hyperreactive to monocytic accessory signals. Clin Immunol Immunopathol. 1993;69:180–188. doi: 10.1006/clin.1993.1168. [DOI] [PubMed] [Google Scholar]
- 24.Vázquez-Vela MEF, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39:715–728. doi: 10.1016/j.arcmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Hug C, Lodish HF. Visfatin: a new adipokine. Science. 2005;307:366–367. doi: 10.1126/science.1106933. [DOI] [PubMed] [Google Scholar]
- 26.Gea-Sorlí S, Closa D. Role of macrophages in the progression of acute pancreatitis. World J Gastrointest Pharmacol Ther. 2010;1:107. doi: 10.4292/wjgpt.v1.i5.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Alessio FR, Tsushima K, Aggarwal NR, Mock JR, Eto Y, Garibaldi BT, Files DC, Avalos CR, Rodriguez JV, Waickman AT. Resolution of experimental lung injury by monocyte-derived inducible nitric oxide synthase. J Immunol. 2012;189:2234–2245. doi: 10.4049/jimmunol.1102606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiyama H, Savill JS, Kitamura M, Zhao L, Stylianou E. Selective sensitization to tumor necrosis factor-α-induced apoptosis by blockade of NF-κB in primary glomerular mesangial cells. J Biol Chem. 1999;274:19532–19537. doi: 10.1074/jbc.274.28.19532. [DOI] [PubMed] [Google Scholar]


