Abstract
Littoral cell angioma (LCA) is an extremely rare benign splenic tumor with typical histomorphologic features, which is difficult to be distinguished preoperatively from other benign or malignant splenic tumors. It is a unique vascular tumor of the spleen, with abdominal pain or as an incidental finding when undergoing physical examination. In this paper, we reported three cases of LCA treated in our hospital. Their clinical symptoms, pathological features, clinical treatment as well as the prognosis are discussed.
Keywords: Littoral cell angioma, LCA, splenic tumor, vascular neoplasm
Introduction
Primary vessel tumors are the most common type of non-lymphomatous tumor of the spleen, most of which are benign, mainly arising from the blood vessels especially veins, and less commonly originating from red-pulp sinuses that are lined by littoral cells. The angioma originating from the cells of red-pulp sinuses have intermediate features between those of endothelial and macrophagic cells. Primary vessel tumors are characterized by expression of typical endothelial antigen [1].
Littoral cell angioma (LCA), first described by Falk et al in 1991 [2], is a rare vascular tumor of the spleen, which may be found in patients with abdominal pain or found by accident [3]. Till now, only a few cases of this tumor have been reported. In this paper, three cases of LCA diagnosed in our hospital were reported.
Materials and methods
Case presentation
Case 1
A 41-year-old man complaining of non-specific abdominal pain under no obvious inducement was admitted to hospital. He complained no weight loss, fever, emesis, or any changes in bowel habits, but only slight abdominal distension, which took place intermittently. Through abdominal ultrasound scanning, multiple hemangiomas were observed in the spleen, additionally, MRI detected multiple space-occupying lesions in the spleen. The patient received a total splenectomy and recovered two weeks later.
Case 2
A 62-year-old man had a sudden abdominal pain after intense exercise, and recovered 20 minutes later without any intervention. But the pain reoccurred after lunch, which was associated with sustained severe abdominal colic and intensified in the following five to six hours. Meanwhile, the patient also complained his back was uncomfortable. Then, he was diagnosed with ‘acute pancreatitis’ because of the increasing amylase level in the blood. The amylase level decreased to normal after treatment. However, a later blood test suggested thrombocytopenia, which had not been cured until recently. Abdominal MRI revealed diffuse signal abnormalities in the spleen (Figure 1). In addition, a cyst was found in the right lobe of the liver and the left kidney, separately. A total splenectomy was also performed.
Figure 1.
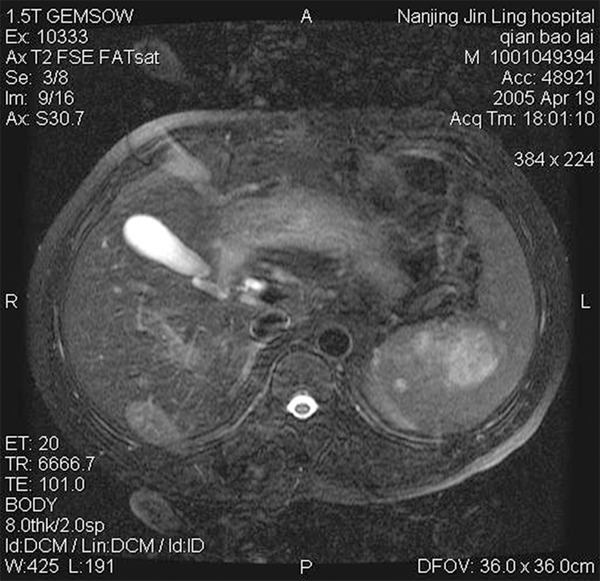
MRI show diffuse hypo-dense splenic masses.
Case 3
A 58-year-old man was admitted to our hospital for “30 years of splenomegalia, liver cirrhosis and abdominal distension”, and was clinically diagnosed of “high pressure of door pulse and liver cirrhosis”. CT showed splenomegalia and some focal calcification. Color Doppler ultrasound showed multiple strong echoes within the spleen, the largest of which was 4 × 5 mm. TIPSS surgery was operated and one week later “portaazygous devascularization and splenectomy cholecystectomy” was performed jointly on the patient.
Methods
For the above three cases, the fresh specimen were first fixed with 10% formaldehyde and then embedded in paraffin, followed by sectioning. The sections prepared were later stained with hematoxylin and eosin, and finally observed under a microscope. Meanwhile, immunohistochemistry staining was performed using CD8, CD68, FVIII lysozyme, CD31, CD34 and D2-40. Simultaneously, electron microscope observation was assisted.
Results
Gross pathologic findings
Gross pathologic examination of case 1 revealed that the spleen measured 15.5 × 13 × 5 cm, which was moderately enlarged. The cut surface of the spleen showed two sponge-like vascular spaces measuring 3.5 × 2 × 1.5 cm and 2.5 × 2.5 × 2 cm, being 5 cm and 2 cm away from the hilum respectively. The spleen of case 2 weighed 350 g and measured 13 × 10 × 4 cm, which was slightly enlarged. The cut surface was dark red and showed multifocal tiny nodules (Figure 2). In case three, the spleen was slightly enlarged, which weighed 420 g and measured 13.5 × 5 × 3 cm. The cut surface was gray red with medium texture, widened trabeculae and accompanied with calcification. Two grayish-red nodules measuring 1.8 cm and 0.8 cm in diameter were observed respectively.
Figure 2.
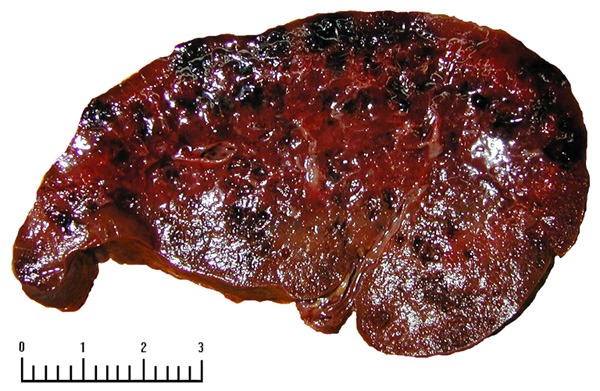
Multiple hemorrhagic focus and capsular space in the incisional surface.
Histological findings
Microscopically, the tumors had clear boundaries but no fibrous capsule. The lesions consisted of anastomosing vascular channels resembling splenic sinusoids (Figure 3). The channels had irregular lumina, with papillary pattern and cyst-like spaces, which were lined with endothelial cells that shaped cuboid or elongated and sometimes showed hemophagocytosis (Figure 4). Comparatively, the extension of blood vessels in case 3 was more obvious.
Figure 3.
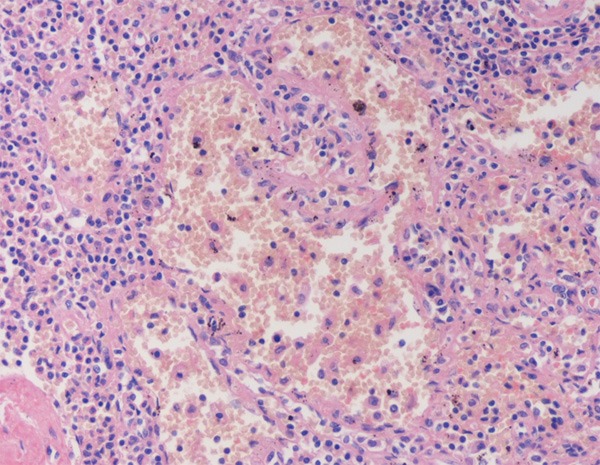
Large sinus-like anastomosing channels, with deciduous endothelial cells in the channel.
Figure 4.
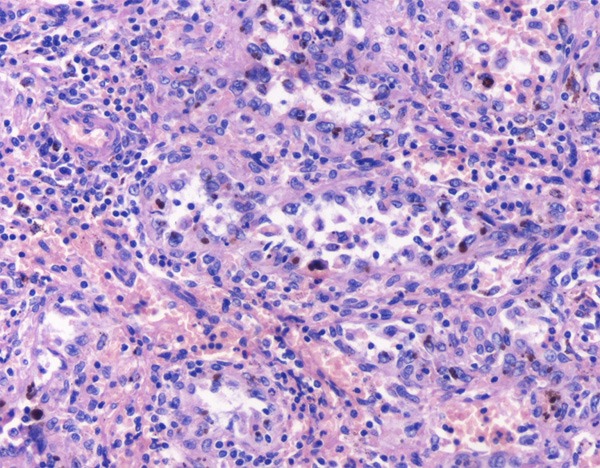
Anastomosing channels lined by tall and plump cells, showing micropapillary architecture in some regions, but without nuclear atypia or mitotic activity. Deposition of hemosiderin can be seen in some cells.
Immunohistochemical results showed that the littoral cells were strongly positive for CD31 (Figure 5), but negative for CD34 (Figure 6); some lining cells were weakly and focally positive for CD68 (Figure 7) and FVIII (Figure 8); CD8 and lysozyme were also detected in littoral cells. Periodic Acid-Schiff (PAS) staining was positive in many lining cells of blood vessels (Figure 9). The histological and immunohistochemical results confirmed the diagnosis of LCA consistently.
Figure 5.
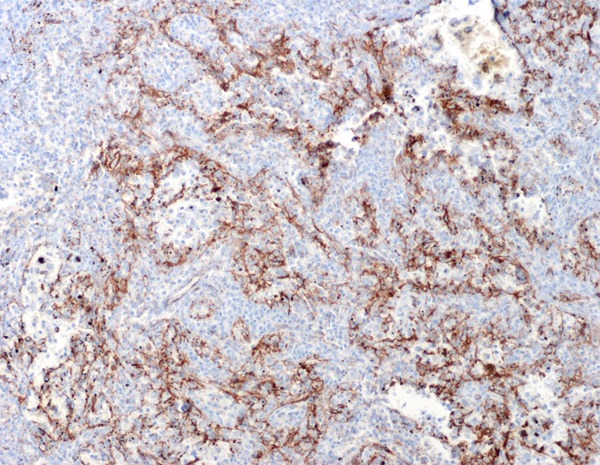
The anastomosing channels lined cells express antigen CD31.
Figure 6.
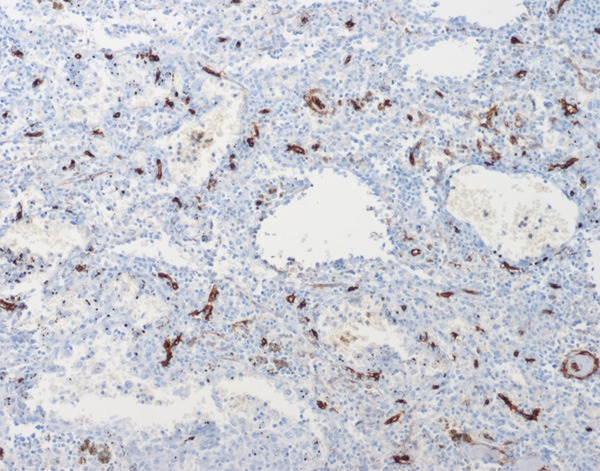
Endothelium of peripheral vessels express antigen CD34, but not the anastomosing channels lined cells.
Figure 7.
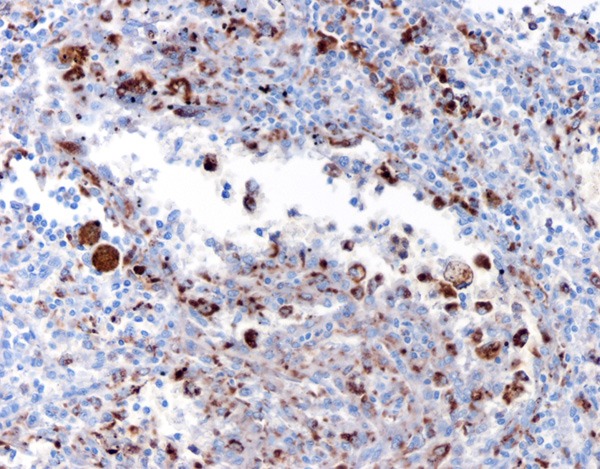
CD68 were positively expressed.
Figure 8.
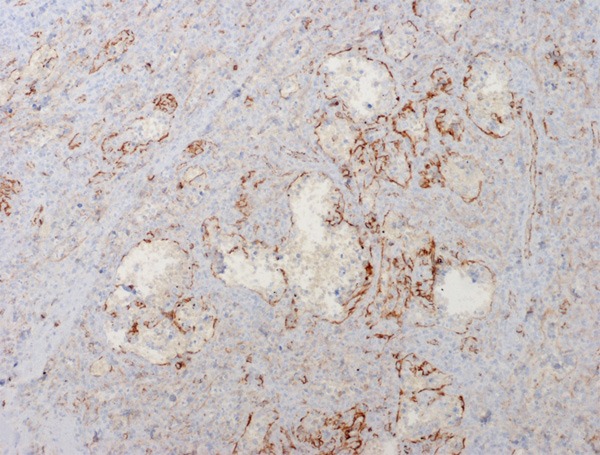
The anastomosing channels lined cells express antigen FVIII.
Figure 9.
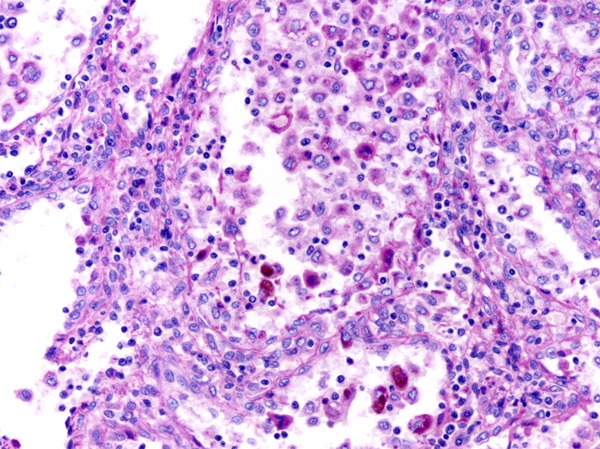
Some lined cells of channels were positive for PAS stain.
Electron microscope findings
By using electron microscope, it was observed that tumor cells were polygonal, and surrounded by blood vessels. No smooth muscle could be found outside the vessel wall, but just a layer of homogeneous, basilar membrane-like materials with medium electron density (Figure 10). Tumor cells were relatively large, with abundant cytoplasm. Irregular and thick cytoplasm was observed, as well as dysplastic desmosome-like structure between cells. The cytoplasm was rich of mitochondria, rough endoplasmic reticulum, and partly with plenty of glycogen (Figure 11). Bundles of intermediate filaments were distributed in some cells (Figure 12), and more notably, the cytoplasm was rich of lysosomes and lipofuscin bodies.
Figure 10.
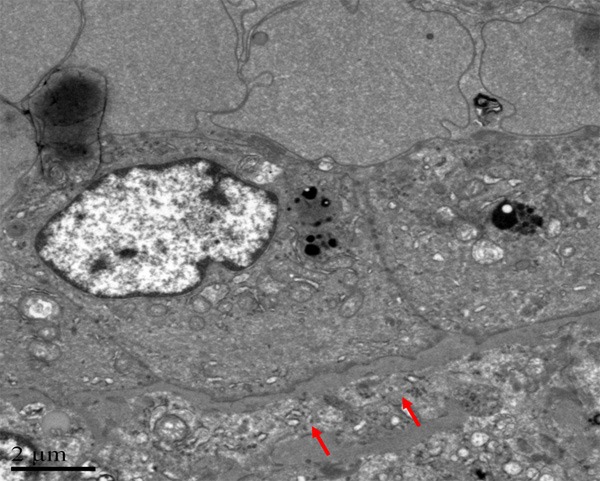
Electron microscopy, no smooth muscle can be found outside the vessel wall, but a layer of homogeneous, which is a kind of basilar membrane-like materials with medium electronics density.
Figure 11.
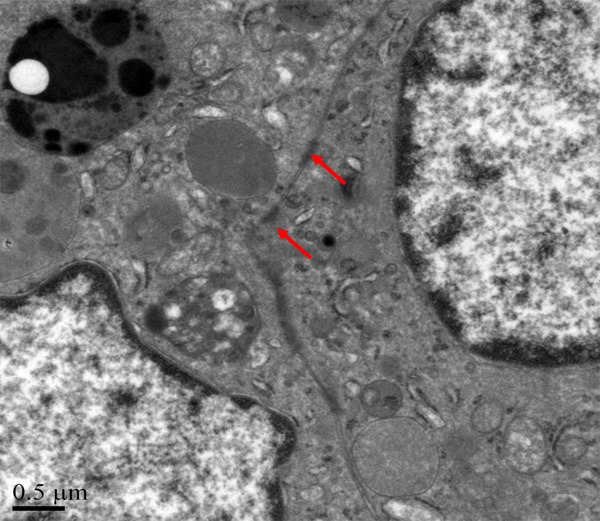
The cytoplasm is rich of mitochondria, rough endoplasmic, and partly with plenty of glycogen.
Figure 12.
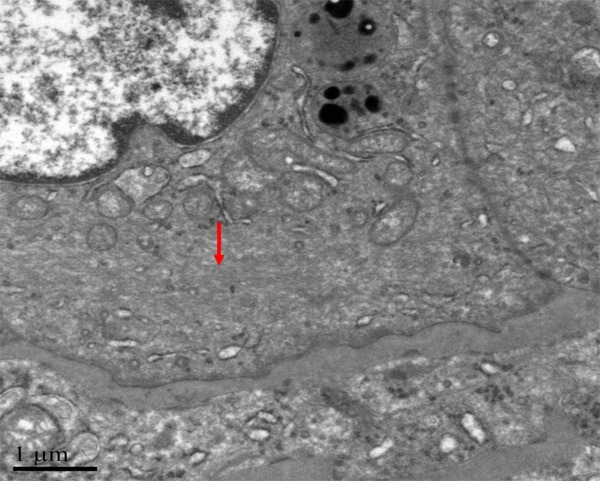
Bundles of intermediate filament distribute in some cells.
Discussion
LCA was first described by Falk et al early in 1991 [2]. In their study, 17 LCA patients, including eight males and nine females (3-77 years of age, median 49) were reported among the 200 benign splenic tumors, which presented different pathological features that had never been reported before. The lesions had nodules of various sizes, which were composed of vascular channels lined by endothelial cells expressing both endothelial (FVIII) and histiocytic antigens (normal endothelial cells only expressing endothelial antigens).
Histopathologically, LCA has morphologic and immunophenotypic features that reflect the dual endothelial and histiocytic potential of splenic sinus-lining cells, which is different from other angioma. Some exfoliated cells may also be found within the vascular spaces. The immunohistochemical profile of the lining cells is characterized by coexpression of both macrophage markers (CD68 and lysozyme), and some endothelial markers (FVIII and CD31) (while normal endothelial cells only express endothelial antigens). It has been proposed, based on the expression of CD68, that this tumor may have its origin in the lining cells or littoral cells of splenic sinus [4].
Littoral cell angioma affects both men and women equally without specific age predilection, but mostly occurs in middle-aged adults, and occasionally in children [4-6]. In this study, we report three cases of male patients in their middle ages. Patients in case 1, 2 and 3 showed the similar histological features. The lesions do not have a capsule but are well delineated from the surrounding splenic parenchyma. In most of the LCA cases, multiple nodules of varying sizes have been detected in the spleen [7,8], and focal angioma has also been reported in two of the cases [9,10]. Splenomegalia can be observed in almost all of the LCA patients, including the three cases reported in this article; additionally, splenomegalia is accompanied with thrombocytopenia in case 2; liver hepatitis and cirrhosis are also observed in case 2 and 3, separately.
At present, radiological methods can hardly achieve a definitive diagnosis of LCA because of its similar appearance to both benign splenic neoplasms, such as hemangiomatosis, lymphangiomatosis, hamartoma, hemangiopericytoma, hemangioendothelioma, and angiosarcoma, as well as malignant tumors such as metastasis, lymphoma, and Kaposi sarcoma [11]. Sonography is rarely helpful because the findings vary widely. The sonographic features that can be seen include heterogeneous echotexture without any definite nodules, or the lesions may appear isoechoic, hypoechoic, or hyperechoic [7]. MRI of the spleen may further help in the diagnosis by showing hypodense lesions on both T1-weighted and T2-weighted scans because of the hemosiderin content of the tumor cells.
Currently, the main treatment is open splenectomy or hand-assisted laparoscopic total splenectomy, which is necessary mainly because of the lesion’s large size or diffuse nature and the purpose to preserve tissue for histological analysis. As far as we know, there has been only one case of partial splenectomy reported till now [10], which was because the patient’s tumors were localized and could be removed completely for histological analysis. Except for the localized lesion, a pure laparoscopic procedure has not been advised, but it has been performed for other lesions of the spleen. The advantage of partial splenectomy lies in that it leaves the patient’s immune function intact, which is especially important for the patient.
Disclosure of conflict of interest
None.
References
- 1.Ziske C, Meybehm M, Sauerbruch T, Schmidt-Wolf IG. Littoral cell angioma as a rare cause of splenomegaly. Ann Hematol. 2001;80:45–48. doi: 10.1007/s002770000223. [DOI] [PubMed] [Google Scholar]
- 2.Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15:1023–1033. [PubMed] [Google Scholar]
- 3.Kutok JL, Fletcher CD. Splenic vascular tumors. Semin Diagn Pathol. 2003;20:128–139. doi: 10.1016/s0740-2570(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 4.Meybehm M, Fischer HP. Littoral zellangiosarkom der Milz Morphologische, immunhistochemische Befunde und Überlegungen zur Histogenese eines seltenen Milztumors. Pathologe. 1997;18:401–405. doi: 10.1007/s002920050233. [DOI] [PubMed] [Google Scholar]
- 5.Ertan G, Tekes A, Mitchell S, Keefer J, Huisman TA. Pediatric littoral cell angioma of the spleen: multimodality imaging including diffusion-weighted imaging. Pediatr Radiol. 2009;39:1105–1109. doi: 10.1007/s00247-009-1339-x. [DOI] [PubMed] [Google Scholar]
- 6.Antón-Pacheo J, Ayuso RMF, Cano I, Martinez MA, Cuadros J, Berchi FJ. Splenic littoral cell angioma in an infant. J Pediatr Surg. 2000;35:508–509. doi: 10.1016/s0022-3468(00)90225-2. [DOI] [PubMed] [Google Scholar]
- 7.Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24:1137–1163. doi: 10.1148/rg.244045006. [DOI] [PubMed] [Google Scholar]
- 8.Shah S, Wasnik A, Pandya A, Bude RO. Multimodality imaging findings in image-guided biopsy proven splenic littoral cell angioma: series of three cases. Abdom Imaging. 2011;36:735–738. doi: 10.1007/s00261-011-9697-x. [DOI] [PubMed] [Google Scholar]
- 9.Kranzfelder M, Bauer M, Richter T, Rudelius M, Huth M, Wagner P, Friess H, Stadler J. Littoral Cell Angioma and Angiosarcoma of the Spleen: Report of Two Cases in Siblings and Review of the Literature. J Gastrointest Surg. 2012;16:863–867. doi: 10.1007/s11605-011-1773-6. [DOI] [PubMed] [Google Scholar]
- 10.Mac New HG, Fowler CL. Partial splenectomy for littoral cell angioma. J Pediatr Surg. 2008;43:2288–2290. doi: 10.1016/j.jpedsurg.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 11.Gaetke-Udager K, Wasnik AP, Kaza RK, Al-Hawary MM, Maturen KE, Udager AM, Azar SF, Francis IR. Multimodality imaging of splenic lesions and the role of non-vascular, image-guided intervention. Abdom Imaging. 2014;39:570–587. doi: 10.1007/s00261-014-0080-6. [DOI] [PubMed] [Google Scholar]


